Fabrication and Characterization of Nanoporous Niobia, and Nanotubular Tantala, Titania and Zirconia via Anodization
Abstract
:1. Introduction
2. Results and Discussion
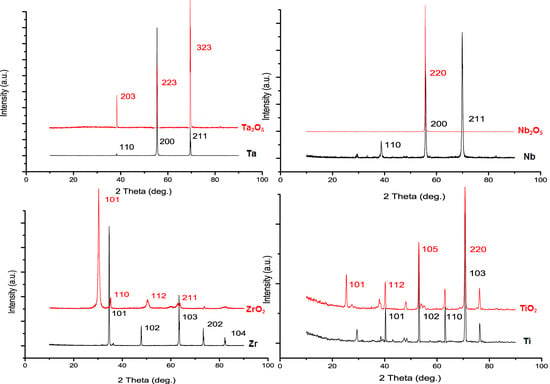
2.1. The Dynamics of the Anodization Process for Tantala (Ta2O5), Niobia (Nb2O5) and Zirconia (ZrO2)


2.2. Physical Characteristics of Nanoporous Ta2O5 and Nb2O5 and Nanotubular ZrO2 Layers

| Material | Surface area index (SI) | Volume index (VI) | Sa(μm) | Sq(μm) | Sskw | Sku |
|---|---|---|---|---|---|---|
| Nanoporous Ta2O5; As formed | 1.34 ± 0.01 | 18393 ± 86 | 0.27 ± 0.01 | 0.35 ± 0.01 | 0.60 ± 0.23 | 4.82 ± 0.52 |
| Nanoporous Ta2O5; Annealed (10 min at 290 °C) | 1.27 ± 0.01 | 1838 ± 132 | 0.25 ± 0.01 | 0.32 ± 0.01 | 0.72 ± 0.03 | 3.90 ± 0.17 |
| Bare tantalum foil (0.1 mm) | 1.06 ± 0.00 | 18229 ± 0 | 0.23 ± 0.00 | 0.29 ± 0.00 | 0.06 ± 0.00 | 2.79 ± 0.00 |
| Nanoporous Nb2O5; As formed | 1.50 ± 0.00 | 18398 ± 134 | 0.26 ± 0.00 | 0.34 ± 0.00 | 0.64 ± 0.03 | 3.78 ± 0.21 |
| Nanoporous Nb2O5; Annealed (10 min at 290 °C) | 1.38 ± 0.00 | 18357 ± 144 | 0.26 ± 0.01 | 0.33 ± 0.01 | 0.39 ± 0.02 | 3.28 ± 0.05 |
| Bare niobium foil (0.05 mm) | 1.04 ± 0.00 | 18327 ± 0 | 0.31 ± 0.00 | 0.38 ± 0.00 | 0.22 ± 0.00 | 2.68 ± 0.00 |
| ZrO2 Nanotube; As formed | 2.44 ± 0.30 | 18422 ± 12 | 0.56 ± 0.05 | 0.71 ± 0.05 | −0.86 ± 0.018 | 3.75 ± 0.49 |
| ZrO2 Nanotube; Annealed (10 min at 290 °C) | 2.94 ± 0.15 | 18428 ± 79 | 0.61 ± 0.01 | 0.76 ± 0.00 | −0.57 ± 0.20 | 3.30 ± 0.35 |
| Bare zirconium foil (0.05 mm) | 1.03 ± 0.00 | 18531 ± 0.00 | 0.12 ± 0.00 | 0.20 ± 0.00 | −4.27 ± 0.00 | 39.31 ± 0.00 |

| Material | Contact angle (θ/°) | (mJ·m−2) | (mJ·m−2) | (mJ·m−2) |
|---|---|---|---|---|
| Nanoporous Ta2O5; As formed | 26.0 ± 5.0 | 11.8 | 55.2 | 67.0 |
| Nanoporous Ta2O5; Annealed (10 min at 290 °C) | 12.1 ± 1.6 | 15.2 | 56.7 | 71.9 |
| Bare tantalum foil (0.1 mm) | 47.9 ± 1.2 | 5.1 | 49.5 | 54.6 |
| Nanoporous Nb2O5; As formed | 25.0 ± 7.0 | 11.02 | 56.94 | 67.96 |
| Nanoporous Nb2O5; Annealed (10 min at 290 °C) | 15.5 ± 1.4 | 18.11 | 52.20 | 70.31 |
| Bare niobium foil (0.05 mm) | 61.0 ± 0.0 | 6.44 | 34.94 | 41.38 |
| ZrO2 Nanotube; As formed | 17.0 ± 1.0 | 11.56 | 60.04 | 71.60 |
| ZrO2 Nanotube; Annealed (10 min at 290 °C) | 8.5 ± 1.0 | 12.84 | 60.77 | 73.61 |
| Bare zirconium foil (0.05 mm) | 58.7 ± 1.3 | 5.82 | 38.042 | 43.87 |


2.3. Bioactivity of Nanoporous and Nanotubular Metal Oxide Layer


3. Experimental Section
3.1. Substrate Preparation
3.2. Surface Characterization
4. Conclusions
- The nanoporous Ta2O5 layer and nanotubular ZrO2 and TiO2 layer exhibited a higher roughness than their respective bare metals but the nanoporous Nb2O5 layer exhibited a lower roughness than its bare metal.
- The nanoporous layers of Ta2O5 and Nb2O5 and the nanotubular layers of ZrO2 and TiO2 revealed an increase in hydrophilic property and surface energy compared to their respective bare metals.
- The hydrophilic property and the surface energy of the nanoporous layers of Ta2O5 and Nb2O5 and the nanotubular layers of ZrO2 and TiO2 were increased after annealing.
- After annealing the pore size of nanoporous Ta2O5 decreased, whereas the pore size of nanoporous Nb2O5 increased. This is due to their different crystalline phases before and after annealing which possess different lattice parameters. The inner diameter of ZrO2 nanotubes did not show any obvious change after annealing because the crystalline phase did not change, whilst annealing led to an increase in the inner diameter of anatase TiO2 nanotubes.
- As formed Ta2O5 and ZrO2 exhibited a good bioactivity similar to TiO2. Annealed Ta2O5 showed a high level of bioactivity, which is promising for biomedical applications. Nb2O5 did not show bioactivity before annealing; but was bioactive after annealing.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ishikawa, M.; Sugimoto, K. Multiple layer protective coating composed of Ta2O5, Cr2O3 and Al2O3 produced by the MO-CVD technique. Corros. Eng. 1989, 38, 619–630. [Google Scholar]
- Arnould, C.; Delhalle, J.; Mekhalif, Z. Corrigendum to “Multifunctional hybrid coating on titanium towards hydroxyapatite growth: Electrodeposition of tantalum and its molecular functionalization with organophosphonic acids films”. [electrochim. Acta 53 (2008) 5632–5638] (doi:10.1016/j.Electacta.2008.03.003). Electrochim. Acta 2009, 54, 2402. [Google Scholar] [CrossRef]
- Arnould, C.; Delhalle, J.; Mekhalif, Z. Multifunctional hybrid coating on titanium towards hydroxyapatite growth: Electrodeposition of tantalum and its molecular functionalization with organophosphonic acids films. Electrochim. Acta 2008, 53, 5632–5638. [Google Scholar] [CrossRef]
- Arnould, C.; Volcke, C.; Lamarque, C.; Thiry, P.A.; Delhalle, J.; Mekhalif, Z. Titanium modified with layer-by-layer sol-gel tantalum oxide and an organodiphosphonic acid: A coating for hydroxyapatite growth. J. Colloid Interface Sci. 2009, 336, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Cava, R.F.; Peck, W.F.; Krajewski, J.J. Enhancement of the dielectric-constant of Ta2O5 through substitution with TiO2. Nature 1995, 377, 215–217. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Sieber, I.; Kannan, B.; Schmuki, P. Self-assembled porous tantalum oxide prepared in H2SO4/HF electrolytes. Electrochem. Solid-State Lett. 2005, 8, J10–J12. [Google Scholar] [CrossRef]
- Sieber, I.; Hildebrand, H.; Friedrich, A.; Schmuki, P. Initiation of tantalum oxide pores grown on tantalum by potentiodynamic anodic oxidation. J. Electroceram. 2006, 16, 35–39. [Google Scholar] [CrossRef]
- Sieber, I.V.; Schmuki, P. Porous tantalum oxide prepared by electrochemical anodic oxidation. J. Electrochem. Soc. 2005, 152, C639–C644. [Google Scholar] [CrossRef]
- Wei, W.; MacAk, J.M.; Shrestha, N.K.; Schmuki, P. Thick self-ordered nanoporous Ta2O5 films with long-range lateral order. J. Electrochem. Soc. 2009, 156, K104–K109. [Google Scholar] [CrossRef]
- Wei, W.; Macak, J.M.; Schmuki, P. High aspect ratio ordered nanoporous Ta2O5 films by anodization of Ta. Electrochem. Commun. 2008, 10, 428–432. [Google Scholar] [CrossRef]
- Aagard, R.L. Optical waveguide characteristics of reactive DC-sputtered niobium pentoxide films. Appl. Phys. Lett. 1975, 27, 605–607. [Google Scholar] [CrossRef]
- Velten, D.; Eisenbarth, E.; Schanne, N.; Breme, J. Biocompatible Nb2O5 thin films prepared by means of the sol-gel process. J. Mater. Sci. Mater. Med. 2004, 15, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Mozalev, A.; Sakairi, M.; Saeki, I.; Takahashi, H. Nucleation and growth of the nanostructured anodic oxides on tantalum and niobium under the porous alumina film. Electrochim. Acta 2003, 48, 3155–3170. [Google Scholar] [CrossRef]
- Sieber, I.; Hildebrand, H.; Friedrich, A.; Schmuki, P. Formation of self-organized niobium porous oxide on niobium. Electrochem. Commun. 2005, 7, 97–100. [Google Scholar] [CrossRef]
- Lu, Q.; Hashimoto, T.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Nanoporous anodic niobium oxide formed in phosphate/glycerol electrolyte. Electrochem. Solid-State Lett. 2005, 8, B17–B20. [Google Scholar] [CrossRef]
- Chambon, L.; Maleysson, C.; Pauly, A.; Germain, J.P.; Demarne, V.; Grisel, A. Investigation, for NH3 gas sensing applications, of the Nb2O5 semiconducting oxide in the presence of interferent species such as oxygen and humidity. Sens. Actuators B Chem. 1997, 45, 107–114. [Google Scholar] [CrossRef]
- Tanabe, K.; Okazaki, S. Various reactions catalyzed by niobium compounds and materials. Appl. Catal. A Gen. 1995, 133, 191–218. [Google Scholar] [CrossRef]
- Pawlicka, A.; Atik, M.; Aegerter, M.A. Synthesis of multicolor Nb2O5 coatings for electrochromic devices. Thin Solid Films 1997, 301, 236–241. [Google Scholar] [CrossRef]
- Choi, J.; Lim, J.H.; Lee, S.C.; Chang, J.H.; Kim, K.J.; Cho, M.A. Porous niobium oxide films prepared by anodization in HF/H3PO4. Electrochim. Acta 2006, 51, 5502–5507. [Google Scholar] [CrossRef]
- Choi, J.; Lim, J.H.; Lee, J.; Kim, K.J. Porous niobium oxide films prepared by anodization–annealing–anodization. Nanotechnology 2007, 18. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T. Application of ZrO2 as a catalyst and a catalyst support. Catal. Today 1994, 20, 199–217. [Google Scholar] [CrossRef]
- Bethke, K.A.; Kung, M.C.; Yang, B.; Shah, M.; Alt, D.; Li, C.; Kung, H.H. Metal oxide catalysts for lean NOx reduction. Catal. Today 1995, 26, 169–183. [Google Scholar] [CrossRef]
- Hahn, R.; Berger, S.; Schmuki, P. Bright visible luminescence of self-organized ZrO2 nanotubes. J. Solid State Electrochem. 2010, 14, 285–288. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Schmuki, P. Thick self-organized porous zirconium oxide formed in H2SO4/NH4F electrolytes. Electrochem. Commun. 2004, 6, 1131–1134. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Taveira, L.; Schmuki, P. Fabrication and characterization of smooth high aspect ratio zirconia nanotubes. Chem. Phys. Lett. 2005, 410, 188–191. [Google Scholar] [CrossRef]
- Schmuki, P.; Tsuchiya, H.; Macak, J.; Taveira, L. Formation of self-organized zirconia nanostructure. ECS Trans. 2006, 1, 351–357. [Google Scholar]
- Berger, S.; Faltenbacher, J.; Bauer, S.; Schmuki, P. Enhanced self-ordering of anodic ZrO2 nanotubes in inorganic and organic electrolytes using two-step anodization. Phys. Status Solidi RRL 2008, 2, 102–104. [Google Scholar] [CrossRef]
- Berger, S.; Jakubka, F.; Schmuki, P. Formation of hexagonally ordered nanoporous anodic zirconia. Electrochem. Commun. 2008, 10, 1916–1919. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, S. A freestanding membrane of highly ordered anodic ZrO2 nanotube arrays. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Kaneko, H.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Mechanism of apatite formation on bioactive tantalum metal in simulated body fluid. Key Eng. Mater. 2003, 240–242, 11–14. [Google Scholar] [CrossRef]
- Nagarajan, S.; Raman, V.; Rajendran, N. Synthesis and electrochemical characterization of porous niobium oxide coated 316l ss for orthopedic applications. Mater. Chem. Phys. 2010, 119, 363–366. [Google Scholar] [CrossRef]
- Wang, X.J.; Xiong, J.Y.; Li, Y.C.; Hodgson, P.D.; Wen, C.E. Apatite formation on nano-structured titanium and niobium surface. Mater. Sci. Forum 2009, 614, 85–92. [Google Scholar] [CrossRef]
- Tang, Z.; Xie, Y.; Yang, F.; Huang, Y.; Wang, C.; Dai, K.; Zheng, X.; Zhang, X. Porous tantalum coatings prepared by vacuum plasma spraying enhance bmscs osteogenic differentiation and bone regeneration in vitro and in vivo. PLoS ONE 2013, 8, e66263. [Google Scholar] [CrossRef] [PubMed]
- Godley, R.; Starosvetsky, D.; Gotman, I. Bonelike apatite formation on niobium metal treated in aqueous NaOH. J. Mater. Sci. Mater. Med. 2004, 15, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, J.; Wang, X.; Xu, R.; Lu, Z.; Li, Y. Bioactivity of zirconia nanotube arrays fabricated by electrochemical anodization. Mater. Sci. Eng. C 2009, 29, 1174–1177. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, J.; Wang, X.; Xu, X.; Liu, H.; Li, Y. Structure and bioactivity of zirconia nanotube arrays fabricated by anodization. Int. J. Appl. Ceram. Technol. 2009, 6, 636–641. [Google Scholar] [CrossRef]
- Wang, L.N.; Adams, A.; Luo, J.L. Enhancement of the capability of hydroxyapatite formation on Zr with anodic ZrO2 nanotubular arrays via an effective dipping pretreatment. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Gomez Sanchez, A.; Ballarre, J.; Orellano, J.C.; Duffó, G.; Ceré, S. Surface modification of zirconium by anodisation as material for permanent implants: In vitro and in vivo study. J. Mater. Sci. Mater. Med. 2013, 24, 161–169. [Google Scholar]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Vanysek, P. Chemical properties of materials. In CRC Materials Science and Engineering Handbook, 3rd ed.; Shackelford, J.F., Alexander, W., Eds.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Lynch, C.T. Thermodynamic and kinetic data. In CRC Materials Science and Engineering Handbook, 3rd ed.; Shackelford, J.F., Alexander, W., Eds.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Minagar, S.; Berndt, C.C.; Gengenbach, T.; Wen, C. Fabrication and characterization of TiO2-ZrO2-ZrTiO4 nanotubes on TiZr alloy manufactured via anodization. J. Mater. Chem. B 2014, 2, 71–83. [Google Scholar] [CrossRef]
- Yasuda, K.; MacAk, J.M.; Berger, S.; Ghicov, A.; Schmuki, P. Mechanistic aspects of the self-organization process for oxide nanotube formation on valve metals. J. Electrochem. Soc. 2007, 154, C472–C478. [Google Scholar] [CrossRef]
- Guidance on the Measurement of Wettability of Insulator Surfaces; SAI Global: Sydney, NSW, Australia, 2005; p. 15.
- Kokubo, T. Apatite formation on surfaces of ceramics, metals and polymers in body environment. Acta Mater. 1998, 46, 2519–2527. [Google Scholar] [CrossRef]
- Kim, H.M.; Himeno, T.; Kawashita, M.; Kokubo, T.; Nakamura, T. The mechanism of biomineralization of bone-like apatite on synthetic hydroxyapatite: An in vitro assessment. J. Royal Soc. Interface 2004, 1, 17–22. [Google Scholar] [CrossRef]
- Ma, Q.; Li, M.; Hu, Z.; Chen, Q.; Hu, W. Enhancement of the bioactivity of titanium oxide nanotubes by precalcification. Mater. Lett. 2008, 62, 3035–3038. [Google Scholar] [CrossRef]
- Kunze, J.; Muller, L.; Macak, J.; Greil, P.; Schmuki, P.; Muller, F. Time-dependent growth of biomimetic apatite on anodic TiO2 nanotubes. Electrochim. Acta 2008, 53, 6995–7003. [Google Scholar] [CrossRef]
- Kosmulski, M. Points of zero charge. In Chemical Properties of Material Surfaces; Hubbard, A.T., Ed.; CRC Press: New York, NY, USA, 2001; pp. 731–744. [Google Scholar]
- Kosmulski, M. Surface charging in absence of strongly adsorbing species. In Chemical Properties of Material Surfaces; Hubbard, A.T., Ed.; CRC Press: New York, NY, USA, 2001; pp. 65–309. [Google Scholar]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Dann, J.R. Forces involved in the adhesive process. I. Critical surface tensions of polymeric solids as determined with polar liquids. J. Colloid Interface Sci. 1970, 32, 302–320. [Google Scholar] [CrossRef]
- Oyane, A.; Onuma, K.; Ito, A.; Kim, H.M.; Kokubo, T.; Nakamura, T. Formation and growth of clusters in conventional and new kinds of simulated body fluids. J. Biomed. Mater. Res. A 2003, 64, 339–348. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minagar, S.; Berndt, C.C.; Wen, C. Fabrication and Characterization of Nanoporous Niobia, and Nanotubular Tantala, Titania and Zirconia via Anodization. J. Funct. Biomater. 2015, 6, 153-170. https://doi.org/10.3390/jfb6020153
Minagar S, Berndt CC, Wen C. Fabrication and Characterization of Nanoporous Niobia, and Nanotubular Tantala, Titania and Zirconia via Anodization. Journal of Functional Biomaterials. 2015; 6(2):153-170. https://doi.org/10.3390/jfb6020153
Chicago/Turabian StyleMinagar, Sepideh, Christopher C. Berndt, and Cuie Wen. 2015. "Fabrication and Characterization of Nanoporous Niobia, and Nanotubular Tantala, Titania and Zirconia via Anodization" Journal of Functional Biomaterials 6, no. 2: 153-170. https://doi.org/10.3390/jfb6020153