Preliminary Investigation of the Dissolution Behavior, Cytocompatibility, Effects of Fibrinogen Conformation and Platelet Adhesion for Radiopaque Embolic Particles
Abstract
:1. Introduction
- Examining materials response (generation of dissolution by-products) after short-term exposure to a simulated physiological environment;
- Evaluating the cytotoxicity of serial extract dilutions (elution assay), and substantiate the identification of a preferable composition for pre-clinical evaluation;
- Examining the likely host response, by examining the state of fibrinogen (Fg) conformation with regards the dissolution by-products and the haemocompatbility (i.e., platelet adhesion) of the materials particle surface.
2. Results and Discussion
2.1. Material Synthesis
| Embolic designation | Variable concentrations | |||
|---|---|---|---|---|
| ZnO | La2O3 | SiO2 | TiO2 | |
| ORP1 | 0.137 | 0.137 | 0.553 | 0.033 |
| ORP2 | 0.240 | 0.000 | 0.570 | 0.050 |
| ORP3 | 0.213 | 0.068 | 0.537 | 0.042 |
| ORP5 | 0.188 | 0.068 | 0.562 | 0.042 |
| ORP6 | 0.068 | 0.188 | 0.562 | 0.042 |
| ORP7 | 0.213 | 0.068 | 0.562 | 0.017 |
| ORP9 | 0.290 | 0.000 | 0.520 | 0.050 |
| ORP11 | 0.290 | 0.000 | 0.570 | 0.000 |
2.2. Material Morphology and Particle Size Distribution
2.3. Material Response
2.3.1. In Vitro Dissolution

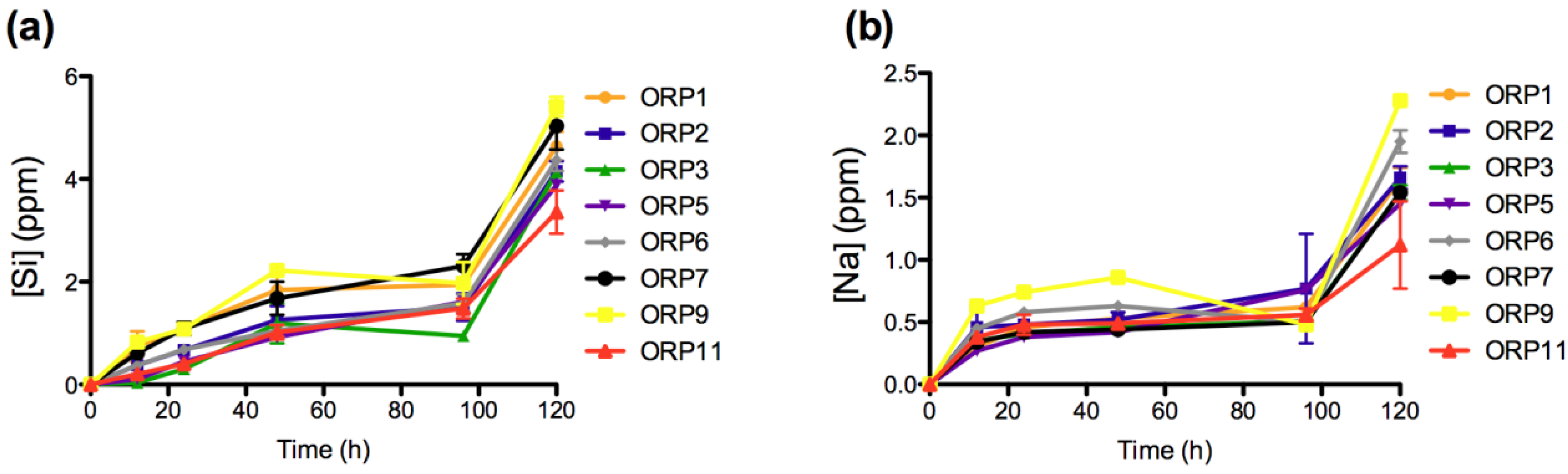
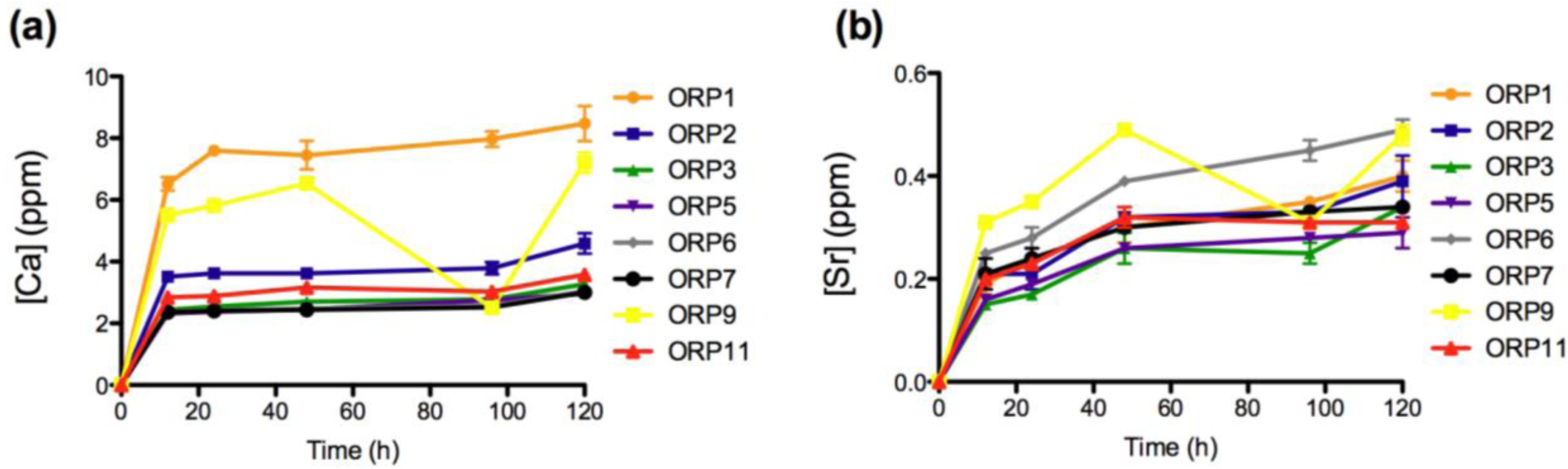
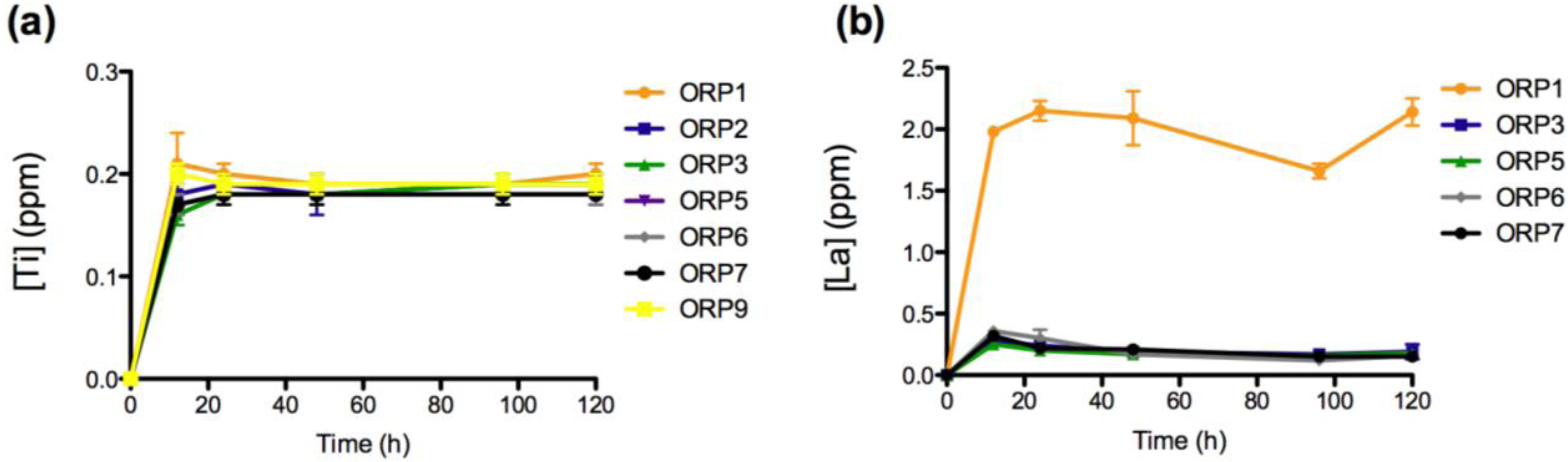
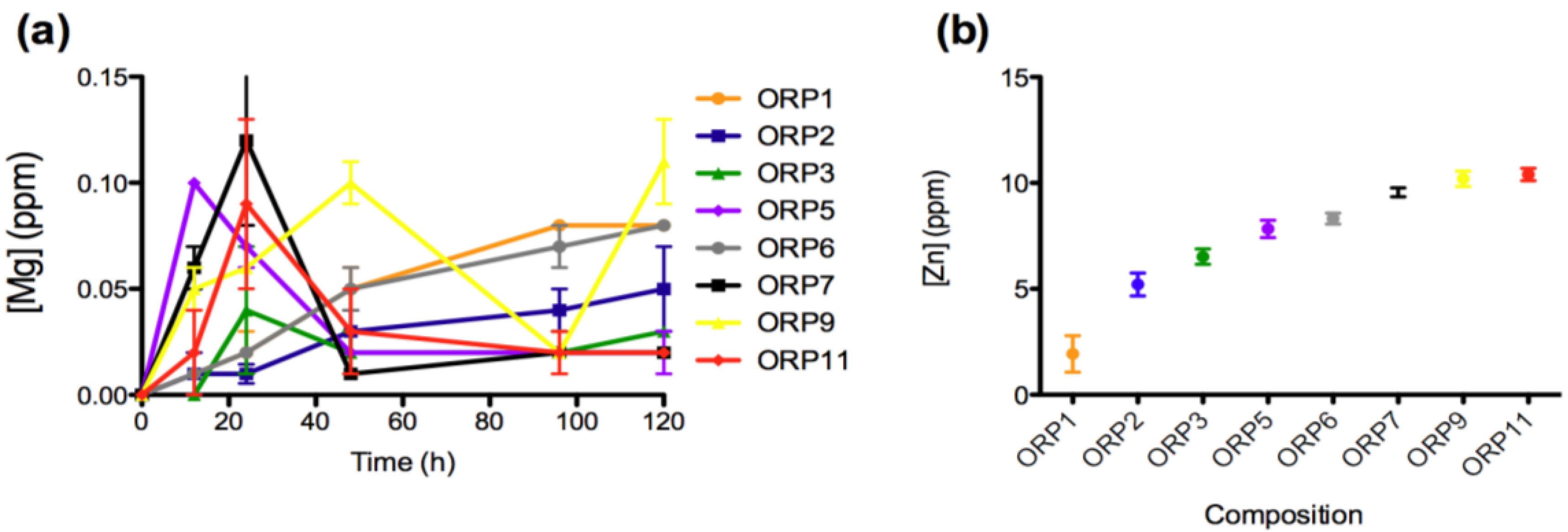
2.3.2. Cell Viabilities (MTT Assay) of the Embolic Particles
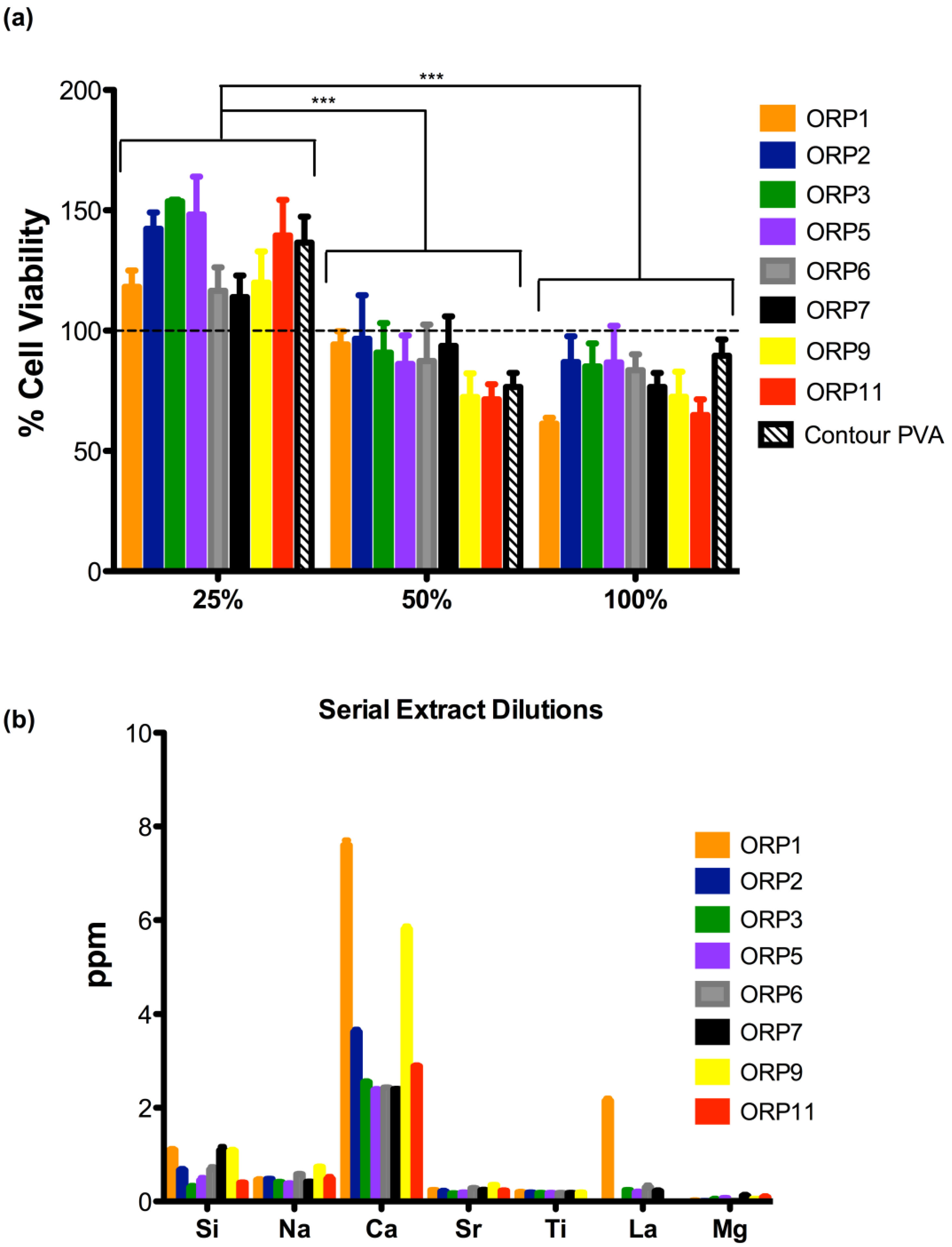
2.4. Host Response
2.4.1. Fg Conformational Changes in Dissolution Extracts of the Embolic Particles
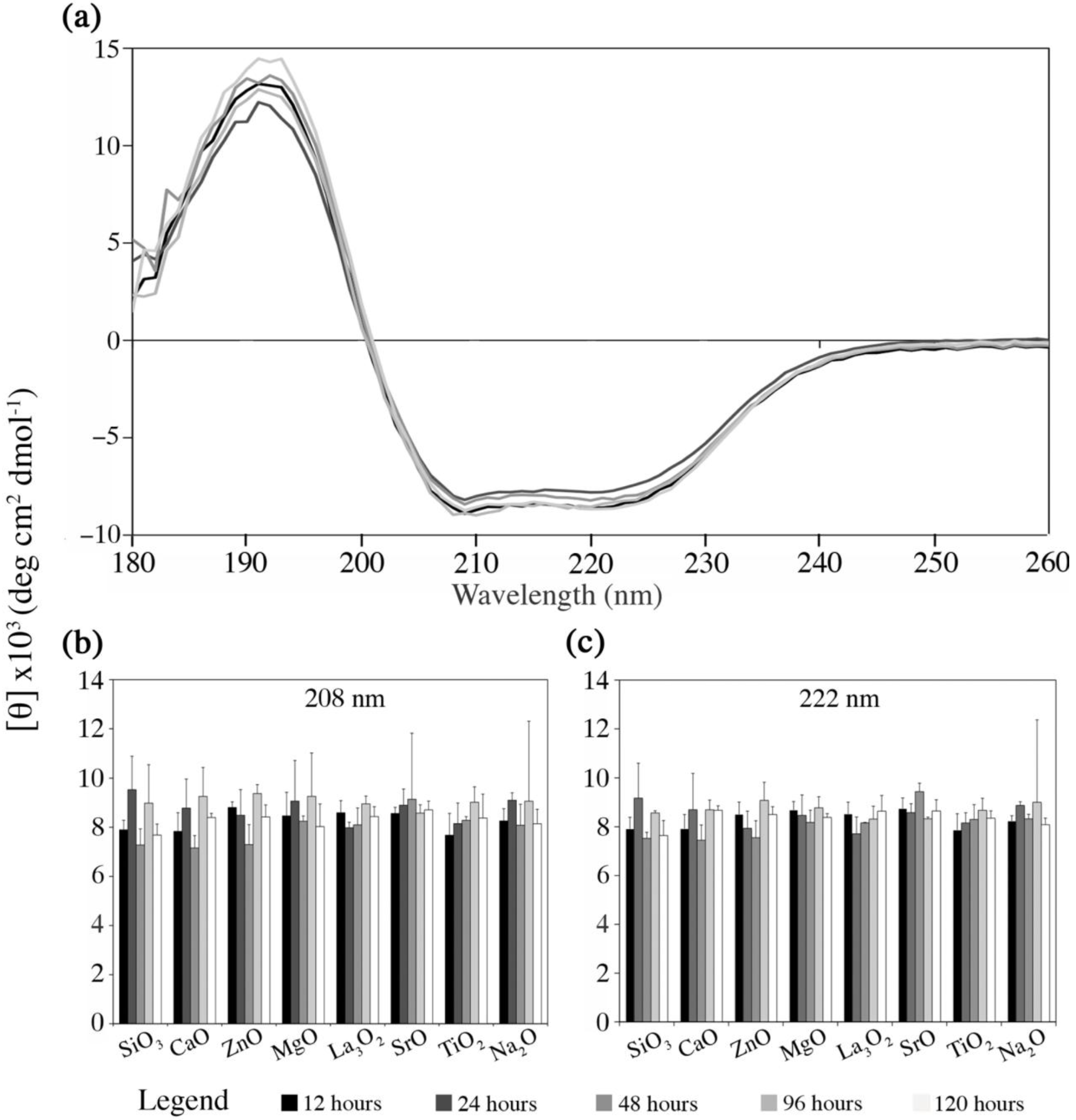
- (i)
- Mimicking the protein environment using PBS solution inherently draws limitations, since its use as a medium has an insufficient buffer capacity in terms of the medium pH and Ca2+ concentration, for which these factors in the real physiological environment are kept homeostatic via the circulatory system. PBS instead of simulated body fluid (SBF) was used in order to avoid the interference with UV signals, which is derived from the organic contents in SBF (Tris);
- (ii)
- In addition, protein complexation within the physiological environment was extensively simplified by using diluted mono-protein solution (Fg). This is due to the difficulties involved in separating and quantifying mixed protein solution.
2.4.2. Platelet Lactate Dehydrogenase (LDH) of the Embolic Particles
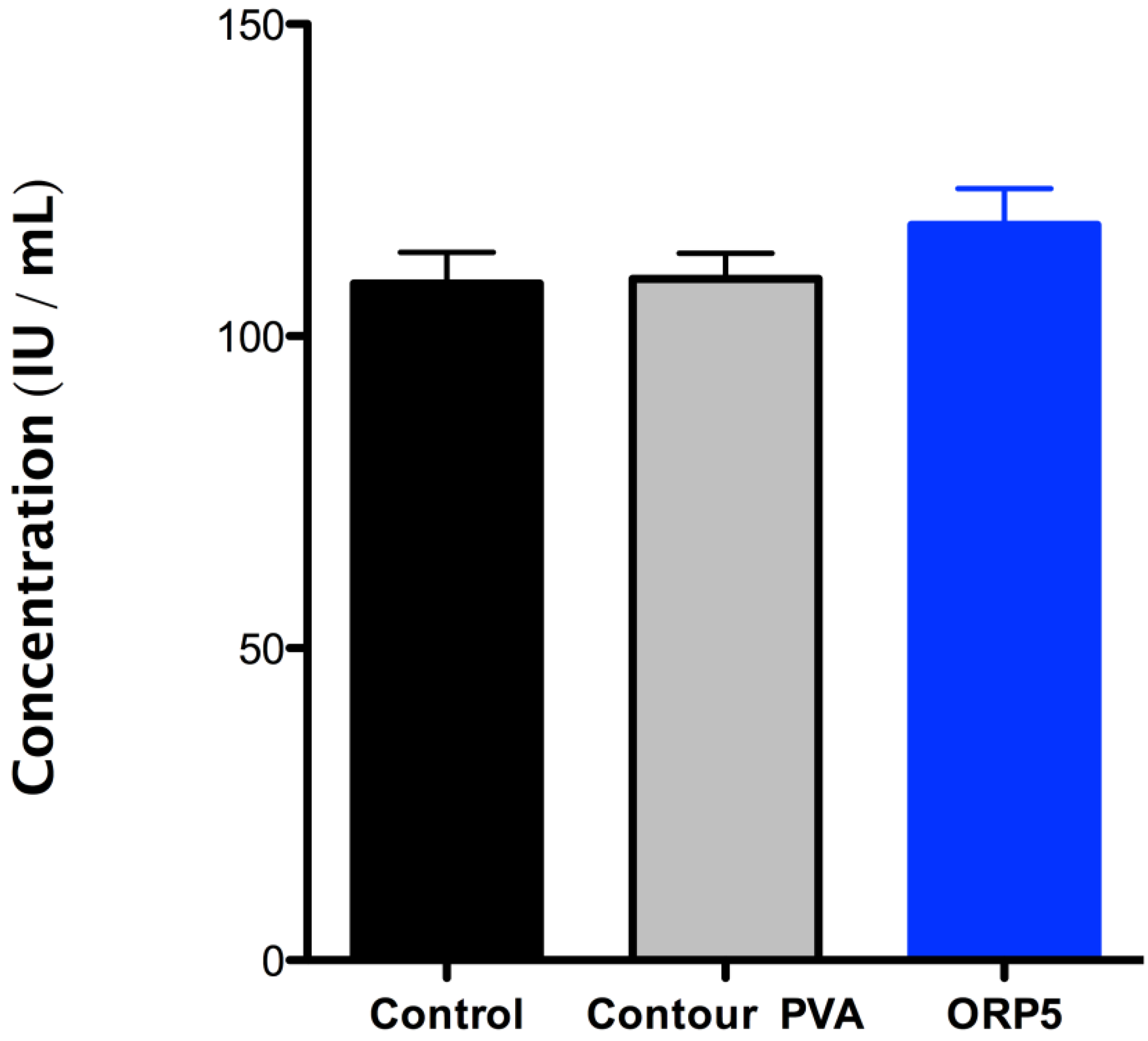
- (i)
- It should be noted that the mechanisms of degradation may not be the same for all materials at low pH as they are at blood pH (approximately pH 7.35 to pH 7.45).
- (ii)
- While it is recognized that additional biological factors such as enzymes and proteins can alter the rate of degradation, degradation by such outside factors is not addressed in this Part of ISO 10993.
3. Experimental Section
3.1. Materials Synthesis
Commercial Control
3.2. Material Morphology and Particle Size Distribution
3.3. Material Response Evaluation
3.3.1. Preparation of Embolic Extracts
3.3.2. Ionic Content Analysis
| Element | Absorption wavelength | Lower limit | Upper limit | Background correction |
|---|---|---|---|---|
| Si4+ | 288.158 | 288.073 | 288.256 | ±0.026 |
| Na+ | 330.237 | 330.136 | 330.348 | ±0.030 |
| Ca2+ | 396.847 | 396.679 | 397.039 | ±0.072 |
| Zn2+ | 334.501 | 334.400 | 334.614 | ±0.031 |
| Ti4+ | 337.279 | 335.188 | 334.810 | ±0.031 |
| La3+ | 407.735 | 407.971 | 407.596 | ±0.075 |
| Mg2+ | 279.553 | 279.646 | 279.399 | ±0.026 |
| Sr2+ | 421.552 | 421.759 | 421.371 | ±0.078 |
| Standard | Chemical element | |||
| Si4+ (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Zn2+ (mg/L) | |
| 1 | 2 | 1 | 0.5 | 1 |
| 2 | 4 | 2 | 1 | 2 |
| 3 | 10 | 4 | 3 | 4 |
| Standard | Ti4+ (mg/L) | La3+ (mg/L) | Mg2+ (mg/L) | Sr2+ (mg/L) |
| 4 | 0.1 | 0.1 | 0.1 | 0.1 |
| 5 | 1 | 1 | 1 | 1 |
| 6 | 10 | 10 | 10 | 10 |
3.3.3. In Vitro Biological Evaluation of Materials
3.3.4. Fibroblast Cell Culture
3.3.5. Assessment of Cell Viability (MTT Assay)
3.4. Host Response Evaluation
3.4.1. Circular Dichroism Spectropolarimetry
3.4.2. Platelet Lactate Dehydrogenase (LDH) Activity
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Hsu, C.; Kwan, G.; Thompson, S.; Evans-Barns, H.; van Driel, M. Embolisation for pulmonary arteriovenous malformation. Cochrane Database Syst. Tev. 2012, 8. [Google Scholar] [CrossRef]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; Benhamou, Y.; Avajon, Y.; Gruenberger, T.; Pomoni, M.; Langenberger, H.; Schuchmann, M.; Dumortier, J.; Mueller, C.; Chevallier, P.; Lencioni, R. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef]
- The REST Investigators. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N. Engl. J. Med. 2007, 356, 360–370. [CrossRef]
- Hehenkamp, W.; Volkers, N.; Birnie, E.; Reekers, J.; Ankum, W. Symptomatic uterine fibroids: Treatment with uterine artery embolization or hysterectomy—Results from the randomized clinical Embolisation versus Hysterectomy (EMMY) Trial. Radiology 2008, 246, 823–832. [Google Scholar] [CrossRef]
- Spies, J.B. What evidence should we demand before accepting a new embolic material for uterine artery embolization? J. Vas. Interv. Radiol. 2009, 20, 567–570. [Google Scholar] [CrossRef]
- Spies, J.B.; Allison, S.; Flick, P.; Cramp, M.; Bruno, J.; Jha, R.C.; Ascher, S.A. Spherical polyvinyl alcohol versus tris-acryl gelatin microspheres for uterine artery embolization for leiomyomas: Results of a limited randomized comparative study. J. Vas. Interv. Radiol. 2005, 16, 1431–1437. [Google Scholar] [CrossRef]
- Fatal nontarget embolization via an intrafibroid arterial venous fistula during uterine fibroid embolization. J. Vas. Interv. Radiol. 2009, 20, 419–420. [CrossRef]
- McCullough, P.A.; Adam, A.; Becker, C.R.; Davidson, C.; Larneire, N.; Stacul, F.; Tumlin, J. Risk prediction of contrast-induced nephropathy. Am. J. Cardiol. 2006, 98, 27K–36K. [Google Scholar]
- McCullough, P.A. Contrast-induced acute kidney injury. J. Am. Coll. Cardiol. 2008, 51, 1419–1428. [Google Scholar] [CrossRef]
- McCullough, P.A.; Adam, A.; Becker, C.R.; Davidson, C.; Lameire, N.; Stacul, F.; Tumlin, J. Epidemiology and prognostic implications of contrast-induced nephropathy. Am. J. Cardiol. 2006, 98, 5k–13k. [Google Scholar]
- Saralidze, K.; Knetsch, M.L.W.; van Berkel, R.G.M.; Mostert, C.; Koole, L.H. Radiopaque microspheres for improved transarterial chemical embolization (TACE). J. Control. Release 2011, 152, E74–E75. [Google Scholar] [CrossRef]
- Mottu, F.; Rufenacht, D.; Laurent, A.; Doelker, E. Iodine-containing cellulose mixed esters as radiopaque polymers for direct embolization of cerebral aneurysms and arteriovenous malformations. Biomaterials 2002, 23, 121–131. [Google Scholar]
- Barnett, B.; Hughes, A.; Lin, S.; Arepally, A.; Gailloud, P. In vitro assessment of EmboGel and UltraGel radiopaque hydrogels for the endovascular treatment of aneurysms. J. Vasc. Interv. Radiol. 2009, 20, 507–512. [Google Scholar] [CrossRef]
- Fatimi, A.; Chabrot, P.; Berrahmoune, S.; Coutu, J.; Soulez, G.; Lerouge, S. A new injectable radiopaque chitosan-based sclerosing embolizing hydrogel for endovascular therapies. Acta Biomater. 2012, 8, 2712–2721. [Google Scholar] [CrossRef]
- Sharma, K.V.; Dreher, M.R.; Tang, Y.; Pritchard, W.; Chiesa, O.A.; Karanian, J.; Peregoy, J.; Orandi, B.; Woods, D.; Donahue, D.; Esparza, J.; Jones, G.; Willis, S.L.; Lewis, A.L.; Wood, B.J. Development of “imageable” beads for transcatheter embolotherapy. J. Vasc. Interv. Radiol. 2010, 21, 865–876. [Google Scholar] [CrossRef]
- Thanoo, B.C.; Jayakrishnan, A. Barium sulphate-loaded p(HEMA) microspheres as artificial emboli: Preparation and properties. Biomaterials 1990, 11, 477–481. [Google Scholar] [CrossRef]
- Thanoo, B.C.; Sunny, M.C.; Jayakrisnnan, A. Tantalum-loaded polyurethane microspheres for particulate embolization: Preparation and properties. Biomaterials 1991, 12, 525–528. [Google Scholar]
- Kehoe, S.; Tonkopi, E.; Abraham, R.J.; Boyd, D. Novel radiopaque embolic agent for uterine fibroid embolization: Determination of radiopacity and biological evaluation; cytocompatibility, intracutaneous reactivity and local effects after implantation. J. Vasc. Interv. Radiol. 2012, 23, S33. [Google Scholar]
- Kehoe, S.; Langman, M.; Zwanziger, U.W.; Abraham, R.J.; Boyd, D. Mixture designs to assess composition–structure–property relationships in SiO2–CaO–ZnO–La2O3–TiO2–MgO–SrO–Na2O glasses: Potential materials for embolization. J. Biomater. Appl. 2012. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22863846.
- Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within A Risk Management Process, ISO 10993-1, Association for the Advancement of Medical Instrumentation/International Organization for Standardization: Geneva, Switzerland, 2009.
- Biological Evaluation of Medical Devices—Part 14: Identification and Quantification of Degradation Products from Ceramics, ISO10993-14, Association for the Advancement of Medical Instrumentation/International Organization for Standardization: Geneva, Switzerland, 2001.
- Owen, R.; Nation, P.; Polakowski, R.; Biliske, J.; Tiege, P.; Griffith, I. A preclinical study of the safety and efficacy of occlusinTM 500 artificial embolization device in sheep. Cardiovasc. Interv. Radiol. 2012, 35, 636–644. [Google Scholar] [CrossRef]
- Latour, R.A.; Sivaraman, B. The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen. Biomaterials 2010, 31, 832–839. [Google Scholar] [CrossRef]
- Santore, M.M.; Wertz, C.F. Adsorption and relaxation kinetics of albumin and fibrinogen on hydrophobic surfaces: Single-species and competitive behavior. Langmuir 1999, 15, 8884–8894. [Google Scholar] [CrossRef]
- Simon, V.; Vanea, E.; Magyari, K. Protein attachment on aluminosilicates surface studied by XPS and FTIR spectroscopy. J. Optoelectron. Adv. Mater. 2010, 12, 1206–1212. [Google Scholar]
- Kamarg, S.; Lip, G.Y.H. Fibrinogen: Biochemistry, epidemology and determinants. Q. J. Med. 2003, 96, 711–729. [Google Scholar] [CrossRef]
- Greenfield, N.J. Analysis of circular dichroism data. Numer. Comput. Methods 2004, 383, 282–317. [Google Scholar] [CrossRef]
- Zhang, X.F.; Kehoe, S.; Adhi, S.K.; Ajithkumar, T.G.; Moane, S.; O’Shea, H.; Boyd, D. Composition-structure-property (Zn2+ and Ca2+ ion release) evaluation of Si–Na–Ca–Zn–Ce glasses: Potential components for nerve guidance conduits. Mater. Sci. Eng. C 2011, 31, 669–676. [Google Scholar] [CrossRef]
- Zhang, X.F.; Coughlan, A.; O’Shea, H.; Towler, M.R.; Kehoe, S.; Boyd, D. Experimental composite guidance conduits for peripheral nerve repair: An evaluation of ion release. Mater. Sci. Eng. C 2012, 32, 1654–1663. [Google Scholar] [CrossRef]
- Kang, B.T.; Lee, J.H.; Jung, D.I.; Park, C.; Gu, S.H.; Jeon, H.W.; Jang, D.P.; Lim, C.Y.; Quan, F.S.; Kim, Y.B.; Cho, Z.H.; Woo, E.J.; Park, H.M. Canine model of ischemic stroke with permanent middle cerebral artery occlusion: Clinical and histopathological findings. J. Vet. Sci. 2007, 8, 369–376. [Google Scholar] [CrossRef]
- Purdy, P.D.; Devous, M.D.; White, C.L.; Batjer, H.H.; Samson, D.S.; Brewer, K.; Hodges, K. Reversible middle cerebral-artery embolization in dogs without intracranial surgery. Stroke 1989, 20, 1368–1376. [Google Scholar] [CrossRef]
- Yamauchi, T.; Furui, S.; Irie, T.; Kusano, S. Partial splenic embolization with Y-shaped silicone particles. Acta Radiol. 1994, 35, 335–339. [Google Scholar]
- Anglin, E.J.; Cheng, L.Y.; Freeman, W.R.; Sailor, M.J. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 2008, 60, 1266–1277. [Google Scholar] [CrossRef]
- He, Q.J.; Ma, M.; Wei, C.Y.; Shi, J.L. Mesoporous carbon@silicon-silica nanotheranostics for synchronous delivery of insoluble drugs and luminescence imaging. Biomaterials 2012, 33, 4392–4402. [Google Scholar] [CrossRef]
- Dewardener, H.E.; Clarkson, E.M.; Bitensky, L.; Macgregor, G.A.; Alaghbandzadeh, J.; Chayen, J. Effect of sodium-intake on ability of human-plasma to inhibit renal Na+-K+-adenosine triphosphatase in vitro. Lancet 1981, 1, 411–412. [Google Scholar]
- Lei, C.Z.; Xiang, Y.; Ao, G.K.; Li, L.; Shi, Y.C.; Bao, Y.R.; Xu, C.J.; Hong, H.; Lang, J.H. Impact of uterine fibroid embolization with danazol alginate microsphere on ovarian function and subsequent pregnancy. Zhonghua Fu Chan Ke Za Zhi 2007, 42, 701–704. [Google Scholar]
- Lubarsky, M.; Ray, C.; Funaki, B. Embolization agents-which one should be used when? Part 2: Small-vessel embolization. Semin. Interv. Radiol. 2010, 27, 99–104. [Google Scholar] [CrossRef]
- Cerruti, M.G.; Greenspan, D.; Powers, K. An analytical model for the dissolution of different particle size samples of Bioglass((R)) in TRIS-buffered solution. Biomaterials 2005, 26, 4903–4911. [Google Scholar] [CrossRef]
- Rudd, C.D.; Ahmed, I.; Parsons, A.J.; Palmer, G.; Knowles, J.C.; Walkers, G.S. Weight loss, ion release and initial mechanical properties of a binary calcium phosphate glass fibre/PCL composite. Acta Biomater. 2008, 4, 1307–1314. [Google Scholar] [CrossRef]
- Fox, B.A.; Yee, V.C.; Pedersen, L.C.; Le Trong, I.; Bishop, P.D.; Stenkamp, R.E.; Teller, D.C. Identification of the calcium binding site and a novel ytterbium site in blood coagulation factor XIII by X-ray crystallography. J. Biol. Chem. 1999, 274, 4917–4923. [Google Scholar]
- Dobelbower, R.R. Review of medical physiology. Med. Phys. 1980, 7, 449. [Google Scholar]
- Curtis, C.G.; Brown, K.L.; Credo, R.B.; Domanik, R.A.; Gray, A.; Stenberg, P.; Lorand, L. Calcium-dependent unmasking of active-center cysteine during activation of fibrin stabilizing factor. Biochemistry 1974, 13, 3774–3780. [Google Scholar]
- Muszbek, L.; Haramura, G.; Polgar, J. Transformation of cellular factor-XIII into an active zymogen transglutaminase in thrombin-stimulated platelets. Thromb. Haemost. 1995, 73, 702–705. [Google Scholar]
- McGarry, S.; Morgan, S.J.; Grosskreuz, R.M.; Williams, A.E.; Smith, W.R. Serum titanium levels in individuals undergoing intramedullary femoral nailing with a titanium implant. J. Trauma-Injury Infect. Crit. Care 2008, 64, 430–433. [Google Scholar] [CrossRef]
- Liu, X.Y.; Poon, R.W.Y.; Kwok, S.C.H.; Chu, P.K.; Ding, C.X. Plasma surface modification of titanium for hard tissue replacements. Surf. Coat. Technol. 2004, 186, 227–233. [Google Scholar] [CrossRef]
- Chu, P.K.; Liu, X.Y.; Ding, C.X. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Costantini, A.; Luciani, G.; Branda, F. Thermal properties and devitrification behavior of (2.5–x)CaO center dot x/3La2O3•2SiO2. Thermochim. Acta 2000, 348, 115–120. [Google Scholar] [CrossRef]
- Su, X.G.; Zheng, X.N.; Ni, J.Z. Lanthanum citrate induces anoikis of Hela cells. Cancer Lett. 2009, 285, 200–209. [Google Scholar] [CrossRef]
- Hill, R.G.; Watts, S.J.; O’Donnell, M.D.; Law, R.V. Influence of magnesia on the structure and properties of bioactive glasses. J. Non-Cryst. Solids 2010, 356, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Zafar, S.; Butt, A. Serum magnesium level; comparison between healthy and malnourished children. Prof. Med. J. 2010, 17, 279–285. [Google Scholar]
- Nurdin, N.; Francois, P.; Mugnier, Y.; Krumeich, J.; Moret, M.; Aronsson, B.O.; Descouts, P. Haemocompatibility evaluation of DLC- and SiC-coated surfaces. Eur. Cell Mater. 2003, 5, 17–26. [Google Scholar]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Shechter, M. Magnesium and cardiovascular system. Magnes. Res. 2010, 23, 60–72. [Google Scholar]
- Jaing, T.H.; Hung, I.H.; Chung, H.T.; Lai, C.H.; Liu, W.M.; Chang, K.W. Acute hypermagnesemia: A rare complication of antacid administration after bone marrow transplantation. Clin. Chim. Acta 2002, 326, 201–203. [Google Scholar] [CrossRef]
- Xu, G.H.; Shu, C.; Wenjuan, Z.; Wei, Z.; Wei, J.; Dongmei, W. Dissolution behavior and bioactivity study of glass ceramic scaffolds in the system of CaO-P2O5-Na2O-ZnO prepared by sol-gel technique. Mater. Sci. Eng. C 2010, 30, 105–111. [Google Scholar] [CrossRef]
- Menabue, L.; Lusvardi, G.; Zaffe, D.; Bertoldi, C.; Malavasi, G.; Consolo, U. In vitro and in vivo behaviour of zinc-doped phosphosilicate glasses. Acta Biomater. 2009, 5, 419–428. [Google Scholar] [CrossRef]
- Boyd, D.; Li, H.; Tanner, D.A.; Towler, M.R.; Wall, J.G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sc. Mater. Med. 2006, 17, 489–494. [Google Scholar]
- Aina, V.; Malavasi, G.; Fiorio Pla, A.; Munaron, L.; Morterra, C. Zinc-containing bioactive glasses: Surface reactivity and behaviour towards endothelial cells. Acta Biomater. 2009, 5, 419–428. [Google Scholar] [CrossRef]
- Murphy, S.; Wren, A.; Towler, M.; Boyd, D. The effect of ionic dissolution products of Ca-Sr-Na-Zn-Si bioactive glass on in vitro cytocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 2827–2834. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Chang, J.; Wang, J.Y.; Zhai, W.Y.; Wang, Z. In vitro bioactivity of novel tricalcium silicate ceramics. J. Mater. Sci. Mater. Med. 2007, 18, 917–923. [Google Scholar] [CrossRef]
- Prokopowicz, M.; Lukasiak, J.; Banecki, B.; Przyjazny, A. In vitro measurement of conformational stability of fibrinogen adsorbed on siloxane. Biomacromolecules 2005, 6, 39–45. [Google Scholar] [CrossRef]
- Nair, M.B.; Varma, H.K.; John, A. Platelet-rich plasma and fibrin glue-coated bioactive ceramics enhance growth and differentiation of goat bone marrow-derived stem cells. Tissue Eng. Part A 2009, 15, 1619–1631. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kehoe, S.; Tremblay, M.-L.; Coughlan, A.; Towler, M.R.; Rainey, J.K.; Abraham, R.J.; Boyd, D. Preliminary Investigation of the Dissolution Behavior, Cytocompatibility, Effects of Fibrinogen Conformation and Platelet Adhesion for Radiopaque Embolic Particles. J. Funct. Biomater. 2013, 4, 89-113. https://doi.org/10.3390/jfb4030089
Kehoe S, Tremblay M-L, Coughlan A, Towler MR, Rainey JK, Abraham RJ, Boyd D. Preliminary Investigation of the Dissolution Behavior, Cytocompatibility, Effects of Fibrinogen Conformation and Platelet Adhesion for Radiopaque Embolic Particles. Journal of Functional Biomaterials. 2013; 4(3):89-113. https://doi.org/10.3390/jfb4030089
Chicago/Turabian StyleKehoe, Sharon, Marie-Laurence Tremblay, Aisling Coughlan, Mark R. Towler, Jan K. Rainey, Robert J. Abraham, and Daniel Boyd. 2013. "Preliminary Investigation of the Dissolution Behavior, Cytocompatibility, Effects of Fibrinogen Conformation and Platelet Adhesion for Radiopaque Embolic Particles" Journal of Functional Biomaterials 4, no. 3: 89-113. https://doi.org/10.3390/jfb4030089





