Incorporation of Exogenous RGD Peptide and Inter-Species Blending as Strategies for Enhancing Human Corneal Limbal Epithelial Cell Growth on Bombyx mori Silk Fibroin Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fibroin Solutions
2.3. Preparation of Fibroin Membranes
2.4. Chemical Functionalization of BMSF with an RGD Peptide
2.5. X-ray Photoelectron Spectroscopy (XPS)
2.6. Contact Angle Analysis
2.7. Establishment of Primary HLE Cultures
2.8. Cell Attachment Assay
2.9. Statistical Analysis
3. Results
3.1. Characterization of BMSF/APSF Blends
3.2. Functionalization of BMSF with GRGDSPC
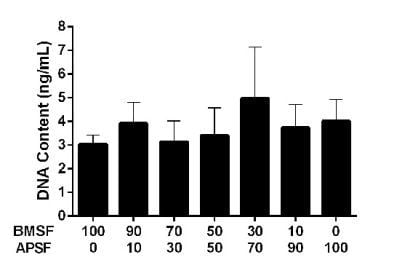
3.3. Response of HLE Cells to BMSF, APSF and Blends
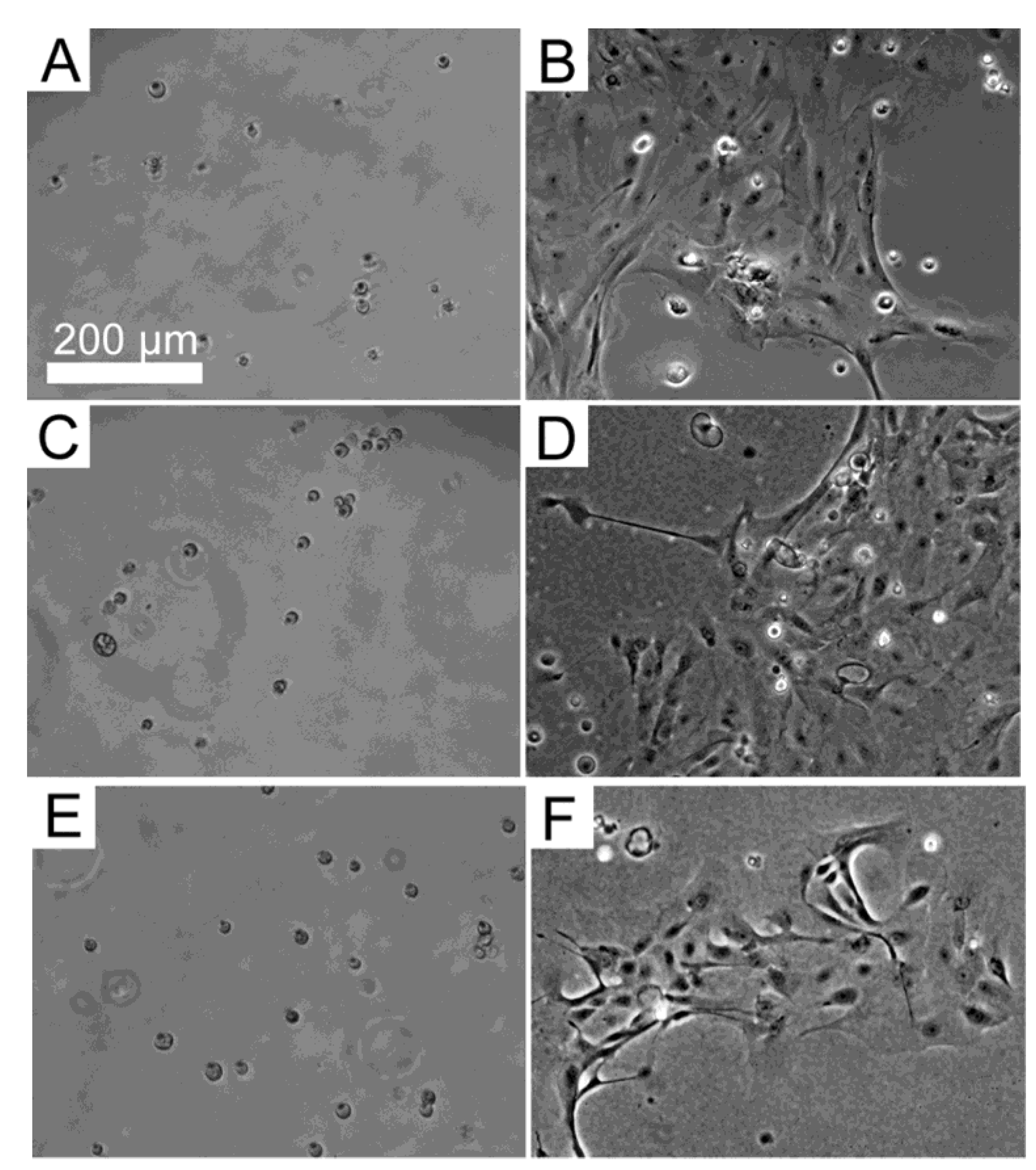
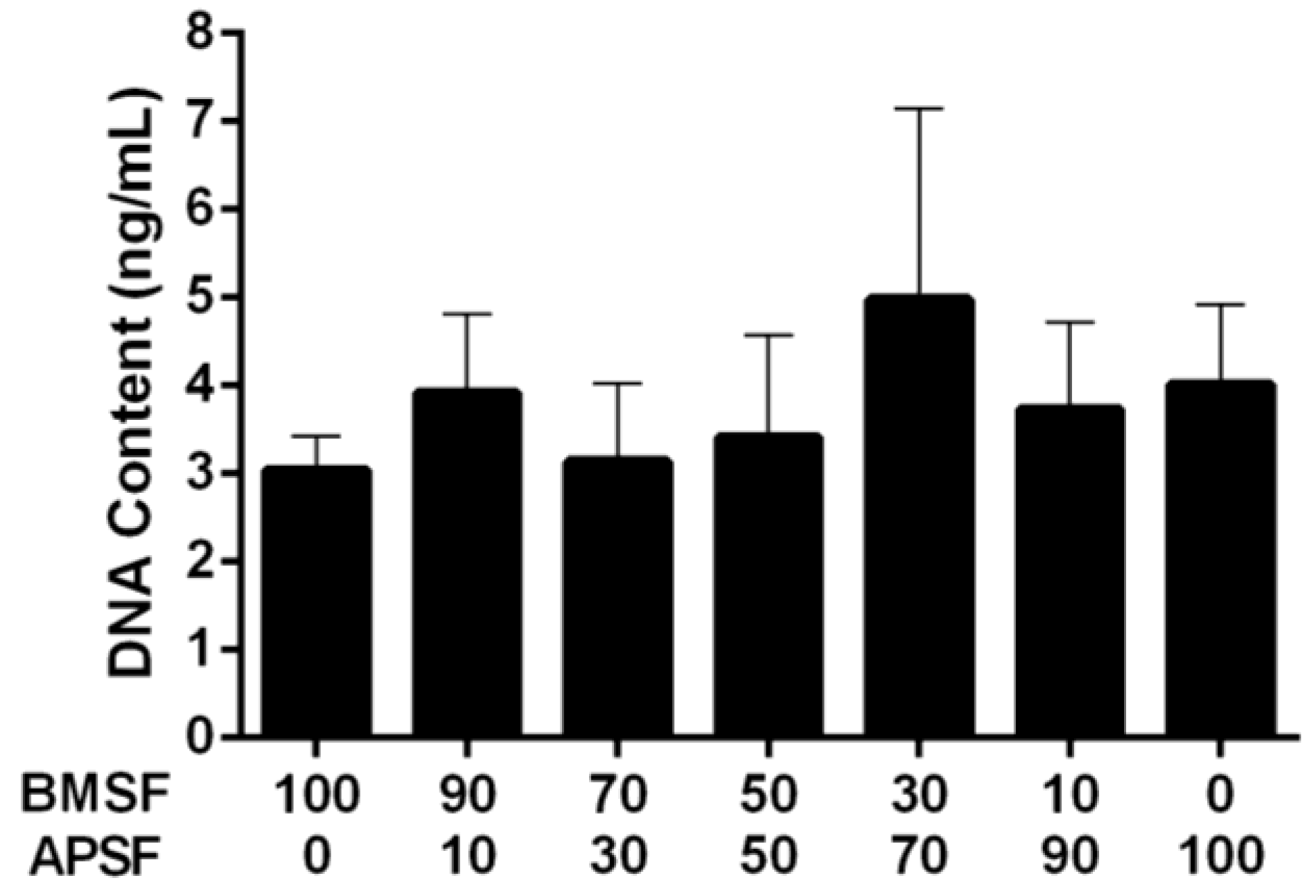
3.4. Response of HLE Cells to RGD-Functionalized BMSF

4. Discussion
5. Conclusions
References
- Minoura, N.; Tsukada, M.; Nagura, M. Physico-chemical properties of silk fibroin membrane as a biomaterial. Biomaterials 1990, 11, 430–434. [Google Scholar] [CrossRef]
- Minoura, N.; Aiba, S.; Higuchi, M.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of fibroblast cells on silk fibroin. Biochem. Biophys. Res. Commun. 1995, 208, 511–516. [Google Scholar] [CrossRef]
- Minoura, N.; Aiba, S.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of cultured fibroblast cells on silk protein matrices. J. Biomed. Mater. Res. 1995, 29, 1215–1221. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Hakimi, O.; Knight, D.P.; Vollrath, F.; Vadgama, P. Spider and mulberry silkworm silks as compatible biomaterials. Composites B 2007, 38, 324–337. [Google Scholar]
- Wang, X.; Cebe, P.; Kaplan, D.L. Silk Proteins—Biomaterials and bioengineering. In Protein Engineering Handbook; Lutz, S., Bornscheuer, U.T., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2009; pp. 939–959. [Google Scholar]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef]
- Hardy, J.G.; Scheibel, T.R. Composite materials based on silk proteins. Prog. Polym. Sci. 2010, 35, 1093–1115. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef]
- Pritchard, E.M.; Kaplan, D.L. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin. Drug Deliv. 2011, 8, 797–811. [Google Scholar] [CrossRef]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control. Rel. 2011, 150, 128–141. [Google Scholar] [CrossRef]
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Armato, U.; Dal Prà, I.; Chiarini, A.; Freddi, G. Will silk fibroin nanofiber scaffolds ever hold a useful place in translational regenerative medicine? Int. J. Burn Trauma 2011, 1, 27–33. [Google Scholar]
- Chirila, T.V.; Barnard, Z.; Zainuddin; Harkin, D.G.; Schwab, I.R.; Hirst, L.W. Bombyx mori silk fibroin membranes as potential substrata for epithelial constructs used in the management of ocular surface disorders. Tissue Eng. Part A 2008, 14, 1203–1211. [Google Scholar] [CrossRef]
- Ghassemifar, R.; Redmond, S.; Zainuddin; Chirila, T.V. Advancing towards a tissue-engineered tympanic membrane: Silk fibroin as a substratum for growing human eardrum keratinocytes. J. Biomater. Appl. 2010, 24, 591–606. [Google Scholar] [CrossRef]
- Madden, P.W.; Lai, J.N.X.; George, K.A.; Giovenco, T.; Harkin, D.G.; Chirila, T.V. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials 2011, 32, 4076–4084. [Google Scholar] [CrossRef]
- Bray, L.J.; George, K.A.; Hutmacher, D.W.; Chirila, T.V.; Harkin, D.G. A dual-layer silk fibroin scaffold for reconstructing the human corneal limbus. Biomaterials 2012, 33, 3529–3538. [Google Scholar] [CrossRef]
- Shadforth, A.M.A.; George, K.A.; Kwan, A.S.; Chirila, T.V.; Harkin, D.G. The cultivation of human retinal pigment epithelial cells on Bombyx mori silk fibroin. Biomaterials 2012, 33, 4110–4117. [Google Scholar] [CrossRef] [Green Version]
- Leal-Egaña, A.; Scheibel, T. Interactions of cells with silk surfaces. J. Mater. Chem. 2012, 22, 14330–14336. [Google Scholar] [CrossRef]
- Lotz, B.; Collona-Cesari, F. The chemical structure and the crystalline structures of Bombyx mori silk fibroin. Biochimie 1979, 61, 205–214. [Google Scholar]
- Mita, K.; Ichimura, S.; James, T.C. Highly repetitive structure and its organization of the silk fibroin gene. J. Mol. Evol. 1994, 38, 583–592. [Google Scholar]
- Zhou, C.-Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucl. Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef]
- Yukuhiro, K.; Kanda, T.; Tamura, T. Preferential codon usage and two types of repetitive motifs in the fibroin gene of the Chinese oak silkworm, Antheraea pernyi. Insect Mol. Biol. 1997, 6, 89–95. [Google Scholar]
- Sezutsu, H.; Yukuhiro, K. Dynamic rearrangement within the Antheraea pernyi silk fibroin gene is associated with four types of repetitive units. J. Mol. Evol. 2000, 51, 329–338. [Google Scholar]
- Hwang, J.-S.; Lee, J.-S.; Goo, T.-W.; Yun, E.-Y.; Lee, K.-S.; Kim, Y.-S.; Jin, B.-R.; Lee, S.-M.; Kim, K.-Y.; Kang, S.-W.; et al. Cloning of the fibroin gene from the oak silkworm, Antheraea yamamai and its complete sequence. Biotechnol. Lett. 2001, 23, 1321–1326. [Google Scholar] [CrossRef]
- Zheng, Z.; Wei, Y.; Yan, S.; Li, M. Preparation of regenerated Antheraea yamamai silk fibroin film and controlled-molecular conformation changes by aqueous ethanol treatment. J. Appl. Polym. Sci. 2010, 116, 461–467. [Google Scholar] [CrossRef]
- Lv, L.; Wei, Y.; Wang, J.; Li, M.; Zhao, H.; Liu, G.; Lv, Q. Preparation and Physical Properties of Antheraea yamamai/Bombyx mori Silk Fibroin Blending Film. In Proceedings of the 4th International Conference on Biomedical Engineering and Informatics (BMEI), Shanghai, China, 15–17 October 2011.
- Acharya, C.; Ghosh, S.K.; Kundu, S.C. Silk fibroin film from non-mulberry tropical tasar silkworms: A novel substrate for in vitro fibroblast culture. Acta Biomater. 2009, 5, 429–437. [Google Scholar] [CrossRef]
- Datta, A.; Ghosh, A.K.; Kundu, S.C. Differential expression of the fibroin gene in developmental stages of silkworm, Antheraea mylitta (Saturniidae). Comp. Biochem. Physiol. Part B 2001, 129, 197–204. [Google Scholar] [CrossRef]
- Gotoh, Y.; Tsukada, M.; Minoura, N. Effect of the chemical modification of the arginyl residue in Bombyx mori silk fibroin on the attachment and growth of fibroblast cells. J. Biomed. Mater. Res. 1998, 39, 351–357. [Google Scholar] [CrossRef]
- Yamada, H.; Igarashi, Y.; Takasu, Y.; Saito, H.; Tsubouchi, K. Identification of fibroin-derived peptides enhancing the proliferation of cultured human skin fibroblasts. Biomaterials 2004, 25, 467–472. [Google Scholar] [CrossRef]
- Sofia, S.; McCarthy, M.B.; Gronowicz, G.; Kaplan, D.L. Functionalized silk-based biomaterials for bone formation. J. Biomed. Mater. Res. 2001, 54, 139–148. [Google Scholar] [CrossRef]
- Kardestuncer, T.; McCarthy, M.B.; Karageorgiou, V.; Kaplan, D.; Gronowicz, G. RGD-tethered silk substrate stimulates the differentiation of human tendon cells. Clin. Orthop. Rel. Res. 2006, 448, 234–239. [Google Scholar] [CrossRef]
- Kim, J.W.; Ki, C.S.; Park, Y.H.; Kim, H.J.; Um, I.C. Effect of RGDS and KRSR peptides immobilized on silk fibroin nanofibrous mats for cell adhesion and proliferation. Macromol. Res. 2010, 18, 442–448. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Zhu, Z.; Kobayashi, I.; Uchino, K.; Tamada, Y.; Tamura, T.; Asakura, T. Improving cell-adhesive properties of recombinant Bombyx mori silk by incorporation of collagen or fibronectin derived peptides produced by transgenic silkworms. Biomacromolecules 2007, 8, 3487–3492. [Google Scholar] [CrossRef]
- Morgan, A.W.; Roskov, K.E.; Lin-Gibson, S.; Kaplan, D.L.; Becker, M.L.; Simon, C.G., Jr. Characterization and optimization of RGD-containing silk blends to support osteoblastic differentiation. Biomaterials 2008, 29, 2556–2563. [Google Scholar] [CrossRef]
- Yang, M.; Tanaka, C.; Yamauchi, K.; Ohgo, K.; Kurokawa, M.; Asakura, T. Silklike materials constructed from sequences of Bombyx mori silk fibroin, fibronectin and elastin. J. Biomed. Mater. Res. 2008, 84A, 353–363. [Google Scholar] [CrossRef]
- Kambe, Y.; Yamamoto, K.; Kojima, K.; Tamada, Y.; Tomita, N. Effects of RGDS sequence genetically interfused in the silk fibroin light chain protein on chondrocyte adhesion and cartilage synthesis. Biomaterials 2010, 31, 7503–7511. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Wang, X.; Rnjak, J.; Weiss, A.S.; Kaplan, D.L. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials 2010, 31, 8121–8131. [Google Scholar] [CrossRef]
- Hu, X.; Park, S.-H.; Gil, E.S.; Xia, X.-X.; Weiss, A.S.; Kaplan, D.L. The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials 2011, 32, 8979–8989. [Google Scholar] [CrossRef]
- Gil, E.S.; Mandal, B.B.; Park, S.-H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 2010, 31, 8953–8963. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Ren, T.; Gu, S.; Tan, Q.; Zhang, L.; Lv, K.; Pan, K.; Jiang, X. Bone marrow stromal cells cultured on poly(lactide-co-glycolide)/nano-hydroxyapatite composites with chemical immobilization of Arg-Gly-Asp peptide and preliminary bone regeneration of mandibular defect thereof. J. Biomed. Mater. Res. 2010, 95, 993–1003. [Google Scholar]
- Bray, L.J.; George, K.A.; Ainscough, S.L.; Hutmacher, D.W.; Chirila, T.V.; Harkin, D.G. Human corneal epithelial equivalents constructed on Bombyx mori silk fibroin membranes. Biomaterials 2011, 32, 5086–5091. [Google Scholar] [CrossRef]
- Suzuki, S.; Bray, L.J.; Edwards, G.A.; Chirila, T.V. Queensland Eye Institute,: Queensland, Australia, Unpublished work. 2013.
- Tretinnikov, O.N.; Tamada, Y. Influence of casting temperature on the near-surface structure and wettability of cast silk fibroin films. Langmuir 2001, 17, 7406–7413. [Google Scholar] [CrossRef]
- Murphy, A.R.; St. John, P.; Kaplan, D.L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 2008, 29, 2829–2838. [Google Scholar] [CrossRef]
- Vepari, C.; Matheson, D.; Drummy, L.; Naik, R.; Kaplan, D.L. Surface modification of silk fibroin with poly(ethylene glycol) for antiadhesion and antithrombotic applications. J. Biomed. Mater. Res. 2010, 93, 595–606. [Google Scholar]
- Sampaio, S.; Miranda, T.M.R.; Santos, J.G.; Soares, G.M.B. Preparation of silk fibroin-poly(ethylene glycol) conjugate films through click chemistry. Polym. Int. 2011, 60, 1737–1744. [Google Scholar] [CrossRef]
- Das, S.; Pati, D.; Tiwari, N.; Nisal, A.; Sen Gupta, S. Synthesis of silk fibroin-glycopolypeptide conjugates and their recognition with lectin. Biomacromolecules 2012, 13, 3695–3702. [Google Scholar] [CrossRef]
- Horbett, T.A.; Klumb, L.A. Cell culturing: Surface aspects and considerations. In Interfacial Phenomena and Bioproducts; Brash, J.L., Wojciechowski, P.W., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 351–445. [Google Scholar]
- De Giglio, E.; Sabbatini, L.; Colucci, S.; Zambonin, G. Synthesis, analytical characterization, and osteoblast adhesion properties on RGD-grafted polypyrrole coatings on titanium substrates. J. Biomater. Sci. Polym. Ed. 2000, 11, 1073–1083. [Google Scholar] [CrossRef]
- Chirila, T.V.; Minamisawa, T.; Keen, I.; Shiba, K. Effect of motif-programmed artificial proteins on the calcium uptake in a synthetic hydrogel. Macromol. Biosci. 2009, 9, 959–967. [Google Scholar] [CrossRef]
- Merrett, K.; Griffith, C.M.; Deslandes, Y.; Pleizier, G.; Sheardown, H. Adhesion of corneal epithelial cells to cell adhesion peptide modified pHEMA surfaces. J. Biomater. Sci. Polym. Ed. 2001, 12, 647–671. [Google Scholar] [CrossRef]
- Kweon, H.; Park, Y.H. Dissolution and characterization of regenerated Antheraea pernyi silk fibroin. J. Appl. Polym. Sci. 2001, 82, 750–758. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Gotoh, Y.; Kasai, N. Physical and chemical properties of tussah silk fibroin films. J. Polym. Sci. Part B 1994, 32, 1407–1412. [Google Scholar] [CrossRef]
- Li, M.; Tao, W.; Lou, S.; Kuga, S. Compliant film of regenerated Antheraea pernyi silk fibroin by chemical crosslinking. Int. J. Biol. Macromol. 2003, 32, 159–163. [Google Scholar] [CrossRef]
- Zuo, B.; Liu, L.; Zhang, F. Structure and properties of regenerated Antheraea pernyi silk fibroin filaments. J. Appl. Polym. Sci. 2009, 113, 2160–2165. [Google Scholar] [CrossRef]
- George, K.; Bray, L.J. Queensland Eye Institute,: Queensland, Australia, Unpublished work. 2012.
- Mandal, B.B.; Kundu, S.C. A novel method for dissolution and stabilization of non-mulberry silk gland protein fibroin using anionic surfactant sodium dodecyl sulfate. Biotechnol. Bioeng. 2008, 99, 1482–1489. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Non-bioengineered silk fibroin protein 3D scaffolds for potential biotechnological and tissue engineering applications. Macromol. Biosci. 2008, 8, 807–818. [Google Scholar] [CrossRef]
- Mandal, B.B.; Das, S.; Choudhury, K.; Kundu, S.C. Implication of silk film RGD availability and surface roughness on cytoskeletal organization and proliferation of primary rat bone marrow cells. Tissue Eng. Part A 2010, 16, 2391–2403. [Google Scholar] [CrossRef]
- Patra, C.; Talukdar, S.; Novoyatleva, T.; Velagala, S.R.; Mühlfeld, C.; Kundu, B.; Kundu, S.C.; Engel, F.B. Silk protein fibroin from Antheraea mylitta for cardiac tissue engineering. Biomaterials 2012, 33, 2673–2680. [Google Scholar] [CrossRef]
- Wu, X.F. The Study of Regenerated Antheraea Pernyi/Bombyx Mori Silk Fibroin Blend Porous Materials. M.S. Thesis, Suzhou University, Suzhou, China, 2009. Available online: http://www.dissertationtopic.net/doc/498028 (accessed on 22 October 2012). [Google Scholar]
- Qu, J.; Xin, L.; Xu, X.; Zhang, F.; Zuo, B.; Zhang, H. Tussah Silk Fibroin Excels Silk Fibroin from the Domesticated Silkworm in Supporting the Development of Neurons. In IFMBE Proceedings (The 6th World Congress of Biomechanics WCB 2010), Singapore, 1–6 August 2010; Lim, C.T., Goh, J.C.H., Eds.; Springer: Heidelberg, Germany, 2010; Volume 31, pp. 1574–1577. [Google Scholar]
- Hakimi, O.; Gheysens, T.; Vollrath, F.; Grahn, M.F.; Knight, D.P.; Vadgama, P. Modulation of cell growth on exposure to silkworm and spider silk fibers. J. Biomed. Mater. Res. 2010, 92, 1366–1372. [Google Scholar]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Massia, S.P.; Hubbell, J.A. Covalent surface immobilization of Arg-Gly-Asp- and Tyr-Ile-Gly-Ser-Arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal. Biochem. 1990, 187, 292–301. [Google Scholar] [CrossRef]
- Massia, S.P.; Hubbell, J.A. Human endothelial cell interactions with surface-coupled adhesion peptides on a nonadhesive glass substrate and two polymeric biomaterials. J. Biomed. Mater. Res. 1991, 25, 223–242. [Google Scholar] [CrossRef]
- Massia, S.P.; Hubbell, J.A. An RGD spacing of 440 nm is sufficient for integrin αvβ3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 1991, 114, 1089–1100. [Google Scholar] [CrossRef]
- Hern, D.L.; Hubbell, J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J. Biomed. Mater. Res. 1998, 39, 266–276. [Google Scholar] [CrossRef]
- Elbert, D.L.; Hubbell, J.A. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomaterials 2001, 22, 430–441. [Google Scholar]
- Huang, J.; Gräter, S.V.; Corbellini, F.; Rinck, S.; Bock, E.; Kemkemer, R.; Kessler, H.; Ding, J.; Spatz, J.P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009, 9, 1111–1116. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bray, L.J.; Suzuki, S.; Harkin, D.G.; Chirila, T.V. Incorporation of Exogenous RGD Peptide and Inter-Species Blending as Strategies for Enhancing Human Corneal Limbal Epithelial Cell Growth on Bombyx mori Silk Fibroin Membranes. J. Funct. Biomater. 2013, 4, 74-88. https://doi.org/10.3390/jfb4020074
Bray LJ, Suzuki S, Harkin DG, Chirila TV. Incorporation of Exogenous RGD Peptide and Inter-Species Blending as Strategies for Enhancing Human Corneal Limbal Epithelial Cell Growth on Bombyx mori Silk Fibroin Membranes. Journal of Functional Biomaterials. 2013; 4(2):74-88. https://doi.org/10.3390/jfb4020074
Chicago/Turabian StyleBray, Laura J., Shuko Suzuki, Damien G. Harkin, and Traian V. Chirila. 2013. "Incorporation of Exogenous RGD Peptide and Inter-Species Blending as Strategies for Enhancing Human Corneal Limbal Epithelial Cell Growth on Bombyx mori Silk Fibroin Membranes" Journal of Functional Biomaterials 4, no. 2: 74-88. https://doi.org/10.3390/jfb4020074