Control of Scar Tissue Formation in the Cornea: Strategies in Clinical and Corneal Tissue Engineering
Abstract
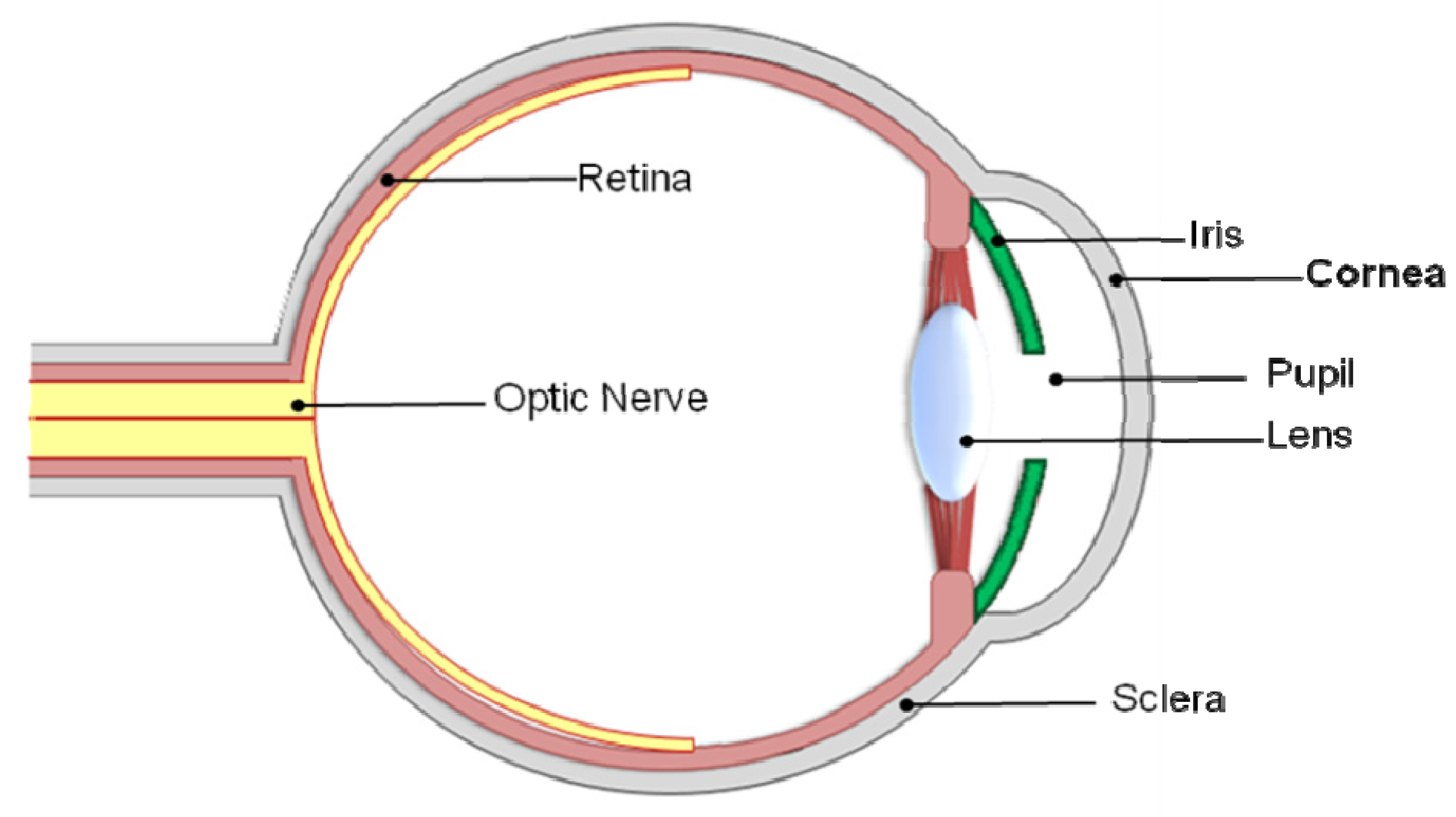
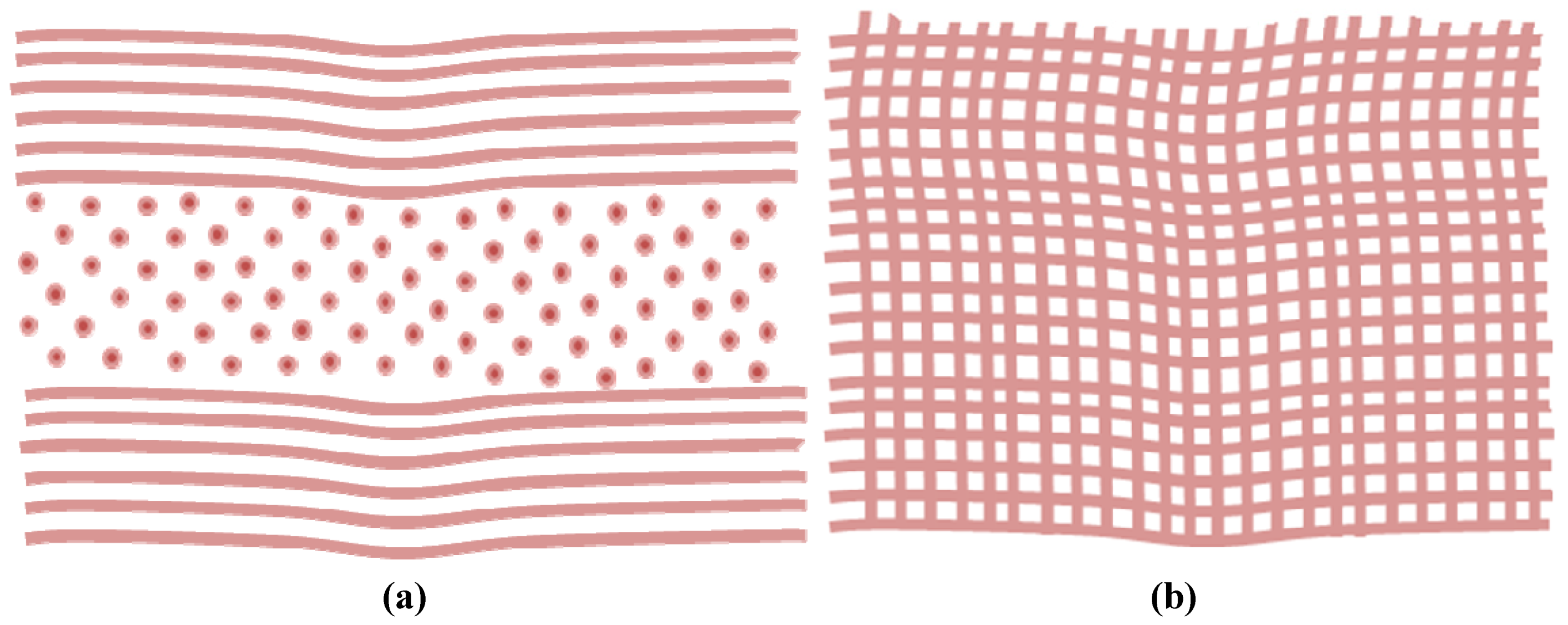
:1. The Importance and Correlation of Transparency and Scar Tissue

2. Diseases Linking to Scar Tissue Formation in the Cornea
| Disease | Epidemiology | Causes | Treatment | Further information |
|---|---|---|---|---|
| Fuchs dystrophy | Deterioration of endothelial cells. | |||
| Loss in efficiency of pumping water from stroma. | ||||
| Swelling and distortion of cornea. | Thought to be inherited, autosomal dominant trait [32]. | Salt solutions such as sodium chloride drops or ointment are often prescribed to draw fluid from the cornea and reduce swelling. | Short-term success with transplantation, but long-term survival is a problem. | |
| Changes in the cornea’s curvature. | Gene mutation strongly suspected. | Contact lenses. | Cannot be cured. | |
| Hazing. | Hair dryer to dry out corneal blisters. | |||
| Tiny blisters on corneal surface. | ||||
| Glare and light sensitivity. | ||||
| Usually affects both eyes. | ||||
| Ocular trauma and ulceration | Unilateral vision loss. | |||
| Ulceration. | Mechanical trauma, debris entering the eye, chemical and thermal burns. | |||
| Corneal perforation Endophthalmitis. | Workplace activities, such as mining injuries, agriculture and warfare. | Corneal transplant. | Ocular traumas are becoming more prevalent causes of scarring and blindness. | |
| Phthisis. | Road accidents [33]. | Antibiotic and antifungal treatments although visual outcome usually poor [30]. | Worldwide, half a million people are blind as the result of trauma [30,33]. | |
| Blindness. | Domestic accidents [33]. | |||
| Hyphaemas [33]. | ||||
| Ruptured globes. | ||||
| Opthalmia Neonatorum (conjunctivitis of the newborn) | Bilateral scarring. | Infection caused by Neisseria gonorrhoeae. | Saline washes. | Blindness risk is reduced when opthalmia neonatorum is caused by less virulent pathogens such as Chlamydia trachomatis [30]. |
| Blindness. | Herpes simplex virus (HSV) can also cause childhood corneal blindness by causing opthalmia and xerophthalmia, although such infections are infrequent in infants. | Antibacterial eye ointments. | ||
| Affects both eyes. | Treatments with tetracyanite/erythromycin/silver nitrate ointments. | |||
| Stevens-Johnson Syndrome (SJS) also known as Erytheme multiform | Subepithelial bullae. | Withdrawal of all potential causative drugs [35]. | ||
| Scarring. | Drugs including sulphonamides, anticonvulsants, salicytes, NSAIDs, penicillin [34]. | Intravenous fluid replacement. | <100 drugs associated with SJS [34]. | |
| Keratinocyte apoptosis of the surrounding skin and epidermal necrolysis. | Infection, such as HSV. | Immunosupressive therapy. | Most severe cases referred to as toxic epidermal necrolysis (TEN) | |
| Erosion of mucous membranes [34]. | Streptociocci, adenovirus and microplasma [35]. | Corneal stem cell transplant. | Transplanted tissue often rejected. | |
| Scarring. | Corneal transplant. | |||
| Bilateral blindness [35]. | ||||
| Xerophthalmia (dry eye syndrome) | Night blindness. | 70% of cases are due to a vitamin A deficiency [30,36]. | Artificial tears. | |
| Xerosis. | Increase humidity of surroundings. | Xerophthalmia patients are predominantly infants or young children, with a peak age of approximately 2.5 years [36]. | ||
| Corneal perforation. Scarring. | Vitamin A supplementation. | All but disappeared in Western Europe [36]. | ||
| Irreversible blindness [36]. | ||||
| Trachoma | Vascularization. | Bacterial infection trachoma caused by Chlamydia trachomatis [30]. | Corneal transplant. | World’s leading cause of ocular morbidity and blindness [30]. |
| Ocular surface problems Entropion. | Infection can be transmitted from eye to eye via contaminated fingers, clothes, make-up and flies. | The disease is preventable via antibiotic treatment with azithromycin [37]; however more antibiotic treatment is still required to prevent further progression of the infection to corneal blindness in previously infected individuals [30]. | ||
| Trichiasis. | ||||
| Onchocerciasis (river blindness) | Destructive chorioretinitis. Blinding keatitis | Caused by a parasite Onchocerca volvulus [30]. | Invermectin kills the microfilaria (larval form) and sterilizes the adult worm to prevent spread in infected individuals. | Incidences of onchoceriasis have decreased since the introduction of invermectin in the 1980s. |
| Acute corneal scarring. | Collagenase secretion, caused by the influx of inflammatory cells is believed to be responsible for the rapid destruction of the xerophthalmic cornea [36]. | |||
| Vascularization. | ||||
| Leprosy | Blindness. | Mycobacterium laprae usually affects the anterior segment of the eye. | Multi drug therapy using dapsone, rifampicin and clofazamine. | Corneal complications caused by leprosy are a significant cause of corneal blindness globally affecting 250,000 people, predominantly in Africa and Southern India. |
| Chronic uveitis. | ||||
| Cataract formation. Exposure keratitis. Reoccurring corneal ulcers. Corneal scarring. | ||||
| Vascularization. |
3. Mechanisms of Wound Healing in the Cornea
3.1. Regeneration
3.2. Scar Formation
4. Effectors to Control the Outcome of Corneal Wound Healing
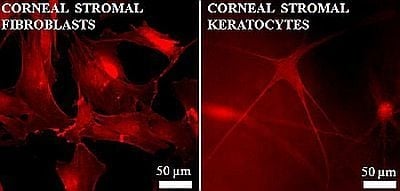
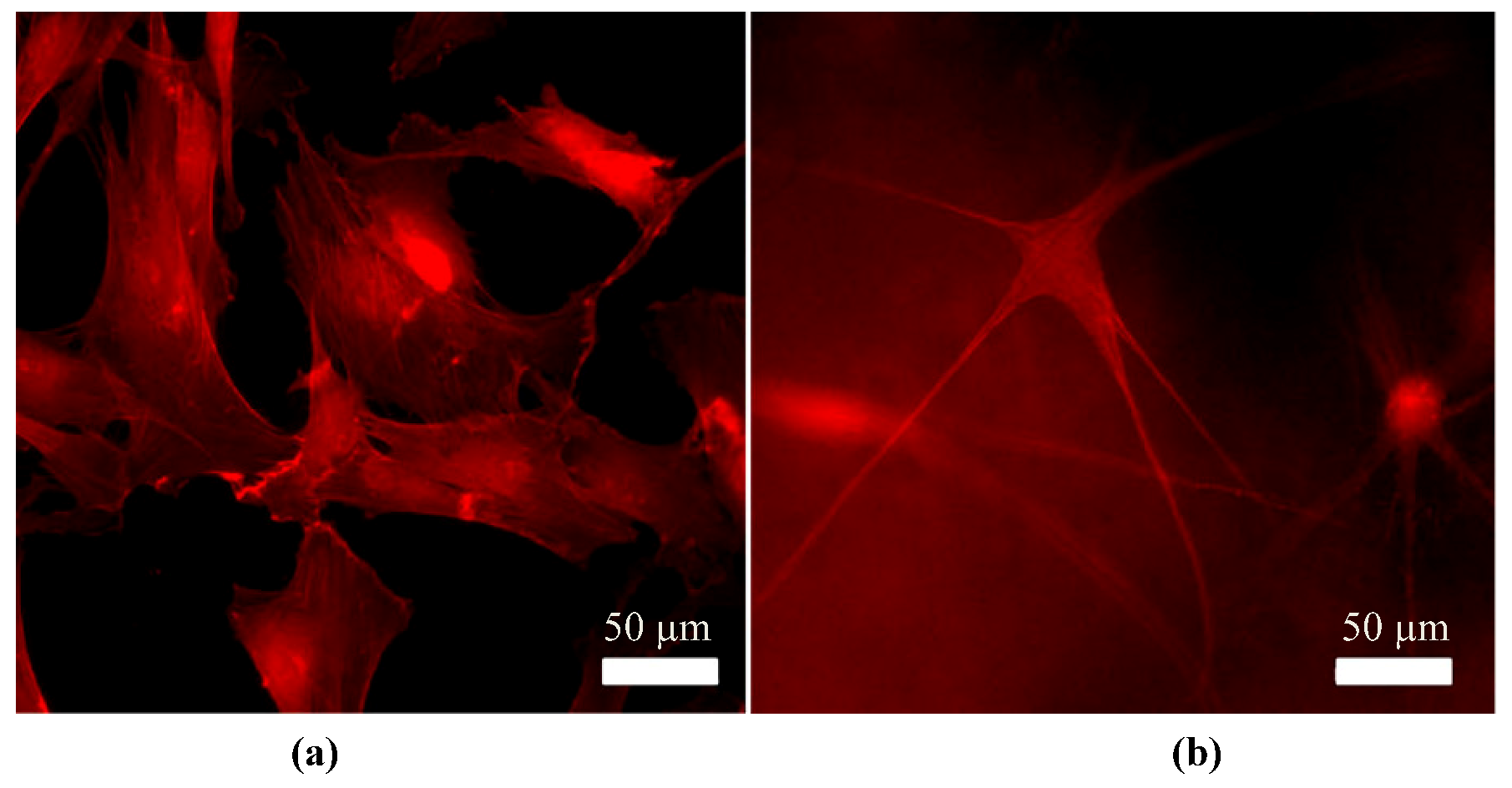
4.1. Phenotype Differentiation in the Corneal Stroma
4.2. Growth Factors
4.3. Matrix Components
4.4. Mechanical Stress
5. Refractive Surgery and Scar Tissue
5.1. Refractive Surgery Techniques
5.2. Associated Scar Tissue Formation
5.3. Summary
6. Corneal Grafts and Corneal Tissue Engineering
6.1. Corneal Allografting
6.2. Xenografts
6.3. Keratoprostheses
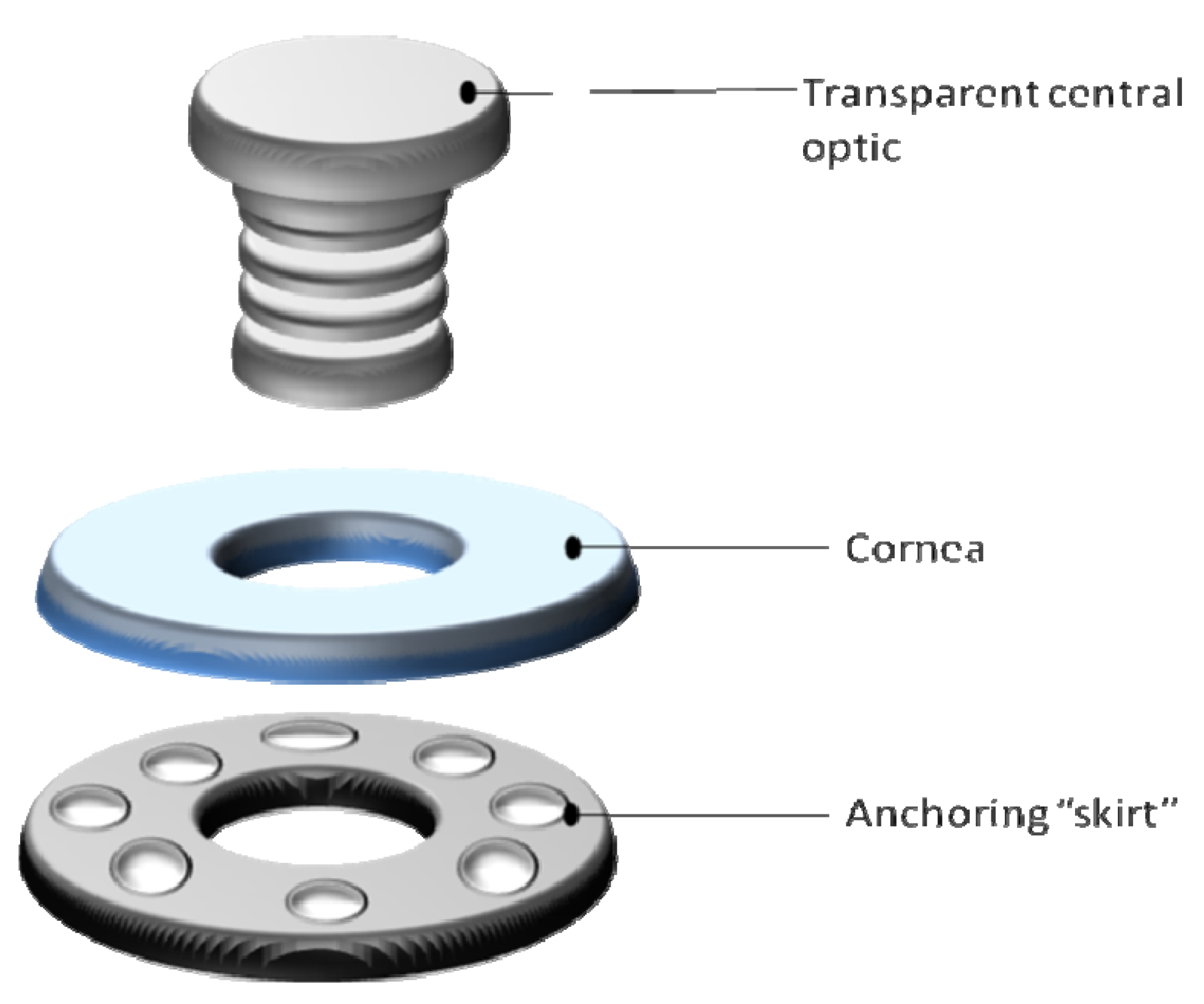
6.4. Tissue Engineered Corneas
6.4.1. Manipulation of Chemical Cues
6.4.2. Utilizing Topographic Cues
6.4.3. Micro- and Nano-Patterning
6.4.4. Magnetically Aligned Collagen
6.4.5. Electrospinning of Nanofibers
6.4.6. Co-Culture Approaches
6.4.7. Growth Factors
6.5. Summary
7. Conclusions
8. Perspectives
Acknowledgements
References
- Ruberti, J.W.; Zieske, J.D.; Trinkaus-Randall, V. Corneal-tissue replacement. In Principles of Tissue Engineering, 3rd; Lanza, R.P., Langer, R., Vacanti, J., Eds.; Elsevier Academic Press: Waltham, MA, USA, 2007; pp. 1025–1066. [Google Scholar]
- Anderson, K.; El-Sheikh, A.; Newson, T. Application of structural analysis to the mechanical behaviour of the cornea. J. R. Soc. Interface 2004, 1, 3–15. [Google Scholar] [CrossRef]
- Fullwood, N.J. Collagen fibril orientation and corneal curvature. Structure 2004, 12, 169–170. [Google Scholar]
- Levin, A.; Nilsson, F.E.; ver Hoeve, J.; Wu, S.; Kaufman, P.L.; Alm, A. Alder's Physiology of the Eye, 11th ed; Saunders Elsevier: Philadelphia, PA, USA, 2011; pp. 1–40. [Google Scholar]
- Griffith, M.; Osborne, R.; Munger, R.; Xiong, X.J.; Doillon, C.J.; Laycock, N.L.C.; Hakim, M.; Song, Y.; Watsky, M.A. Functional human corneal equivalents constructed from cell lines. Science 1999, 286, 2169–2172. [Google Scholar] [CrossRef]
- McLaughlin, C.R.; Tsai, R.J.F.; Latorre, M.A.; Griffith, M. Bioengineered corneas for transplantation and in vitro toxicology. Front. Biosci. 2009, 14, 3326–3337. [Google Scholar]
- Glass, D.H.; Roberts, C.J.; Litsky, A.S.; Weber, P.A. A viscoelastic biomechanical model of the cornea describing the effect of viscosity and elasticity on hysteresis. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3919–3926. [Google Scholar] [CrossRef]
- Ahearne, M. Mechanical characterisation of cornea and corneal stromal equivalents.
- Ruberti, J.W.; Zieske, J.D. Prelude to corneal tissue engineering—Gaining control of collagen organization. Prog. Retin. Eye Res. 2008, 27, 549–577. [Google Scholar] [CrossRef]
- West-Mays, J.A.; Dwivedi, F.A.U.; Dhruva, J.; Dwivedi, D.J. The keratocyte, corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1652–1631. [Google Scholar]
- Sutphin, J.E. External Disease and Cornea. In 2011–2012 Basic and Clinical Science Course, 1st ed; American Academy of Opthalmology: San Fransisco, CA, USA, 2011. [Google Scholar]
- Germain, L.; Carrier, P.; Auger, F.A.; Salesse, C.; Guerin, S.L. Can we produce a human corneal equivalent by tissue engineering? Prog. Retin. Eye Res. 2000, 19, 497–527. [Google Scholar] [CrossRef]
- Musselmann, K. Developing culture conditions to study keratocyte phenotypes in Vitro.
- Jakus, M. Studies on the cornea. II. The fine structure of Descemet’s membrane. J. Biophys. Biochem. Cytol. 1956, 2, 243–252. [Google Scholar] [CrossRef]
- Kaufman, D.S.; Hanson, E.T.; Lewis, R.L.; Auerbach, R.; Thomson, J.A. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 10716–10721. [Google Scholar]
- Meek, K.M.; Boote, C. The use of x-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog. Retin. Eye Res. 2009, 28, 369–392. [Google Scholar] [CrossRef]
- Kim, A.; Zhou, C.; Lakshman, N.; Petrol, W.M. Corneal stromal cells use both high- and low-contractility migration mechanisms in 3-D collagen matrices. Exp. Cell Res. 2012, 318, 741–752. [Google Scholar] [CrossRef]
- Wray, L.S.; Orwin, E.J. Recreating the microenvironment of the native cornea for tissue engineering applications. Tissue Eng. Part A 2009, 15, 1463–1472. [Google Scholar] [CrossRef]
- Bron, A. The architecture of the corneal stroma. Br. J. Ophthalmol. 2001, 85, 379–381. [Google Scholar] [CrossRef]
- Skuta, G.L.; Cantor, L.B.; Weiss, J.S. Refractive Surgery, Section 13 Basic and Clinical Science Course, 1st ed; American Academy of Opthamology: San Fransisco, CA, USA, 2011; pp. 2–44. [Google Scholar]
- Boote, C.; Dennis, S.; Huang, Y.F.; Quantock, A.J.; Meek, K.M. Lamellar orientation in human cornea in relation to mechanical properties. J. Struct. Biol. 2005, 149, 1–6. [Google Scholar] [CrossRef]
- Lim, M.; Ye, H.; Panoskaltsis, N.; Drakakis, E.M.; Yue, X.C.; Cass, A.E.G.; Radomska, A.; Mantalaris, A. Intelligent bioprocessing for haemotopoietic cell cultures using monitoring and design of experiments. Biotechnol. Adv. 2007, 25, 353–368. [Google Scholar] [CrossRef]
- Dupps, W.J.; Wilson, S.E. Biomechanics and wound healing in the cornea. Exp. Eye Res. 2006, 83, 709–720. [Google Scholar] [CrossRef]
- Meek, K.M.; Boote, C. The organization of collagen in the corneal stroma. Exp. Eye Res. 2004, 73, 503–512. [Google Scholar] [CrossRef]
- Maurice, D.M. Mechanics of the cornea. In The Cornea, Transactions of the World Congress on the Cornea III, 8th; Cavanagh, H.D., Ed.; Raven Press: New York, NY, USA, 1988; pp. 187–193. [Google Scholar]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggieo, F.; Hulmes, D.J.S. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Q. An active artificial cornea with the function of inducing new corneal tissue generation in vivo—A new approach to corneal tissue engineering. Biomed. Mater. 2007, 2, 121–125. [Google Scholar] [CrossRef]
- Allan, B. Artificial corneas—Risks of complications are high now, but better materials are on the way. Br. Med. J. 1999, 318, 821–822. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Li, F.; Shimmura, S.; Griffith, M. Bioengineered corneas, how close are we? Curr. Opin. Ophthalmol. 2003, 14, 192–197. [Google Scholar] [CrossRef]
- Whitcher, J.; Srinivasan, M.; Upadhyay, M. Corneal blindness, a global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Miller, S.J.H. Parsons’ Diseases of the Eye, 18th ed; Churchill Livingstone, Elsevier: Philadelphia, PA, USA, 2002. [Google Scholar]
- Gottsch, J.D.; Bowers, A.L.; Margulies, E.H.; Seitzman, G.D.; Kim, S.W.; Saha, S.; Jun, A.S.; Stark, W.J.; Liu, S.H. Serial analysis of gene expression in the corneal endothelium of Fuchs’ dystrophy. Invest. Ophthalmol. Vis. Sci. 2003, 44, 594–599. [Google Scholar] [CrossRef]
- Thylefors, B. Epidemiologic patterns of ocular trauma. Aust. N. Z. J. Ophthalmol. 1992, 20, 95–98. [Google Scholar] [CrossRef]
- Roujeau, J.; Kelly, J.; Naldi, L.; Rzany, B.; Stern, R.; Anderson, T.; Auquier, A.; Bastujigarins, S.; Correia, O.; Locati, F.; Mockenhaupt, M.; Paoletti, C.; Shapiro, S.; Shear, N.; Schopf, E.; Kaufman, D. Medication use and the risk of stevens-johnson syndrome or toxic epidermal necrolysis. N. Engl. J. Med. 1995, 333, 1600–1607. [Google Scholar]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Sotozono, C.; Kinoshita, S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 2001, 108, 1569–1574. [Google Scholar] [CrossRef]
- Pirie, A. Xerophthalmia. Invest. Ophthalmol. 1976, 15, 417–422. [Google Scholar]
- Kanski, J.J. Clinical Ophthalmology, ASystematic Approach, 6th ed; Elsevier Health Sciences: Philadelphia, PA, USA, 2007. [Google Scholar]
- Etheredge, L.; Kane, B.P.; Hassell, J.R. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3128–3136. [Google Scholar] [CrossRef]
- Steele, C. Corneal wound healing: A review. Optom. Today 1999, 40, 28–32. [Google Scholar]
- Agrawal, V.B.; Tsai, R.J.F. Corneal epithelial wound healing. Indian J. Ophthalmol. 2003, 51, 5–15. [Google Scholar]
- Eraslan, M.; Toker, E. Mechanisms of corneal wound healing and its modulation follwing refractive surgery. Marmara Med. J. 2009, 22, 169–178. [Google Scholar]
- Zieske, J.; Takahashi, H.; Hutcheon, A.; Dalbone, A. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1346–1355. [Google Scholar]
- Fini, M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 1999, 18, 529–551. [Google Scholar] [CrossRef]
- Mulholland, B.; Tuft, S.; Khaw, P. Matrix metalloproteinase distribution during early corneal wound healing. Eye 2005, 19, 584–588. [Google Scholar] [CrossRef]
- Wang, L.; Ko, C.; Meyers, E.E.; Pedroja, B.S.; Pelaez, N.; Bernstein, A.M. Concentration-dependent effects of transforming growth factor beta 1 on corneal wound healing. Mol. Vis. 2011, 17, 2835–2846. [Google Scholar]
- Gabison, E.E.; Huet, E.; Baudouin, C.; Menashi, S. Direct epithelial-stromal interaction in corneal wound healing: Role of EMMPRIN/CD147 in MMPs induction and beyond. Prog. Retin. Eye Res. 2009, 28, 19–33. [Google Scholar] [CrossRef]
- Tuft, S.; Gartry, D.; Rawe, I.; Meek, K. Photorefractive keratectomy—Implications of corneal wound-healing. Br. J. Ophthalmol. 1993, 77, 243–247. [Google Scholar] [CrossRef]
- Ainscough, S.L.; Linn, M.L.; Barnard, Z.; Schwab, I.R.; Harkin, D.G. Effects of fibroblast origin and phenotype on the proliferative potential of limbal epithelial progenitor cells. Exp. Eye Res. 2011, 92, 10–19. [Google Scholar] [CrossRef]
- Bernstein, A.M.; Twining, S.S.; Warejcka, D.J.; Tall, E.; Masur, S.K. Urokinase receptor cleavage, a crucial step in 14 fibroblast-to-myofibroblast differentiation. Mol. Biol. Cell 2007, 18, 2716–2727. [Google Scholar] [CrossRef]
- Akhtar, S.; Schonthaler, H.B.; Bron, A.J.; Dahm, R. Formation of stromal collagen fibrils and proteoglycans in the developing zebrafish cornea. Acta Ophthalmol. 2008, 86, 655–665. [Google Scholar] [CrossRef]
- Quantock, A.J.; Young, R.D. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev. Dyn. 2008, 237, 2607–2621. [Google Scholar] [CrossRef]
- Baldwin, H.; Marshall, J. Growth factors in corneal wound healing following refractive surgery, a review. Acta Ophthalmol Scand 2002, 80, 238–247. [Google Scholar] [CrossRef]
- Schultz, G.; Khaw, P.; Oxford, K.; Macauley, S.; Vansetten, G.; Chesini, N. Growth-factors and ocular wound-healing. Eye 1994, 8, 184–187. [Google Scholar] [CrossRef]
- Kamma-Lorge, C.S.; Boote, C.; Hayes, S.; Albon, J.; Boulton, M.E.; Meek, K.M. Collagen ultrastructural changes during stromal wound healing in organ cultured bovine corneas. Exp. Eye Res. 2009, 88, 953–959. [Google Scholar] [CrossRef]
- Jester, J.V.; Jin, H.C. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef]
- Builles, N.; Bechetoille, N.; Justin, V.; Ducerf, A.; Auxenfans, C.; Burillon, C.; Sergent, M.; Damour, O. Development of an optimised culture medium for keratocytes in monolayer. Biomed. Mater. Eng. 2006, 16, 95–104. [Google Scholar]
- Berryhill, B.L.; Kader, R.; Kane, B.; Birk, D.E.; Teng, J.; Hassell, A.R. Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest. Ophthalmol. Vis. Sci. 2002, 43, 3416–3421. [Google Scholar]
- Pei, Y.; Sherry, D.M.; McDermott, A.M. Thy-1 distinguishes human corneal fibroblasts and myofibroblasts from keratocytes. Exp. Eye Res. 2004, 79, 705–712. [Google Scholar] [CrossRef]
- Jester, J.V.; Petroll, W.M.; Barry, P.A.; Cavanagh, H.D. Expression of alpha-smooth muscle (alpha-sm) actin during corneal stromal wound-healing. Invest. Ophthalmol. Vis. Sci. 1995, 36, 809–819. [Google Scholar]
- Matsuda, S.; Hisama, M.; Shibayama, H.; Itou, N.; Iwaki, M. Application of the reconstructed rabbit corneal epithelium model to assess the in vitro eye irritancy test of chemicals. J. Pharm. Soc. Jpn. 2009, 129, 1113–1120. [Google Scholar] [CrossRef]
- Garana, R.; Petroll, W.; Chen, W.; Herman, I.; Barry, P.; Andrews, P.; Cavanagh, H.; Jester, J. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest. Ophthalmol. Vis. Sci. 1992, 33, 3271–3282. [Google Scholar]
- Beales, M.P.; Funderburgh, J.L.; Jester, J.V.; Hassell, J.R. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts, Maintenance of the keratocyte phenotype in culture. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1658–1663. [Google Scholar]
- Funderburgh, J.L.; Mann, M.M.; Funderburgh, M.L. Keratocyte phenotype mediates proteoglycan structure—A role for fibroblasts in corneal fibrosis. J. Biol. Chem. 2003, 278, 45629–45637. [Google Scholar] [CrossRef]
- Helary, C.; Ovtracht, L.; Coulomb, B.; Godeau, G.; Giraud-Guille, M.M. Dense fibrillar collagen matrices, A model to study myofibroblast behaviour during wound healing. Biomaterials 2006, 27, 4443–4452. [Google Scholar] [CrossRef]
- Nakamura, K.; Kurosaka, D.; Yoshino, M.; Oshima, T.; Kurosaka, H. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2002, 4, 2603–2608. [Google Scholar]
- Netto, M.V.; Mohan, R.R.; Sinha, S.; Sharma, A.; Dupps, W.; Wilson, S.E. tromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res. 2006, 82, 788–797. [Google Scholar] [CrossRef]
- Wilson, S.L.; Wimpenny, I.; Ahearne, M.; Rauz, S.; El Haj, A.J.; Yang, Y. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv. Func. Mater. 2012, 22, 3641–3649. [Google Scholar] [CrossRef]
- Lakshman, N.; Petroll, W.M. Growth Factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1077–1086. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Klenkler, B.; Sheardown, H. Growth factors in the anterior segment: Role in tissue maintenance, wound healing and ocular pathology. Exp. Eye Res. 2004, 79, 677–688. [Google Scholar] [CrossRef]
- Karamichos, D.; Hutcheon, A.E.K.; Zieske, J.D. Transforming growth factor-beta 3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J. Tissue Eng. Regen. Med. 2011, 5, 228–238. [Google Scholar] [CrossRef]
- Carrington, L.; Albon, J.; Anderson, I.; Kamma, C.; Boulton, M. Differential regulation of key stages in early corneal wound healing by TGF-beta Isoforms and their inhibitors. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1886–1894. [Google Scholar] [CrossRef]
- Petroll, W.; Jester, J.; Bean, J.; Cavanagh, H. Myofibroblast transformation of cat corneal endothelium by transforming growth factor-beta(1), -beta(2), and -beta(3). Invest. Ophthalmol. Vis. Sci. 1998, 39, 2018–2032. [Google Scholar]
- Funderburgh, J.; Funderburgh, M.; Mann, M.; Corpuz, L.; Roth, M. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J. Biol. Chem. 2001, 276, 44173–44178. [Google Scholar]
- Karamichos, D.; Guo, X.Q.; Hutcheon, A.E.K.; Zieske, J.D. Human corneal fibrosis: An in vitro model. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1382–1388. [Google Scholar]
- Grinnell, F. Fibroblast mechanics in three-dimensional collagen matrices. J. Bodyw. Mov. Ther. 2008, 12, 191–193. [Google Scholar] [CrossRef]
- Wilson, S.E.; Liu, J.J.; Mohan, R.R. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 1999, 18, 293–309. [Google Scholar] [CrossRef]
- Bussolino, F.; Direnzo, M.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P. Hepatocyte Growth-factor is a potent angiogenic factor which stimulates endothelial-cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef]
- Carlson, E.; Wang, I.; Liu, C.; Brannan, P.; Kao, C.; Kao, W. Altered KSPG expression by keratocytes following corneal injury. Mol. Vis. 2003, 9, 615–623. [Google Scholar]
- Hayashi, Y.; Call, M.K.; Chikama, T.; Liu, H.; Carlson, E.C.; Sun, Y.; Pearlman, E.; Funderburgh, J.L.; Babcock, G.; Liu, C.; Ohashi, Y.; Kao, W.W. Lumican is required for neutrophil extravasation following corneal injury and wound healing. J. Cell Sci. 2010, 123, 2987–2995. [Google Scholar] [CrossRef]
- Mohan, R.R.; Tovey, J.C.K.; Gupta, R.; Sharma, A.; Tandon, A. Decorin Biology, Expression, Function and Therapy in the Cornea. Curr. Mol. Med. 2011, 11, 110–128. [Google Scholar] [CrossRef]
- Funderburgh, J.; Hevelone, N.; Roth, M.; Funderburgh, M.; Rodrigues, M.; Nirankari, V.; Conrad, G. Decorin and biglycan of normal and pathologic human corneas. Invest. Ophthalmol. Vis. Sci. 1998, 39, 1957–1964. [Google Scholar]
- Mohan, R.R.; Gupta, R.; Mehan, M.K.; Cowden, J.W.; Sinha, S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp. Eye Res. 2010, 91, 238–245. [Google Scholar] [CrossRef]
- Cintron, C.; Hong, B.; Covington, H.; MacCarak, E. Heterogeneity of collagens in Rabbit cornea-type-III collagen. Invest. Ophthalmol. Vis. Sci. 1988, 29, 767–775. [Google Scholar]
- Conrad, G.; Dessau, W.; VonDerMark, K. Synthesis of Type-Iii collagen by fibroblasts from the embryonic chick cornea. J. Cell. Biol. 1980, 84, 501–512. [Google Scholar] [CrossRef]
- Ahearne, M.; Liu, K.; El Haj, A.J.; Then, K.K.; Rauz, S.; Yang, Y. Online monitoring of the mechanical behavior of collagen hydrogels, influence of corneal fibroblasts on elastic modulus. Tissue Eng. Part C Methods 2010, 16, 319–327. [Google Scholar] [CrossRef]
- Ahearne, M.; Siamantouras, E.; Yang, Y.; Liu, K.K. Mechanical characterization of biomimetic membranes by micro-shaft poking. J. R. Soc. Interface 2009, 6, 471–478. [Google Scholar]
- Ahearne, M.; Yang, Y.; Then, K.Y.; Liu, K.K. An indentation technique to characterize the mechanical and viscoelastic properties of human and porcine corneas. Ann. Biomed. Eng. 2007, 35, 1608–1616. [Google Scholar] [CrossRef]
- Vrana, N.E.; Elsheikh, A.; Builles, N.; Damour, O.; Hasirci, V. Effect of human corneal keratocytes and retinal pigment epithelial cells on the mechanical properties of micropatterned collagen films. Biomaterials 2007, 28, 4303–4310. [Google Scholar] [CrossRef]
- Komai, Y.; Ushiki, T. The 3-dimensional organization of collagen fibrils in the human cornea and sclera. Invest. Ophthalmol. Vis. Sci. 1991, 32, 2244–2258. [Google Scholar]
- O’Keefe, M.; Kirwan, C. Laser epithelial keratomileusis in 2010—A review. Clin. Exp. Ophthalmol. 2010, 38, 183–191. [Google Scholar] [CrossRef]
- Trokel, S.; Srinivasan, R.; Braren, B. Excimer Laser-Surgery of the Cornea. Am J Ophthalmol 1983, 96, 710–715. [Google Scholar]
- Pang, K.; Du, L.; Wu, X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials 2010, 31, 7257–7265. [Google Scholar] [CrossRef]
- Niederkorn, J. The immune privilege of corneal grafts. J. Leukoc. Biol. 2003, 74, 167–171. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Karimian, F.; Javadi, M.A.; Jafarinasab, M.R.; Nowroozpour, K.; Hosseini, M.; Anisian, A. Corneal graft rejection, incidence and risk factors. Iran. J. Opthalimis Res. 2007, 2, 7–14. [Google Scholar]
- Coster, D.J.; Jessup, C.F.; Williams, K.A. Mechanisms of corneal allograft rejection and regional immunosuppression. Eye 2009, 23, 1894–1897. [Google Scholar] [CrossRef]
- Boneva, R.S.; Folks, T.M. Xenotransplantation and risks of zoonotic infections. Ann. Med. 2004, 36, 504–517. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, C.; Jie, Y.; Wang, N.; Wang, L. WZS-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation 2007, 14, 603–611. [Google Scholar] [CrossRef]
- Larkin, D.F.P.; Williams, K.A. The host response in experimental corneal xenotransplantation. Eye 1995, 9, 254–260. [Google Scholar] [CrossRef]
- Zerbe, B.L.; Belin, M.W.; Ciolino, J.B. Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter boston type 1 keratoprosthesis study. Ophthalmology 2006, 113, 1779–1784. [Google Scholar] [CrossRef]
- Pintucci, S.; Pintucci, F.; Cecconi, M.; Caiazza, S. New dacron tissue colonisable keratoprosthesis-clinical-experience. Br. J. Ophthalmol. 1995, 79, 825–829. [Google Scholar] [CrossRef]
- Orwin, E.J.; Hubel, A. In vitro culture characteristics of corneal epithelial, endothelial, and keratocyte cells in a native collagen matrix. Tissue Eng. 2000, 6, 307–319. [Google Scholar] [CrossRef]
- Hicks, C.R.; Fitton, J.H.; Chirila, T.V.; Crawford, G.J.; Constable, I.J. Keratoprostheses, advancing toward a true artificial cornea. Surv. Ophthalmol. 1997, 42, 175–189. [Google Scholar] [CrossRef]
- Eguchi, H.; Hicks, C.R.; Crawford, G.J.; Tan, D.T.; Sutton, G.R. Cataract surgery with the AlphaCor artificial cornea. J. Cataract. Refract. Surg. 2004, 30, 1486–1491. [Google Scholar] [CrossRef]
- Sandeman, S.R.; Lloyd, A.W.; Tighe, B.J.; Franklin, V.; Li, J.; Lydon, F.; Liu, C.S.C.; Mann, D.J.; James, S.E.; Martin, R. A model for the preliminary biological screening of potential keratoprosthetic biomaterials. Biomaterials 2003, 24, 4729–4739. [Google Scholar] [CrossRef]
- Chirila, T.V. An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application. Biomaterials 2001, 22, 3311–3317. [Google Scholar] [CrossRef]
- Suuronen, E.J.; McLaughlin, C.R.; Stys, P.K.; Nakamura, M.; Munger, R.; Griffith, M. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol. Sci. 2004, 82, 525–533. [Google Scholar] [CrossRef]
- Guo, X.Q.; Hutcheon, A.E.K.; Melotti, S.A.; Zieske, J.D.; Trinkaus-Randall, V.; Ruberti, J.W. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4050–4060. [Google Scholar] [CrossRef]
- Xu, Y.G.; Xu, Y.S.; Huang, C.; Feng, Y.; Li, Y.; Wang, W. Development of a rabbit corneal equivalent using an acellular corneal matrix of a porcine substrate. Mol. Vis. 2008, 14, 2180–2189. [Google Scholar]
- Sachlos, E.; Czernuszka, J.T. Making tissue engineering scaffolds work, Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 29–40. [Google Scholar]
- Mi, S.; Chen, B.; Wright, B.; Connon, C.J. Ex vivo construction of an artificial ocular surface by combination of corneal limbal epithelial cells and a compressed collagen scaffold containing keratocytes. Tissue Eng. Part A 2010, 16, 2091–2100. [Google Scholar] [CrossRef]
- Levis, H.; Daniels, J.T. New technologies in limbal epithelial stem cell transplantation. Curr. Opin. Biotechnol. 2009, 20, 593–597. [Google Scholar] [CrossRef]
- Vrana, N.E.; Builles, N.; Justin, V.; Bednarz, J.; Pellegrini, G.; Ferrari, B.; Damour, O.; Hulmes, D.J.S.; Hasirci, V. Development of a reconstructed cornea from collagen-chondroitin sulfate foams and human cell cultures. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5325–5331. [Google Scholar] [CrossRef]
- Schneider, A.I.; Maier-Reif, K.; Graeve, T. Constructing an in vitro cornea from cultures of the three specific corneal cell types. In Vitro Cell. Dev. Biol. Anim. 1999, 35, 515–526. [Google Scholar] [CrossRef]
- Barry, P.A.; Cavanagh, H.D.; Jester, J.V. Effect of serum, Bfgf, Tgf(beta-1) and heparin on in vitro myofibroblast transformation in rabbit corneal keratocytes. Invest. Ophthalmol. Vis. Sci. 1994, 35, 1356–1356. [Google Scholar]
- Kotecha, A. What biomechanical properties of the cornea are relevant for the clinician? Surv. Ophthalmol. 2007, 52, 109–114. [Google Scholar] [CrossRef]
- Brown, R.; Wiseman, M.; Chuo, C.; Cheema, U.; Nazhat, S. Ultrarapid engineering of biomimetic materials and tissues, Fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Valiron, O.; Peris, L.; Rikken, G.; Schweitzer, A.; Saoudi, Y.; Remy, C.; Job, D. Cellular disorders induced by high magnetic fields. J. Magn. Reson. Imaging 2005, 22, 334–340. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, 89–106. [Google Scholar] [CrossRef]
- Then, K.Y.; Yang, Y.; Ahearne, M.; El Haj, A.J. Effect of microtopographical cues on human keratocyte orientation and gene expression. Curr. Eye Res. 2011, 36, 88–93. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef]
- Minami, Y.; Sugihara, H.; Oono, S. Reconstruction of cornea in 3-Dimensional collagen gel matrix culture. Invest. Ophthalmol. Vis. Sci. 1993, 34, 2316–2324. [Google Scholar]
- Nishimura, T.; Toda, S.; Mitsumoto, T.; Oono, S.; Sugihara, H. Effects of hepatocyte growth factor, transforming growth factor-beta 1 and epidermal growth factor on bovine corneal epithelial cells under epithelial-keratocyte interaction in reconstruction culture. Exp. Eye Res. 1998, 66, 105–116. [Google Scholar] [CrossRef]
- Dayhaw-Barker, P. Corneal wound healing: I. The players. Int. Contact Lens Clin. 1995, 22, 105–109. [Google Scholar] [CrossRef]
- Du, Y.; SundarRaj, N.; Funderburgh, M.L.; Harvey, S.A.; Birk, D.E.; Funderburgh, J.L. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest. Ophthalmol. Vis. Sci. 2007, 48, 5038–5045. [Google Scholar] [CrossRef]
- Kawakita, T.; Espana, E.M.; He, H.; Hornia, A.; Yeh, L.K.; Ouyang, J.; Liu, C.Y.; Tseng, S.C.G. Keratocan expression of murine keratocytes is maintained on amniotic membrane by down-regulating transforming growth factor-beta signaling. J. Biol. Chem. 2005, 280, 27085–27092. [Google Scholar]
- Chan, K.; Haschke, R. Epithelial-stromal interactions-specific stimulation of corneal epithelial-cellgrowth-in vitro by a factor(s) from cultured stromal fibroblasts. Exp. Eye Res. 1983, 36, 231–246. [Google Scholar] [CrossRef]
- Wilson, S.E.; Netto, M.; Ambrósio, R., Jr. Corneal cells: Chatty in development, homeostasis, wound healing, and disease. Am. J. Ophthalmol. 2003, 136, 530–536. [Google Scholar] [CrossRef]
- Hendriks, J.; Riesle, J.; van Blitterswijk, C.A. Co-culture in cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2007, 1, 170–178. [Google Scholar] [CrossRef]
- Hibino, T.; Wada, Y.; Mishima, H.; Otori, T. The effect of corneal epithelial cells on the collagen gel contraction by keratocytes. Jpn. J. Ophthalmol. 1998, 42, 174–179. [Google Scholar] [CrossRef]
- Nakazawa, K.; Takahashi, I.; Ohno, Y.; Sato, M. Modification of proteoglycan synthesis by corneal stromal cells on co-culture with either epithelial or endothelial cells. J. Biochem. 1997, 122, 851–858. [Google Scholar] [CrossRef]
- Lu, L.; Reinach, P.S.; Kao, W.W. Corneal Epithelial Wound Healing. Exp. Biol. Med. 2001, 226, 653–664. [Google Scholar]
- Wilson, S.; He, Y.; Weng, J.; Zieske, J.; Jester, J.; Schultz, G. Effect of epidermal growth-factor, hepatocyte growth-factor, and keratinocyte growth-factor, on proliferation, motility and differentiation of human corneal epithelial-cells. Exp. Eye Res. 1994, 59, 665–678. [Google Scholar] [CrossRef]
- Sotozono, C.; Kinoshita, S.; Kita, M.; Imanishi, J. Paracrine role of keratinocyte growth-factor in rabbit corneal epithelial-cell growth. Exp. Eye Res. 1994, 59, 385–391. [Google Scholar] [CrossRef]
- Dayhaw-Barker, P. Corneal wound healing: II. The process. Int. Contact Lens Clin. 1995, 22, 110–116. [Google Scholar] [CrossRef]
- Hoppenreijs, V.; Pels, E.; Vrensen, G.; Treffers, W. Basic fibroblast growth-factor stimulates corneal endothelial-cell growth and endothelial wound-healing of human corneas. Invest. Ophthalmol. Vis. Sci. 1994, 35, 931–944. [Google Scholar]
- Shah, A.; Brugnano, J.; Sun, S.; Vase, A.; Orwin, E. The development of a tissue-engineered cornea, Biomaterials and culture methods. Pediatr. Res. 2008, 63, 535–544. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, M.; Kinoshita, S. Keratinocyte growth-factor accelerates corneal epithelial wound-healing in vivo. Invest. Ophthalmol. Vis. Sci. 1995, 36, 1524–1529. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wilson, S.L.; El Haj, A.J.; Yang, Y. Control of Scar Tissue Formation in the Cornea: Strategies in Clinical and Corneal Tissue Engineering. J. Funct. Biomater. 2012, 3, 642-687. https://doi.org/10.3390/jfb3030642
Wilson SL, El Haj AJ, Yang Y. Control of Scar Tissue Formation in the Cornea: Strategies in Clinical and Corneal Tissue Engineering. Journal of Functional Biomaterials. 2012; 3(3):642-687. https://doi.org/10.3390/jfb3030642
Chicago/Turabian StyleWilson, Samantha L., Alicia J. El Haj, and Ying Yang. 2012. "Control of Scar Tissue Formation in the Cornea: Strategies in Clinical and Corneal Tissue Engineering" Journal of Functional Biomaterials 3, no. 3: 642-687. https://doi.org/10.3390/jfb3030642