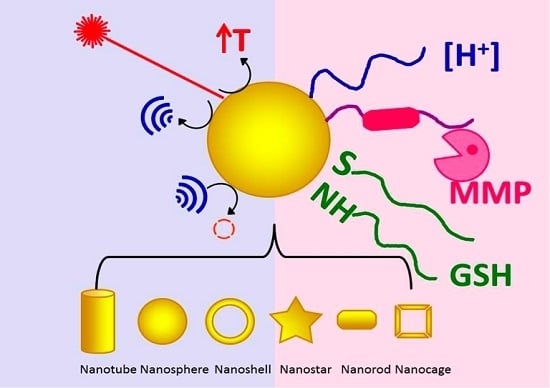
Stimuli-Responsive Gold Nanoparticles for Cancer Diagnosis and Therapy
Abstract
:1. Introduction
2. Intrinsic Stimuli
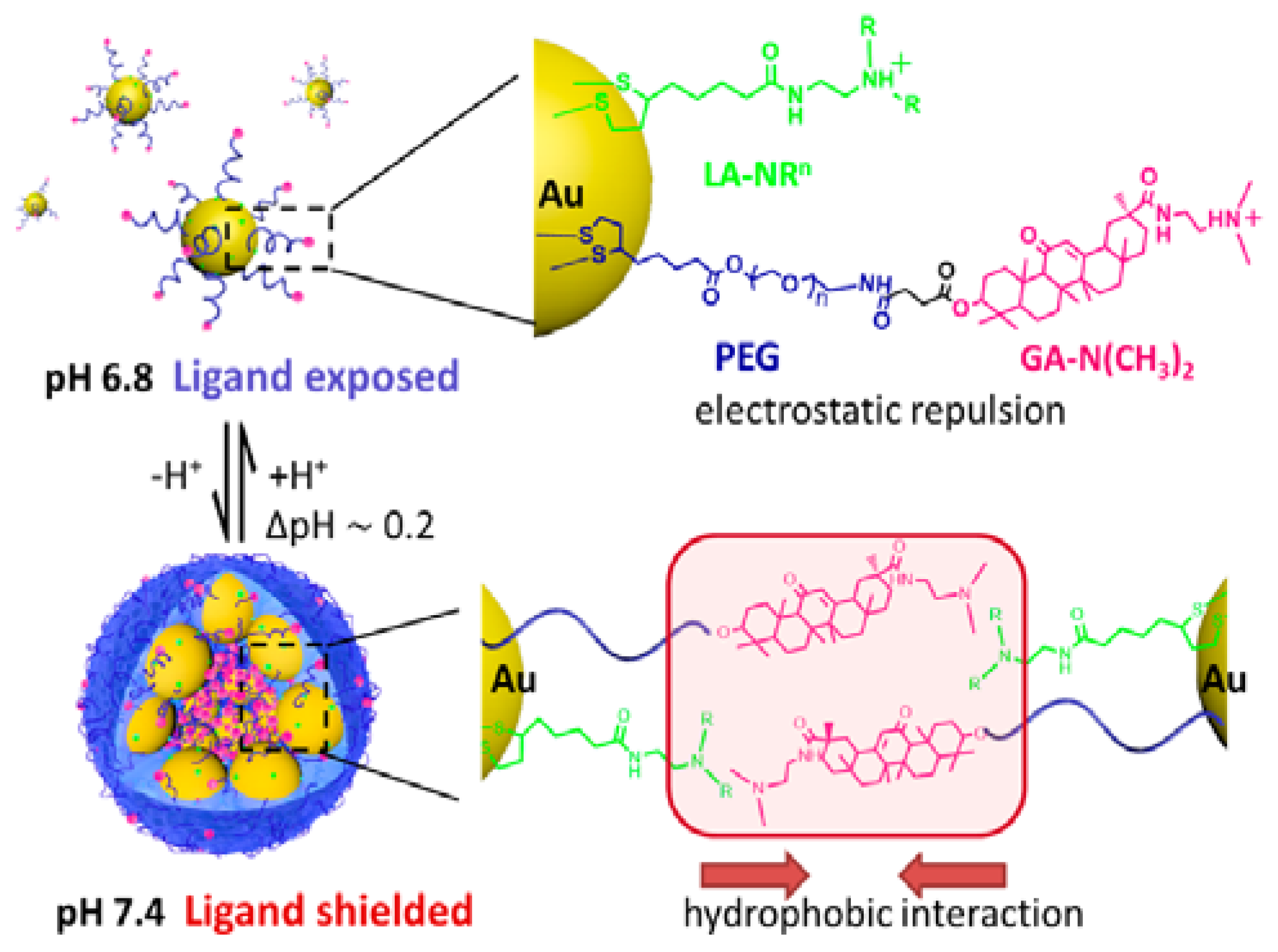
2.1. pH
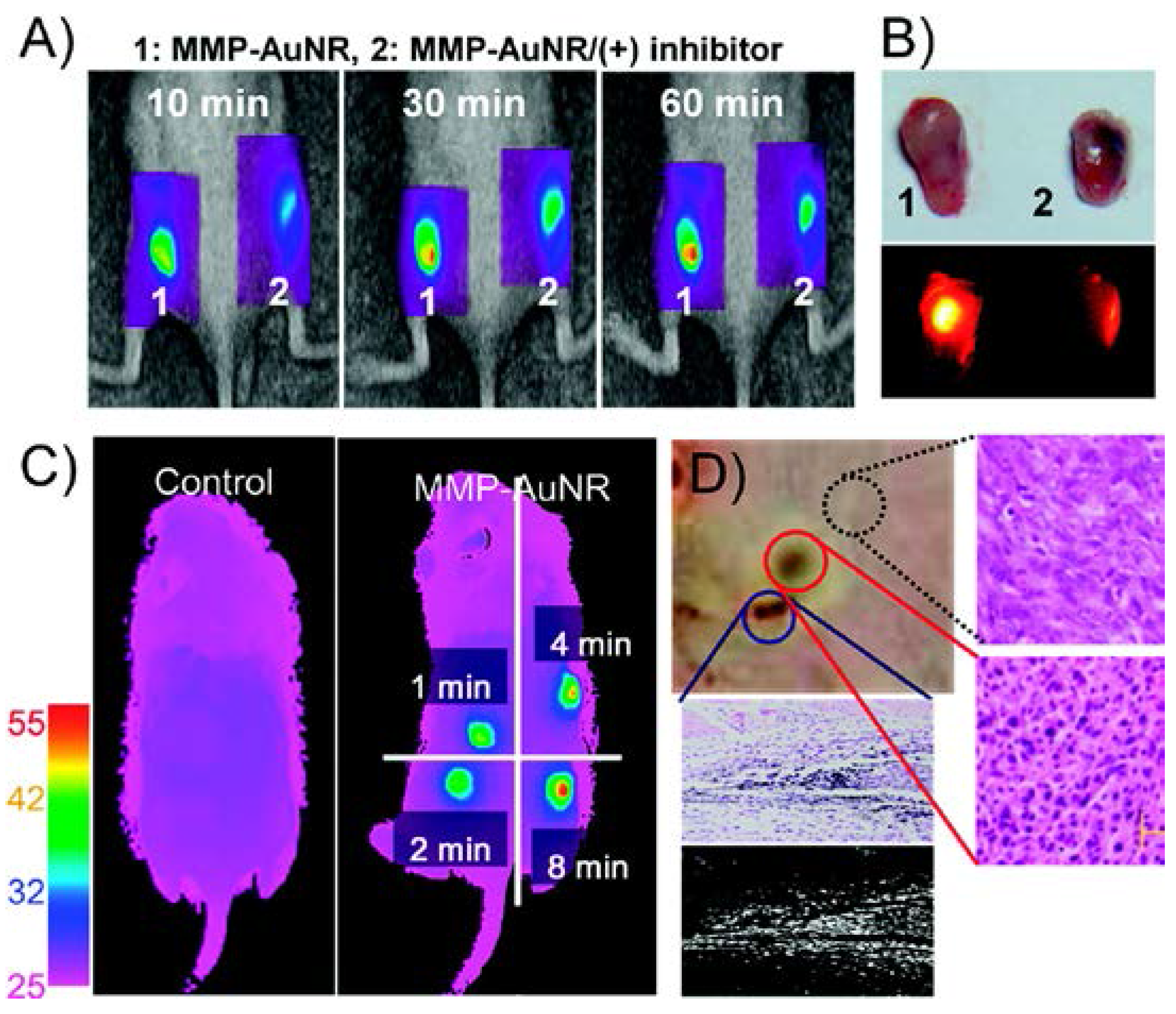
2.2. Matrix Metalloproteinases
2.3. Glutathione
3. External Stimuli
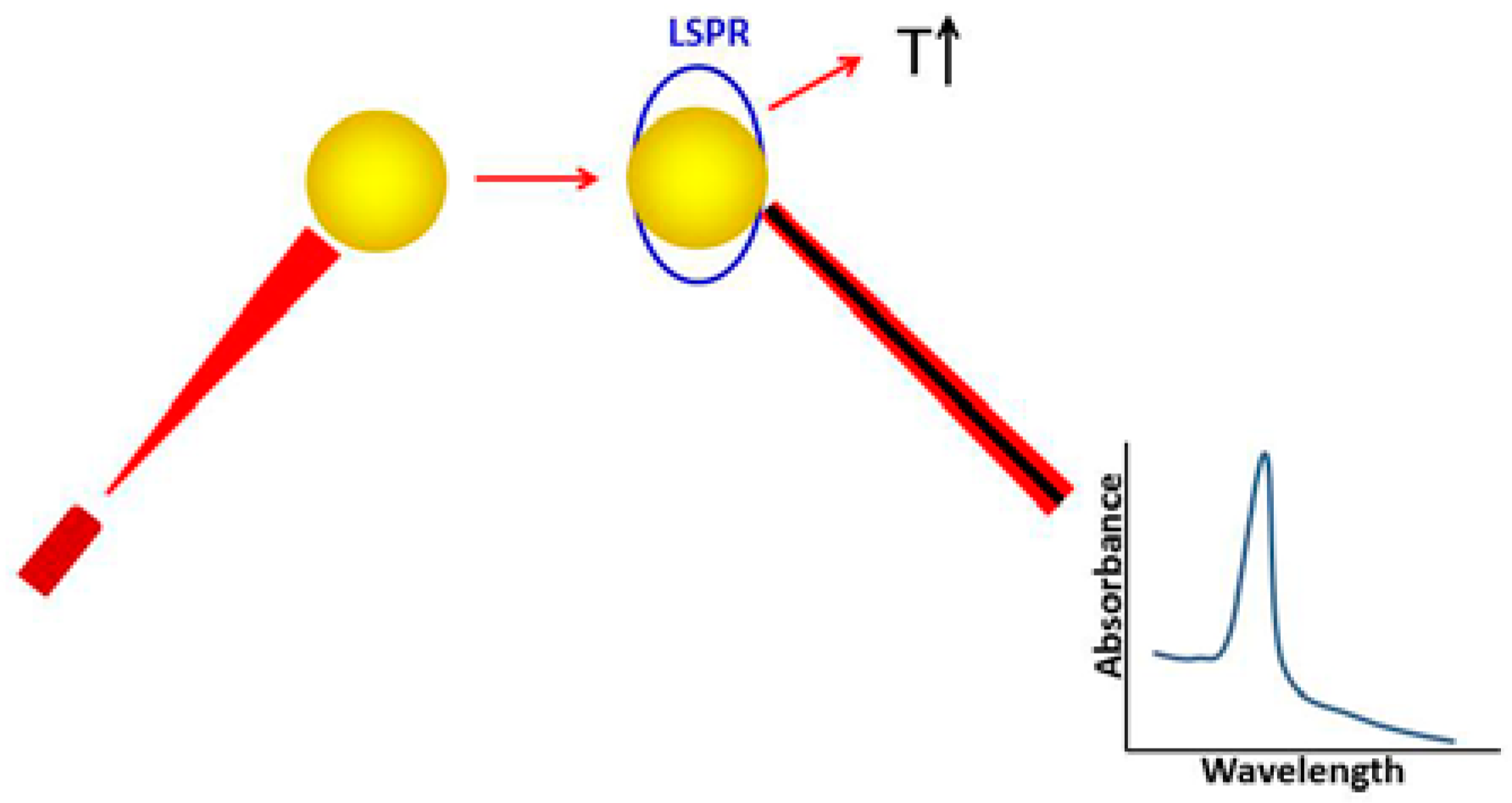
3.1. Laser
3.2. Ultrasound
3.3. Photoacoustic Imaging
3.4. X-ray Radiation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ad | adenovirus |
| Ad-MSCs | adipose-derived mesenchymal cells |
| AFM | atomic-force microscopy |
| AuNP | gold nanoparticle |
| cRGO | cyclic RGD |
| CT | computed tomography |
| CW | continuous wave |
| DLS | dynamic light scattering |
| Dox | doxorubicin |
| EGFR | epidermal growth factor receptor |
| FA | folic acid |
| GFP | green fluorescent protein |
| GGMPN | gold nanoparticles loaded with miR-122 |
| GO | graphene oxide |
| GSH | glutathione |
| HCC | hepatocellular carcinoma |
| HIFU | high intensity focused ultrasound |
| HSBDP | thiolated Bodipy dye |
| iPS | human induced pluripotent stem cells |
| LSPR | localized surface plasmon resonance |
| MB | microbubble |
| miRNA | microRNA |
| MMP | matrix metalloproteinases |
| mPEG | methoxy polyethylene glycol |
| MR | magnetic resonance |
| MTX | methotrexate |
| NIR | near infrared |
| NIRF | near-infrared fluorescence |
| PA | photoacoustic |
| PB | Prussian blue |
| PCM | phase-changing material |
| PDA | polydopamine |
| PF-PTX | paclitaxel loaded Pluronic micelles |
| P-gp | P-glycoprotein |
| PhA | pheophorbide A |
| PHF | perfluorohexane |
| PK | pharmacokinetics |
| PLGA | poly(lactic-co-glycolic acid) |
| PpIX | Protoporphyrin IX |
| rGO | reduced graphene oxide |
| SER | surface-enhanced Raman scattering |
| SERS | surface-enhanced Raman spectroscopy |
| siRNA | short interfering RNA |
| SPIO@AuNPs | superparamagnetic iron oxide-coated gold nanoparticles |
| TAT | TAT peptide |
| TN | therapeutic nanoparticle |
| TTMA | tetra(ethylene glycol)-lyated cationic ligand |
| uPIC | short interfering RNA-loaded unimer polyion complex |
| US | ultrasound |
References
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc.Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Lu, W.; Li, C. Gold-based magneto/optical nanostructures: Challenges for in vivo applications in cancer diagnostics and therapy. MRS Bull. 2009, 34, 415–421. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine (Lond.) 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Ausprunk, D.H.; Folkman, J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc. Res. 1977, 14, 53–65. [Google Scholar] [CrossRef]
- Folkman, J. Proceedings: Tumor angiogenesis factor. Cancer Res. 1974, 34, 2109–2113. [Google Scholar] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1995, 1, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Grunt, T.; Lametschwandtner, A.; Staindl, O. The vascular pattern of basal cell tumors: Light microscopy and scanning electron microscopic study on vascular corrosion casts. Microvasc. Res. 1985, 29, 371–386. [Google Scholar] [CrossRef]
- Shubik, P. Vascularization of tumors: A review. J. Cancer Res. Clin. Oncol. 1982, 103, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Atkuri, K.R.; Herzenberg, L.A.; Herzenberg, L.A. Culturing at atmospheric oxygen levels impacts lymphocyte function. Proc. Natl. Acad. Sci. USA. 2005, 102, 3756–3759. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, Z. Hypoxia or in situ normoxia: The stem cell paradigm. J. Cell. Physiol. 2009, 219, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Talks, K.L.; Turley, H.; Gatter, K.C.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J.; Harris, A.L. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 2000, 157, 411–421. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, M.; Pouyssegur, J. Hypoxia in cancer cell metabolism and pH regulation. Essays Biochem. 2007, 43, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Bobulescu, I.A.; Di Sole, F.; Moe, O.W. Na+/h+ exchangers: Physiology and link to hypertension and organ ischemia. Curr. Opin. Nephrol. Hypertens. 2005, 14, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, L.A.; Fallon, M.; Pisarcik, S.; Wang, J.; Semenza, G.L. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L941–L949. [Google Scholar] [CrossRef] [PubMed]
- Kallinowski, F.; Schlenger, K.; Runkel, S.; Kloes, M.; Stohrer, M.; Okunieff, P.; Vaupel, P. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res. 1989, 49, 3759–3764. [Google Scholar] [PubMed]
- Beaney, R.P.; Brooks, D.J.; Leenders, K.L.; Thomas, D.; Jones, T.; Halnan, K. Blood flow and oxygen utilisation in the contralateral cerebral cortex of patients with untreated intracranial tumours as studied by positron emission tomography, with observations on the effect of decompressive surgery. J. Neurol. Neurosurg. Psychiatry 1985, 48, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Controll. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Volk, T.; Jähde, E.; Fortmeyer, H.; Glüsenkamp, K.; Rajewsky, M. pH in human tumour xenografts: Effect of intravenous administration of glucose. Br. J. Cancer 1993, 68, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.; Leeper, D.; Cater, J.; Thistlethwaite, A.; Tupchong, L.; McFarlane, J. Extracellular pH distribution in human tumours. Int. J. Hyperth. 1995, 11, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yang, C.; Wang, W.; Yuan, Z. Shieldable tumor targeting based on pH responsive self-assembly/disassembly of gold nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 17865–17876. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; La, W.-G.; Hwang, S.; Ha, Y.S.; Park, N.; Won, N.; Jung, S.; Bhang, S.H.; Ma, Y.-J.; Cho, Y.-M. pH-responsive assembly of gold nanoparticles and “spatiotemporally concerted” drug release for synergistic cancer therapy. ACS Nano 2013, 7, 3388–3402. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.; Boron, W.F. Intracellular pH. Physiol. Rev. 1981, 61, 296–434. [Google Scholar] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.-J.; Sun, I.-C.; Lee, S.C.; Kim, K.; Kwon, I.C.; Ahn, C.-H. Development of a pH sensitive nanocarrier using calcium phosphate coated gold nanoparticles as a platform for a potential theranostic material. Macromol. Res. 2012, 20, 319–326. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Furukawa, S.; Kataoka, K. Block copolymer-coated calcium phosphate nanoparticles sensing intracellular environment for oligodeoxynucleotide and sirna delivery. J. Control. Release 2004, 97, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H. Activation mechanisms of matrix metalloproteinases. Biol. Chem. 1997, 378, 151–160. [Google Scholar] [PubMed]
- Nagase, H.; Woessner, J.F. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.; Murray, G. Matrix metalloproteinases: Molecular aspects of their roles in tumour invasion and metastasis. Eur. J. Cancer 2000, 36, 1621–1630. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Bogenrieder, T.; Herlyn, M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene 2003, 22, 6524–6536. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer—Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Kim, K.H.; Park, Y.H.; Ko, S.; Kim, Y.-P. Colorimetric assay of matrix metalloproteinase activity based on metal-induced self-assembly of carboxy gold nanoparticles. Biosens. Bioelectron. 2013, 41, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Ku, M.; Lee, E.; Suh, J.-S.; Huh, Y.-M.; Yoon, D.S.; Yang, J. Localized surface plasmon resonance based nanobiosensor for biomarker detection of invasive cancer cells. J. Biomed. Opt. 2014, 19, 051202. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, Y.; Yan, F.; Ding, L.; Ding, S.; Ju, H.; Yin, Y. Ultrasensitive scanometric strategy for detection of matrix metalloproteinases using a histidine tagged peptide–Au nanoparticle probe. Chem. Commun. 2011, 47, 2877–2879. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.K.; Weng, Y.; Li, Z.; Zerda, R.; Van Haute, D.; Williams, J.C.; Berlin, J.M. Matrix metalloproteinase-triggered denuding of engineered gold nanoparticles for selective cell uptake. J. Mater. Chem. B 2013, 1, 2341–2349. [Google Scholar] [CrossRef]
- Yi, D.K.; Sun, I.-C.; Ryu, J.H.; Koo, H.; Park, C.W.; Youn, I.-C.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.-H. Matrix metalloproteinase sensitive gold nanorod for simultaneous bioimaging and photothermal therapy of cancer. Bioconjugate Chem. 2010, 21, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Xu, X.-D.; Jia, H.-Z.; Lei, Q.; Luo, G.-F.; Cheng, S.-X.; Zhuo, R.-X.; Zhang, X.-Z. Therapeutic nanomedicine based on dual-intelligent functionalized gold nanoparticles for cancer imaging and therapy in vivo. Biomaterials 2013, 34, 8798–8807. [Google Scholar] [CrossRef] [PubMed]
- Arrick, B.A.; Nathan, C.F. Glutathione metabolism as a determinant of therapeutic efficacy: A review. Cancer Res. 1984, 44, 4224–4232. [Google Scholar] [PubMed]
- Soliman, M.; Allen, S.; Davies, M.C.; Alexander, C. Responsive polyelectrolyte complexes for triggered release of nucleic acid therapeutics. Chem. Commun. 2010, 46, 5421–5433. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Kang, H.C.; Bae, Y.H. Bioreducible polymers as a determining factor for polyplex decomplexation rate and transfection. Biomacromolecules 2013, 14, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Manickam, D.S.; Oupický, D.; Mao, G. DNA release dynamics from reducible polyplexes by atomic force microscopy. Langmuir 2008, 24, 12474–12482. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.C.; Lee, M.; Bae, Y.H. Polymeric gene carriers. Crit. Rev. Eukaryot. Gene Expr. 2005, 15, 317–342. [Google Scholar] [CrossRef]
- HeeáKook, Y.; TaxáOh, E.; JooáPark, H. Cyclodextrin-covered gold nanoparticles for targeted delivery of an anti-cancer drug. J. Mater. Chem. 2009, 19, 2310–2315. [Google Scholar]
- Heo, D.N.; Yang, D.H.; Moon, H.-J.; Lee, J.B.; Bae, M.S.; Lee, S.C.; Lee, W.J.; Sun, I.-C.; Kwon, I.K. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 2012, 33, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Han, G.; Fernández, J.M.; Kim, B.-j.; Forbes, N.S.; Rotello, V.M. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006, 128, 1078–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, X.; Hu, J.; Shao, N.; Wang, F.; Zhang, Q.; Xiao, J.; Cheng, Y. Glutathione-triggered “off–on” release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nurunnabi, M.; Nafiujjaman, M.; Lee, Y.-K.; Huh, K.M. GSH-mediated photoactivity of pheophorbide a-conjugated heparin/gold nanoparticle for photodynamic therapy. J. Control. Release 2013, 171, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Takemoto, H.; Yi, Y.; Zheng, M.; Maeda, Y.; Chaya, H.; Hayashi, K.; Mi, P.; Pittella, F.; Christie, R.J. Precise engineering of sirna delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS Nano 2014, 8, 8979–8991. [Google Scholar] [CrossRef] [PubMed]
- Egusa, S.; Ebrahem, Q.; Mahfouz, R.Z.; Saunthararajah, Y. Ligand exchange on gold nanoparticles for drug delivery and enhanced therapeutic index evaluated in acute myeloid leukemia models. Exp. Biol. Med. 2014, 239, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Ock, K.-S.; Ganbold, E.O.; Park, J.; Cho, K.; Joo, S.-W.; Lee, S.Y. Label-free raman spectroscopy for accessing intracellular anticancer drug release on gold nanoparticles. Analyst 2012, 137, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Han, J.; Ye, C.; Thomas, T.; Dou, H. Reduction-responsive gold-nanoparticle-conjugated pluronic micelles: An effective anti-cancer drug delivery system. J. Mater. Chem. 2012, 22, 18864–18871. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.; El-Sayed, I.; El-Sayed, M. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 3, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Tong, L.; Wei, Q.; Wei, A.; Cheng, J.X. Gold nanorods as contrast agents for biological imaging: Optical properties, surface conjugation and photothermal effects. Photochem. Photobiol. 2009, 85, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, A.M.; Zhang, J.Z. Novel optical properties and emerging applications of metal nanostructures. J. Phys. Chem. C 2008, 112, 10323–10337. [Google Scholar] [CrossRef]
- Melancon, M.P.; Zhou, M.; Li, C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc. Chem. Res. 2011, 44, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Bischof, J.C. Thermophysical and biological responses of gold nanoparticle laser heating. Chem. Soc. Rev. 2012, 41, 1191–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wallace, M.; Melancon, M.P. Cancer theranostics with gold nanoshells. Nanomedicine 2014, 9, 2041–2057. [Google Scholar] [CrossRef] [PubMed]
- Singhana, B.; Slattery, P.; Chen, A.; Wallace, M.; Melancon, M.P. Light-activatable gold nanoshells for drug delivery applications. AAPS PharmSciTech 2014, 15, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.B.; Tong, L.; Zhao, Y.; Hansen, M.N.; Cheng, J.-X.; Wei, A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine 2007, 2, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.L.; Melancon, M.P.; Abdelsalam, M.; Figueira, T.A.; Dixon, K.; McWatters, A.; Zhou, M.; Huang, Q.; Mawlawi, O.; Dunner, J. Imaging intratumoral nanoparticle uptake after combining nanoembolization with various ablative therapies in hepatic VX2 rabbit tumors. J. Biomed. Nanotechnol. 2016, 12, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Silva, A.K.A.; Sánchez-Iglesias, A.; Grzelczak, M.; Péchoux, C.; Desboeufs, K.; Liz-Marzán, L.M.; Wilhelm, C. Cancer cell internalization of gold nanostars impacts their photothermal efficiency in vitro and in vivo: Toward a plasmonic thermal fingerprint in tumoral environment. Adv. Healthc. Mater. 2016, 5, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Larson, A.C. Deoxycholate bile acid directed synthesis of branched Au nanostructures for near infrared photothermal ablation. Biomaterials 2015, 56, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Xia, W.; Liu, L.; Niu, L.; Lu, Q. Golden single-walled carbon nanotubes prepared using double layer polysaccharides bridge for photothermal therapy. ACS Appl. Mater. Interfaces 2014, 6, 4989–4996. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Gerlach, W.; Ghandehari, H. Comparative effect of gold nanorods and nanocages for prostate tumor hyperthermia. J. Control. Release 2015, 220, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT peptide-functionalized gold nanostars: Enhanced intracellular delivery and efficient NIR photothermal therapy using ultralow irradiance. J. Am. Chem. Soc. 2012, 134, 11358–11361. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Dai, S.; Ma, Y.; Cui, S.; Achilefu, S.; Gu, Y. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics 2013, 3, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wu, Y.-J.; Chen, J.-J. Gold nanotheranostics: Photothermal therapy and imaging of Mucin 7 conjugated antibody nanoparticles for urothelial cancer. Biomed. Res. Int. 2015, 2015, 813632. [Google Scholar] [CrossRef] [PubMed]
- Mocan, L.; Matea, C.; Tabaran, F.A.; Mosteanu, O.; Pop, T.; Mocan, T.; Iancu, C. Photothermal treatment of liver cancer with albumin-conjugated gold nanoparticles initiates Golgi apparatus-ER dysfunction and caspase-3 apoptotic pathway activation by selective targeting of Gp60 receptor. Int. J. Nanomed. 2015, 10, 5435–5445. [Google Scholar]
- Jing, L.; Liang, X.; Deng, Z.; Feng, S.; Li, X.; Huang, M.; Li, C.; Dai, Z. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials 2014, 35, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Lu, P.-H.; Yang, H.-C.; Lee, G.-D.; Li, H.-R.; Liao, K.-C. Fiber-optic triggered release of liposome in vivo: Implication of personalized chemotherapy. Int. J. Nanomed. 2015, 10, 5171–5184. [Google Scholar]
- Rengan, A.K.; Bukhari, A.B.; Pradhan, A.; Malhotra, R.; Banerjee, R.; Srivastava, R.; De, A. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015, 15, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.Y.; Ren, X.; Sawant, R.R.; Wu, X.; Torchilin, V.P.; Lapotko, D.O. On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nat. Med. 2014, 20, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Wang, Y.; Gong, B.; Xiao, Y.; Chen, Y.; Wang, S.; Li, S.; Huang, F.; Shen, Y.; Xie, A. Reduced graphene oxide/amaranth extract/AuNPs composite hydrogel on tumor cells as integrated platform for localized and multiple synergistic therapy. ACS Appl. Mater. Interfaces 2015, 7, 11246–11256. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Y.; Liu, B.; Wu, H.; Kang, Y.; Li, M.; Zeng, X.; He, N.; Zhang, G. The effects of multifunctional MiR-122-loaded graphene-gold composites on drug-resistant liver cancer. J. Nanobiotechnol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Liu, T.-Y.; Chen, P.-J.; Chang, P.-H.; Chen, S.-Y. A high-sensitivity and low-power theranostic nanosystem for cell SERS imaging and selectively photothermal therapy using anti-EGFR-conjugated reduced graphene oxide/mesoporous silica/AuNPs nanosheets. Small 2016, 12, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Zhang, J.; Zhi, X.; Li, C.; Zhang, C.; Pan, F.; Wang, K.; Yang, Y.; Martinez de la Fuente, J. Human induced pluripotent stem cells for tumor targeted delivery of gold nanorods and enhanced photothermal therapy. ACS Nano 2016, 10, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Vykoukal, J.; Abdelsalam, M.; Recio-Boiles, A.; Huang, Q.; Qiao, Y.; Singhana, B.; Wallace, M.; Avritscher, R.; Melancon, M.P. Stem cell-mediated delivery of SPIO-loaded gold nanoparticles for the theranosis of liver injury and hepatocellular carcinoma. Nanotechnology 2014, 25, 405101. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Mallidi, S.; Mehrmohammadi, M.; Truby, R.; Homan, K.; Joshi, P.; Chen, Y.-S.; Sokolov, K.; Emelianov, S. Magneto-photo-acoustic imaging. Biomed. Opt. Express 2011, 2, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Kennedy, A.M.; Shea, J.E.; Scaife, C.L.; Nam, K.-H. Ultrasonic nanotherapy of pancreatic cancer: Lessons from ultrasound imaging. Mol. Pharm. 2010, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.A.; Singh, A.K.; Sharma, P.; Brown, S.C.; Moudgil, B.M. Nanoparticles as contrast agents for in vivo bioimaging: Current status and future perspectives. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Manthe, R.L.; Foy, S.P.; Krishnamurthy, N.; Sharma, B.; Labhasetwar, V. Tumor ablation and nanotechnology. Mol. Pharm. 2010, 7, 1880–1898. [Google Scholar] [CrossRef] [PubMed]
- Varchi, G.; Foglietta, F.; Canaparo, R.; Ballestri, M.; Arena, F.; Sotgiu, G.; Guerrini, A.; Nanni, C.; Cicoria, G.; Cravotto, G. Engineered porphyrin loaded core-shell nanoparticles for selective sonodynamic anticancer treatment. Nanomedicine 2015, 10, 3483–3494. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.T.; Bao, A.; Brenner, A.J.; Goins, B.A. Image-guided interventional therapy for cancer with radiotherapeutic nanoparticles. Adv. Drug Deliv. Rev. 2014, 76, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.-H.; Ma, M.; Chen, Y.; Jia, X.-Q.; Xu, G.; Xu, H.-X.; Chen, H.-R.; Wu, R. Multifunctional Bi2S3/PLGA nanocapsule for combined HIFU/radiation therapy. Biomaterials 2014, 35, 8197–8205. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Qian, X.; Qian, K.; Zhang, W.; He, W.; Chen, Y.; Han, J.; Zhang, Y.; Yang, X.; Fan, L. Au nanoparticle-coated, PLGA-based hybrid capsules for combined ultrasound imaging and HIFU therapy. J. Mater. Chem. B 2015, 3, 4213–4220. [Google Scholar] [CrossRef]
- Frazier, N.; Ghandehari, H. Hyperthermia approaches for enhanced delivery of nanomedicines to solid tumors. Biotechnol. Bioeng. 2015, 112, 1967–1983. [Google Scholar] [CrossRef] [PubMed]
- Farny, C.H.; Wu, T.; Holt, R.G.; Murray, T.W.; Roy, R.A. Nucleating cavitation from laser-illuminated nano-particles. Acoust. Res. Lett. Online 2005, 6, 138–143. [Google Scholar] [CrossRef]
- Maharajan Sivasubramanian, Y.H.; Lo, L.-W. Nanoparticle-facilitated functional and molecular imaging for the early detection of cancer. Front. Mol. Biosci. 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Sazgarnia, A.; Shanei, A.; Shanei, M.M. Monitoring of transient cavitation induced by ultrasound and intense pulsed light in presence of gold nanoparticles. Ultrason. Sonochem. 2014, 21, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Roy, R.A.; Murray, T.W. Gold nanoparticle targeted photoacoustic cavitation for potential deep tissue imaging and therapy. Biomed. Opt. Express 2013, 4, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Zheng, Y.; Ma, M.; Chen, Y.; Zhang, K.; Zeng, D.; Shi, J. Au-nanoparticle coated mesoporous silica nanocapsule-based multifunctional platform for ultrasound mediated imaging, cytoclasis and tumor ablation. Biomaterials 2013, 34, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Tarapacki, C.; Karshafian, R. Enhancing laser therapy using PEGylated gold nanoparticles combined with ultrasound and microbubbles. Ultrasonics 2015, 57, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Costley, D.; Mc Ewan, C.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sazgarnia, A.; Shanei, A.; Meibodi, N.T.; Eshghi, H.; Nassirli, H. A novel nanosonosensitizer for sonodynamic therapy in vivo study on a colon tumor model. J. Ultrasound Med. 2011, 30, 1321–1329. [Google Scholar] [PubMed]
- Moon, G.D.; Choi, S.-W.; Cai, X.; Li, W.; Cho, E.C.; Jeong, U.; Wang, L.V.; Xia, Y. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J. Am. Chem. Soc. 2011, 133, 4762–4765. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gradilla, V.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Yardımcı, C.; Wiitala, D.; Galarnyk, M.; Wang, J. Ultrasound-propelled nanoporous gold wire for efficient drug loading and release. Small 2014, 10, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, X.; Kim, C.; Sun, G.; Zhang, Y.; Deng, R.; Yang, M.; Chen, J.; Achilefu, S.; Wang, L.V.; et al. Gold nanocages covered with thermally-responsive polymers for controlled release by high-intensity focused ultrasound. Nanoscale 2011, 3, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Carlisle, R.; Laga, R.; Myers, R.; Graham, S.; Cawood, R.; Ulbrich, K.; Seymour, L.; Coussios, C.-C. Increasing the density of nanomedicines improves their ultrasound-mediated delivery to tumours. J Control. Release 2015, 210, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Olafsson, R.; Bauer, D.R.; Montilla, L.G.; Witte, R.S. Real-time, contrast enhanced photoacoustic imaging of cancer in a mouse window chamber. Opt. Express 2010, 18, 18625–18632. [Google Scholar] [CrossRef] [PubMed]
- Conversano, F.; Soloperto, G.; Greco, A.; Ragusa, A.; Casciaro, E.; Chiriacò, F.; Demitri, C.; Gigli, G.; Maffezzoli, A.; Casciaro, S. Echographic detectability of optoacoustic signals from low-concentration PEG-coated gold nanorods. Int. J. Nanomed. 2012, 7, 4373–4389. [Google Scholar]
- Shah, J.; Park, S.; Aglyamov, S.; Larson, T.; Ma, L.; Sokolov, K.; Johnston, K.; Milner, T.; Emelianov, S.Y. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J. Biomed. Opt. 2008, 13, 034024. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, Y.-S.; Luke, G.P.; Emelianov, S.Y. In-vivo ultrasound and photoacoustic image-guided photothermal cancer therapy using silica-coated gold nanorods. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Kim, B.; Wang, L.V.; Lanza, G.M. A brief account of nanoparticle contrast agents for photoacoustic imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 517–543. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast agents for photoacoustic and thermoacoustic imaging: A review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.J.; Hu, S.; Klibanov, A.L.; Hossack, J.A. Oscillatory dynamics and in vivo photoacoustic imaging performance of plasmonic nanoparticle-coated microbubbles. Small 2015, 11, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Pramanik, M.; Senpan, A.; Allen, J.S.; Zhang, H.; Wickline, S.A.; Wang, L.V.; Lanza, G.M. Molecular photoacoustic imaging of angiogenesis with integrin-targeted gold nanobeacons. FASEB J. 2011, 25, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Mesbahi, A. A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep. Pract. Oncol. Radiother. 2010, 15, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Coulter, J.A.; Hounsell, A.R.; Butterworth, K.T.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Zeng, J.; Wang, X.; Yang, X.; Yang, J.; McQuarrie, S.; McEwan, A.; Roa, W.; Chen, J.; Xing, J.Z. Enhancement of radiation cytotoxicity in breast–cancer cells by localized attachment of gold nanoparticles. Small 2008, 4, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.-M.; Liu, P.-X.; Liang, X.-J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [PubMed]
| Structure and Size at Basic/Physiological pH (7.4) | Structure and Size at Acidic pH | In Vitro Effects | In Vivo Effects | Ref. |
|---|---|---|---|---|
 ~80 nm |  50 nm (pH = 6.8) | Increased cellular uptake at pH 6.8 when compared with pH 7.4 | None | [23] |
 10 nm |  ~250 nm (pH 2.0); ~50 nm (pH 5.5) | Synergistic effect of Dox release and photothermal ablation (660-nm laser) | Significant tumor suppression without noticeable damage to other organs | [24] |
 79.8 ± 18.7 nm |  Dissolution of calcium phosphate layer at acidic pH. Size: N/A | Higher cytotoxicity on HeLa cells | None | [27] |
| Structure and Size before MMP Exposure | Structure after MMP Exposure | Targeting MMP Subclass | In Vitro Effects | In Vivo Effects | Ref. |
|---|---|---|---|---|---|
 Mean diameter 21.5 nm |  | MMP-7 | Extinction ratio (E520/E700) as function of MMP concentration | None | [37] |
 Length 35.2 ± 1.5 nm Width 10.8 ± 0.9 nm |  | MT1-MMP | LSPR blueshift after MT1-MMP cleavage. λmax and Δλmax depend on MT1-MMP proteolytic activity in cell lysate. | None | [38] |
 5 nm |  | MMP-7 | Relative intensity of scanometric image as a function of MMP-7 concentration | None | [39] |
 Core 5 nm Whole NP 26 nm |  311 nm | MMP-2 | Enhanced cellular uptake on MDA-MB-231 cells | None | [40] |
 Aspect ratio 2:1 |  | MMP-3, -7, -9, -13 | NIRF imaging of MMP by releasing Cy5.5. Cytotoxicity on HeLa cells by photothermal ablation (671 nm CW laser source) | Maximum NIRF intensity 60 min after injection. Temperature exceeded 45 °C after 4 min of irradiation (SCC-7 tumor xenograft) | [41] |
 Core 13 nm |  | MMP-2 + GSH | Dox release by MMP2 (increased fluorescence), increased cytotoxicity (further intracellular Dox release by GSH) on SCC-7 and HT-29 cells | Increased fluorescence intensity at the tumor site 30 min after injection. Comparable antitumor effect with free Dox, but much lower systemic toxicity and higher animal survival | [42] |
| Structure and Size before GSH Exposure | Structure and Size after GSH Exposure | In Vitro Effects | In Vivo Effects | Ref. |
|---|---|---|---|---|
 Core 27.0 ± 3 nm–27.6 ± 3 nm |  | Release of β-lapachone by GSH. Enhanced cellular uptake by anti-EGFR ligand. Enhanced apoptosis than free β-lapachone (A549 cells). | None | [49] |
 20–40 nm |  | Enhanced cellular uptake on HeLa, A549, and MG63 cell lines. Higher cytotoxicity on HeLa cells as compared with NIH3T3 cells. | None | [50] |
 Core 2 nm |  | Enhanced cellular uptake by TTMA, and HSBDP release by GSH (HepG2 cells). | None | [51] |
 3 nm |  | Improved cytotoxicity as compared with free chemo agent (HeLa cells). | None | [52] |
 Core 31 nm PhA-heparin/AuNP 40 nm |  | Higher cellular uptake and cytotoxicity of PhA-heparin/AuNP as compared with PhA (A549 cells, 670 nm laser source). | Prolonged circulation, improved tumor specificity, reduced tumor size (15 days) (A549 xenograft on SKH1 nude mice). | [53] |
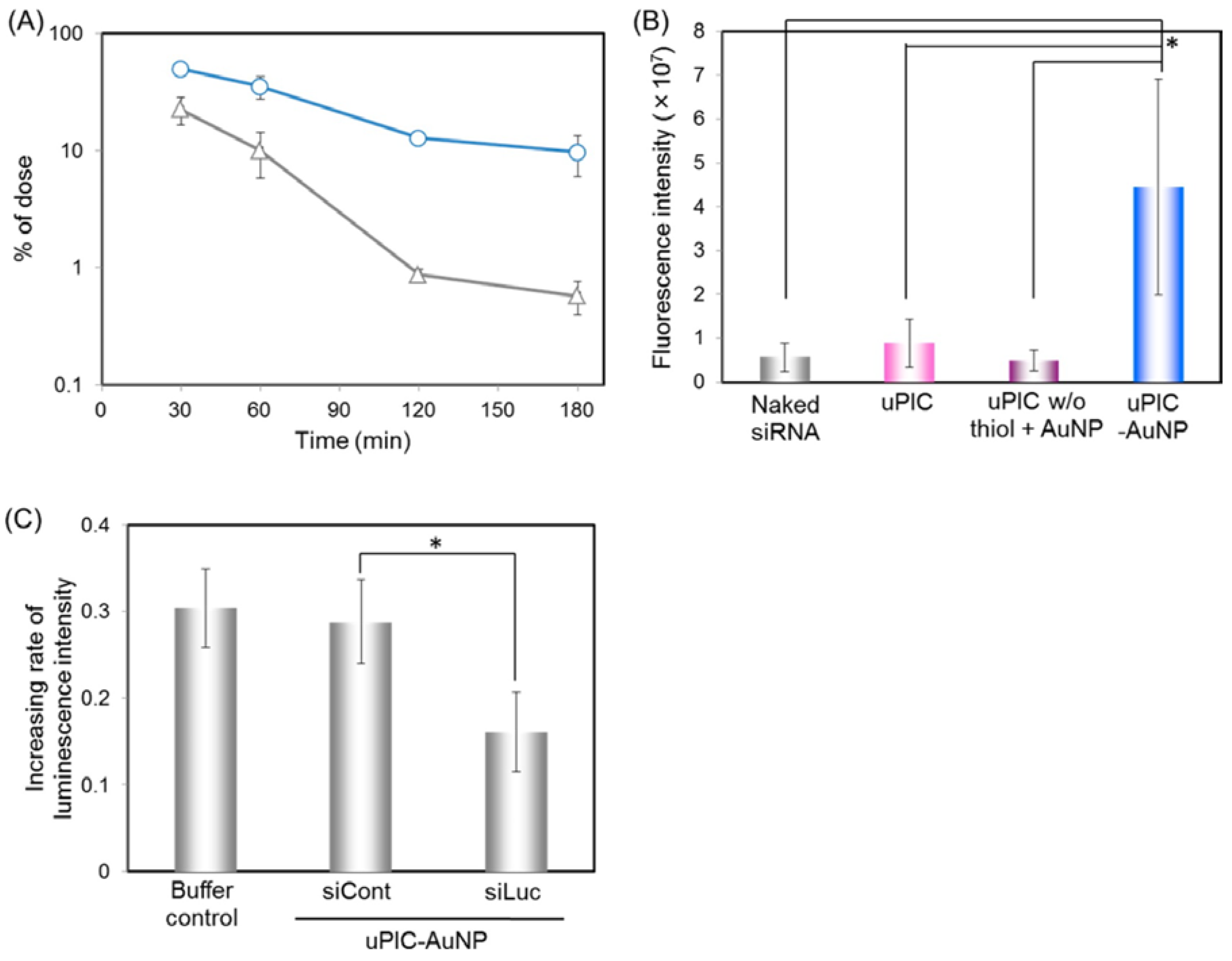
 20 nm uPIC-AuNP 38 nm |  | Reduced luciferase activity from gene silencing by siRNA delivery to luciferase-expressing HeLa (HeLa-Luc) cells. | Significant luciferase silencing on HeLa-Luc xenograft. | [54] |
 Size varies according to different chemo agent AuNP:MTX 2.6 ± 0.7 nm |  | MTX and Au:MTX had similar cytotoxicity on THP-1cell line. | Au:MTX had better leukemia suppression than MTX in a murine xenotransplant model of primary human AML. | [55] |
 ~20 nm |  | AuNPs containing different chemo agent had drug release and SER intensities after cellular uptake (HeLa cells). | None | [56] |
 Size of whole NP varied according to formulation |  | Au-PF-PTX-micelles had higher cytotoxicity on U87 cells pretreated with GSH monoester. | PK and biodistribution studied on BALB/C mice. Au-PF-PTX-micelles preferentially accumulated in spleen and liver. | [57] |
| AuNP Structure and Size | In Vitro Effects | In Vivo Effects | Ref. |
|---|---|---|---|
 25–150 nm | SPR peaked between 500 and 1000 nm. The dependence of heat efficiency on size and wavelength decreased after cellular uptake (PC3 cells). | The dependence of heat efficiency on size and wavelength decreased only after cellular uptake (intratumoral injection of Au stars, PC3 xenograft). | [69] |
 Branched AuNPs Deoxycholate concentration-dependent particle size | cRGD-branched AuNPs decreased cell viability on BxPC3 cells after photothermal ablation (NIR laser source at 808 nm, 1.4 W/cm2, for 3 min). | cRGD-branched AuNPs + NIR laser irradiation had the best antitumor effect on BxPC3 xenograft. | [70] |
 Raw carbon nanotubes 1–2 nm Size of final product varied. | Increased cytotoxicity in combination with NIR irradiation at 808 nm for 15 min (1.6 W, spot size 5 × 20 mm2, HeLa cells). | None | [71] |
 Nanorods 60 × 14.8 ± 6.5 × 2.0 nm  Nanocage edge length 50 ± 7 nm | Compared to nanorod, nanocage had higher light-to-heat transduction efficiencies and higher cellular uptake (HUVEC and DU145 cells). | Compared to nanorod, nanocage had more optimal biodistribution profile over time and higher excretion rate. | [72] |
 | TAT facilitated cellular uptake. Higher photothermolysis efficiency on BT549 breast cancer cells (850 nm pulsed laser source under 0.2 W/cm2 irradiation). | None | [73] |
 Au nanostar 32.6 nm Au-cRCD-Dox 124.7 nm | cRGD facilitated cellular uptake. Synergistic effect of photothermal therapy and chemotherapy (765 nm high power multimode pump laser, 1.0 W/cm2, 10–15 min, MDA-MB-231 and Bel-7402 cells). | Prominent accumulation in tumor and reticuloendothelial system in the liver, and synergistic effect of photothermal therapy and chemotherapy (S180 xenograft). | [74] |
 47 nm | Enhanced photothermal therapy outcome on Mucin-7-expressing MBT2, T24, 9202, and 8301 cells at low energy levels (500 exposures, 532 nm laser) | None | [75] |
 AuNP 9.1 ± 0.64 nm Au@PB NPs 17.8 ± 2.3 nm | Enhanced photothermal cytotoxicity (HeLa cells, NIR 808 nm laser, 1.5W/cm2, 10 min). Concentration dependent X-ray, CT, and photoacoustic signals. | Enabled photothermal ablation and simultaneous photoacoustic/CT bimodal imaging (HT-29 xenograft). | [77] |
 AuNP 3–7 nm AuNP-liposome 100 nm | Laser induced disintegration of liposome and triggered release of fluorescein (fiber-optic guided 65 mW laser, 532 nm). | Higher tumoral retention of fluorescein by liposome as compared to free fluorescein, and fluorescein release triggered by laser (MDA-MB 231 cell xenograft). | [78] |
 LiposAuNPs 100–120 nm | LiposAuNPs were biocompatible on NIT-3T3 cell line, but exhibited cytotoxicity in combination with laser irradiation (MCF-7 and HT1080 cells). | In situ degradation in hepatocytes and clearance through hepatobiliary and renal routes. Complete tumor ablation using NIR laser (750 nm). | [79] |
 60 nm | Intracellular synergy by (1) nanocluster formation after cellular internalization of AuNPs and TNs; (2) release of the chemo agent upon receiving laser pulse by generation of plasmonic nanobubbles; (3) amplification of X-ray. (HN31 cell lines). | Quadrapeutics system including AuNPs, TNs, laser, and X-ray had the most improved efficacy on fast-growing aggressive HN31 xenograft, as compared with standard chemoradiation. | [80] |
 35 nm | Hydrogel shell formation on cells. Enhanced cytotoxicity via combination of photothermal therapy and photodynamic therapy (808 nm, 200 mW/cm2, HeLa and Chinese hamster ovary cells). | None | [81] |
 AuNP 5 nm, FA- miR-122-AuNP 20 nm, GO and GGMPN nanocomposites 500 nm | P-gp antibody and FA facilitated cell targeting. Increased apoptosis on drug-resistant HepG2 cells. | Apoptosis induction and tumor growth inhibition on HepG2 xenograft (semiconductor laser light source, 10 min, every 2 days, 10 treatments). | [82] |
 rGO (< 200 nm) Thickness of GO/silica nanosheets 44 nm, AuNP 4 nm | Anti-EGFR SERS probe nanocomposite. Cancer cell tracking by Raman imaging. Enhanced cytotoxicity by synergistic photothermal effect of AuNP and rGO (808 nm laser, 0.5 W/cm2, A549 cells). | None | [83] |
 Nanorods length 65.0± 7.5 nm and width 12.0 ± 1.5 nm | Loading of nanorods@ SiO2@CXCR4 into human iPS cells. Reservation of viability of iPS cells and photothermal property of Au nanorods. | Stem cell mediated tumoral delivery, MGC803 xenograft. Prolonged tumoral retention confirmed by photoacoustic and two-photon luminescence imaging. Tumor growth inhibition via photothermal therapy (NIR laser at 808 nm 1.5 W/cm2) | [84] |
 SPIO 10 nm SPIO@AuNPs 82 ± 4 nm | Loading of SPIO@AuNPs into AD-MSCs. Reservation of viability of AD-MSCs and photothermal and magnetic properties of SPIO@AuNPs. Photothermal ablation of HepG2 cells by SPIO@AuNP–loaded AD-MSCs. | Homing of AD-MSCs to liver injuries or HCC confirmed by MR imaging and histologic analysis. | [85] |
 30–50 nm | None | Proved NPs interact with ablative techniques differently. Cellular incorporation of NP was only observed after combination with irreversible electroporation. Structural deformation was only observed in combination with laser-induced thermal therapy (808 nm NIR laser). | [68] |
| Structure and Size of AuNP | In Vitro Effects | In Vivo Effects | Ref. |
|---|---|---|---|
 PLGA nanocapsule 690 nm PDA/PLGA 725 nm AuNPs@PDA/PLGA 930 nm | Low cytotoxicity on ECA-109 cells. Increased echogenicity with increased concentration. AuNPs@PDA/PLGA suitable for US imaging. | Highly efficient US guided HIFU ablation ex vivo on degassed bovine liver. | [93] |
 AuNPs 82 ± 6 nm embedded in 7% acrylamide gel phantom | Cavitation activity dependence on HIFU pressure and laser energy was studied. | None | [95] |
 Nanosphere 82 nm  Nanorods 25 nm × 81 nm | PA cavitation vs US pressure and NP concentration was studied. Cavitation threshold fluences decreased with the presence of US. Indicated feasibility of producing PA cavitation in deep tissue within the safe range of US and laser irradiation. | None | [98] |
 7 nm | None | AuNP-PpIX in combination with US had higher antitumor efficacy. | [102] |
 Mesoporous silica nanocapsules 250 nm, with 50 nm shell, AuNP 5–10 nm | Increased cytotoxicity via US induced thermal effect and US induced PFH bubble cavitation and pyrene release (L929 cells). Enhanced grayscale value on US imaging. | Enhanced US imaging of tumor after subcutaneous injection and enhanced HIFU efficacy and accuracy (rabbit VX2 liver tumors). | [99] |
 Nanorods 10 × 41 nm, Aspect ratio 4:1 Definity microbubbles | Additive cytotoxicity of US + microbubble and mPEG-Au nanorods + laser. | None | [100] |
 Nanocage outer edge length 60 ± 11 nm, thickness 7.5 ± 2.8 nm | Dye release by HIFU via thermal phase transition of PCM. | None | [103] |
 Nanoporous Au wire 250 ± 20 nm | US-powered approaching to HeLa cells. NIR triggered release of Dox. | None | [104] |
 Nanocage edge length 52 nm | Triggered release of R6G by HIFU in gelatin phantom. | Controlled release up to 30 mm covering phantom with chicken breast tissue (ex vivo). | [105] |
 AuNP 6.3 nm AuNP-PEG 15 nm Unmodified Ad 117 nm Ad-AuNP-PEG 149 nm | Increased density of Ad-AuNP-PEG increased response to US induced acoustic cavitation | Higher tumoral accumulation and antitumor effect of Ad-AuNP-PEG + US (HepG2 xenograft). | [106] |
| AuNP | In Vitro Effects | In Vivo Effects | Ref. |
|---|---|---|---|
 Nanorod 45 nm × 15 nm, Aspect ratio 3:1 Peak absorption near 780 nm | None | Nanorods improved PA imaging contrast (GFP-expressing PC3N tumor, mouse dorsal window chamber). | [109] |
 Nanorod 10 × 35 nm | Au nanorod-MBs exhibited increased thermal expansion and over 10-fold greater amplitude of PA signal as compared with Au nanorods. Enhanced PA and pulse inversion images as compared with Au nanorods (gelatin flow phantom). | Enhanced PA and pulse inversion images (murine kidney model). | [115] |
 AuNP 3–4 nm Whole NP (αvβ3-Au nanobeacon) 150 nm | Characterization of the size of αvβ3-Au nanobeacon by DLS and AFM. | Sensitive and specific angiogenesis PA imaging (Matrigel-plug mouse model). | [116] |
| AuNP | In Vitro Effects | In Vivo Effects | Ref. |
|---|---|---|---|
 1.9 nm | None | One-year survival rate: 86% with AuNP + X-ray, 20% with X-ray alone, 0% with AuNP alone. | [118] |
 1.9 nm | Greater cellular uptake of AuNP in MDA-MB-231 than in L132 and DU145 cells. Radiation sensitizer enhancement of MV X-ray observed only in MDA-MB-231 cells | None | [119] |
 10.8 nm | Cysteamine-AuNP attached to MCF-7 cell membrane. Glucose-AuNP entered MCF-7 cells. Both AuNPs increased cytotoxicity of 200 kVp X-rays but had no significant effect on cytotoxicity of γ-rays. | None | [120] |
 4.8, 12.1, 27.3 and 46.6 nm | 12.1 and 27.3 nm AuNPs enhanced the radiation cytotoxicity more than 4.8 and 46.6 nm AuNPs in HeLa cells. | When combined withγ-ray, 12.1 and 27.3 nm AuNPs decreased tumor volume and weight more than 4.8 and 46.6 nm AuNPs U14 tumor model. | [121] * |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Lu, L.; Qiao, Y.; Ravi, S.; Salatan, F.; Melancon, M.P. Stimuli-Responsive Gold Nanoparticles for Cancer Diagnosis and Therapy. J. Funct. Biomater. 2016, 7, 19. https://doi.org/10.3390/jfb7030019
Tian L, Lu L, Qiao Y, Ravi S, Salatan F, Melancon MP. Stimuli-Responsive Gold Nanoparticles for Cancer Diagnosis and Therapy. Journal of Functional Biomaterials. 2016; 7(3):19. https://doi.org/10.3390/jfb7030019
Chicago/Turabian StyleTian, Li, Linfeng Lu, Yang Qiao, Saisree Ravi, Ferandre Salatan, and Marites P. Melancon. 2016. "Stimuli-Responsive Gold Nanoparticles for Cancer Diagnosis and Therapy" Journal of Functional Biomaterials 7, no. 3: 19. https://doi.org/10.3390/jfb7030019