Use of Micropatterned Thin Film Nitinol in Carotid Stents to Augment Embolic Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Micropatterned Thin Film Nitinol (M-TFN)
2.2. In Vitro Testing for Assessing the Efficiency of Embolic Protection Capability
2.3. In Vitro Cell Culture Experiment
2.4. Endothelial Cell Growth Behaviour Study
2.4.1. Quantification of Endothelial Cell Elongation and Alignment
2.4.2. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) Endothelial Cell Viability and Proliferation Assay
2.4.3. Morphology and Coverage Percentage of Endothelial Cells
2.5. Statistical Analysis
3. Results and Discussion
3.1. Efficiency of the Embolic Protection Capability (EEPC) of the M-TFN Covered Stents
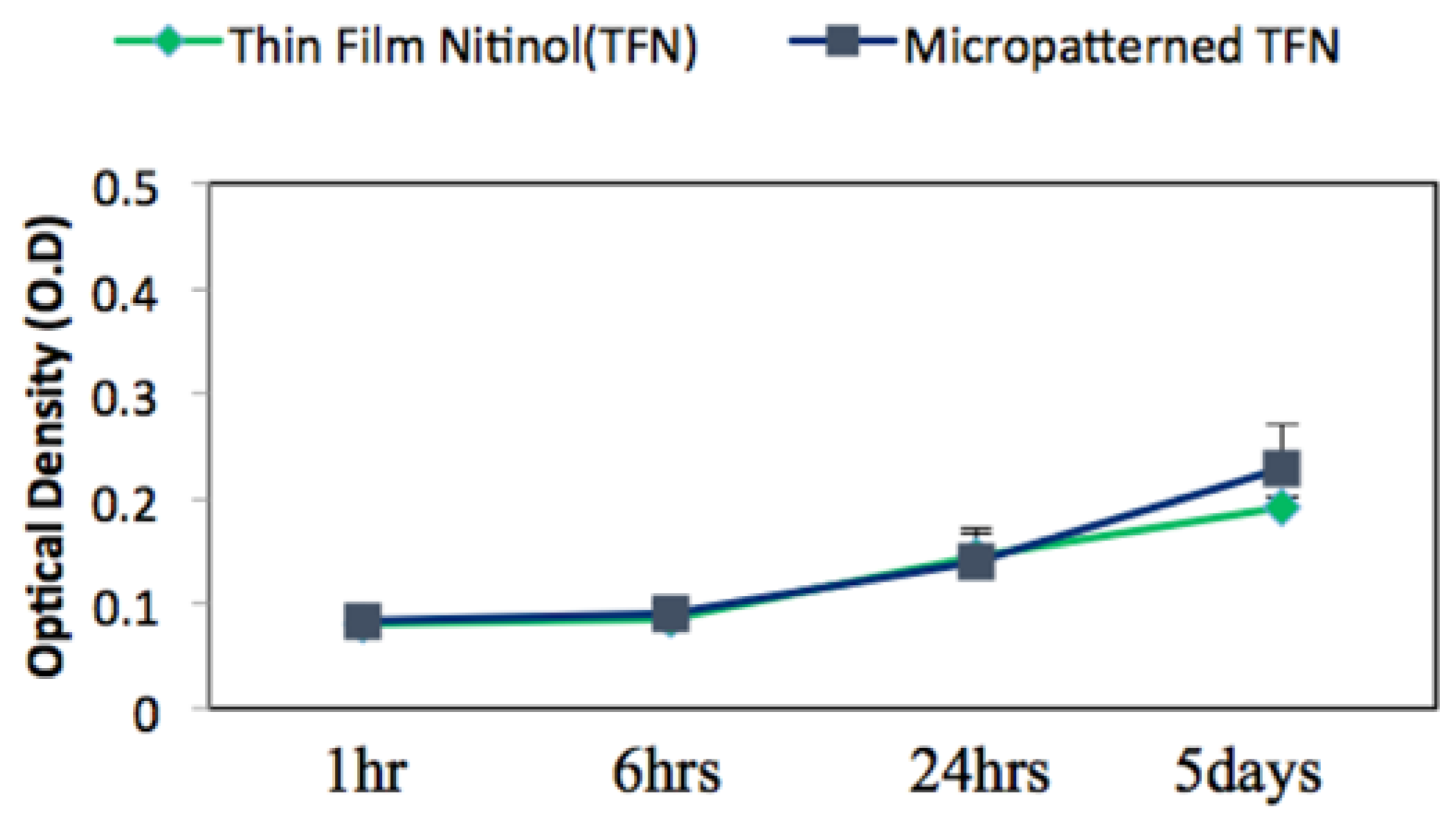
3.2. Biocompatibility Assessment via In Vitro Endothelial Cell Growth Behaviour
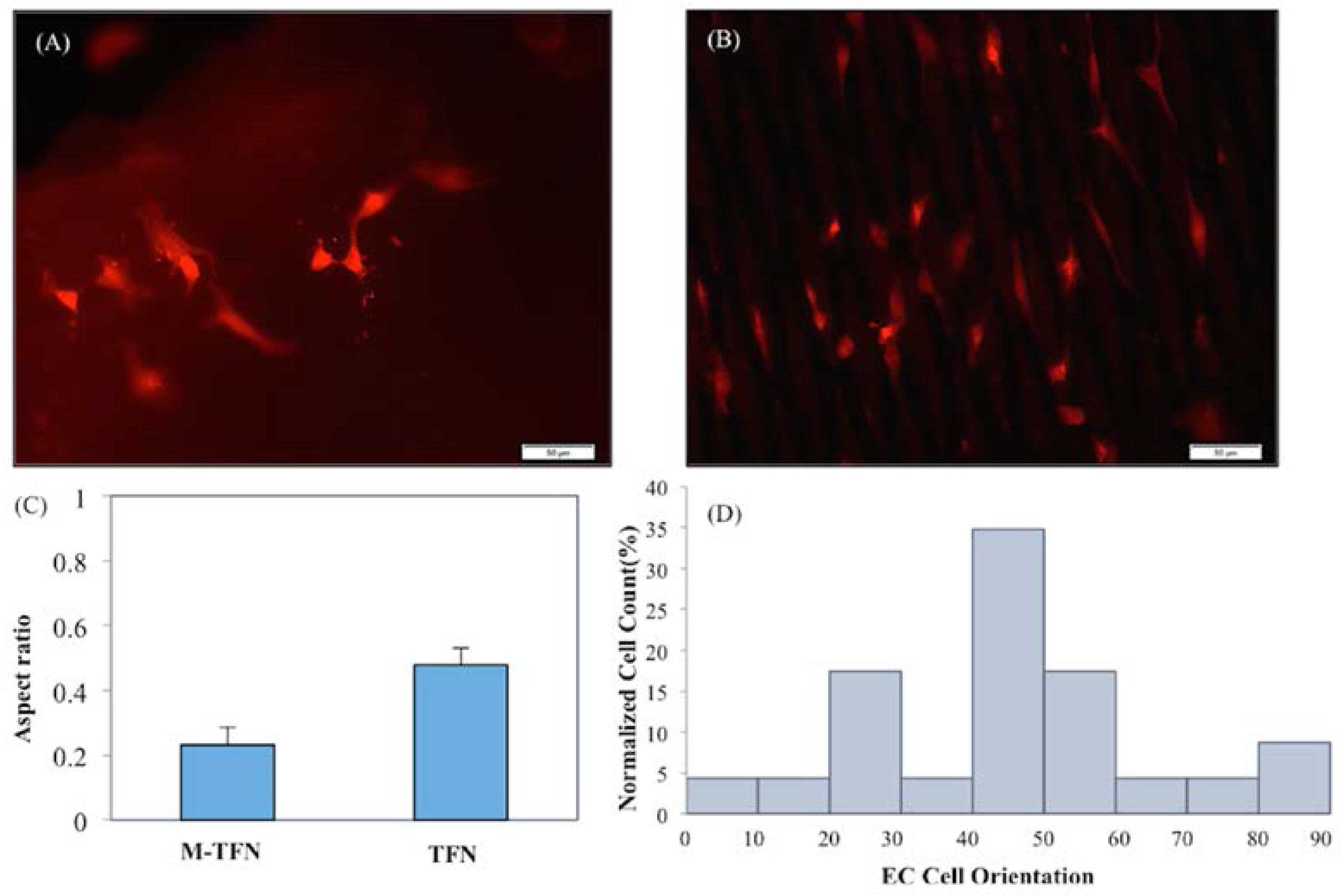
3.3. Quantification of the Endothelial Cell’s Growth Pattern and Orientation
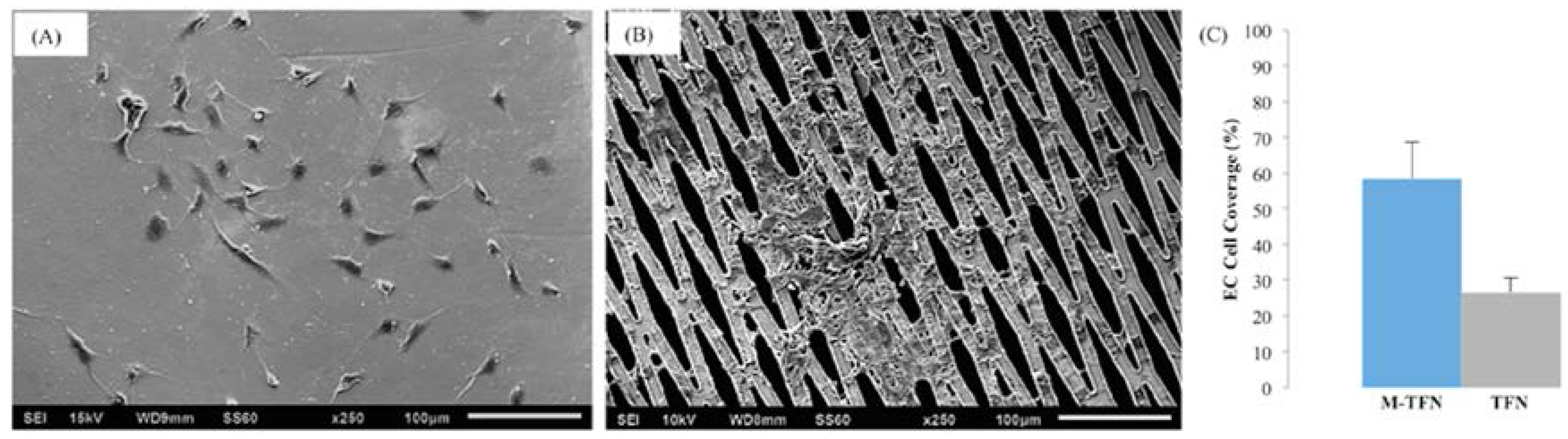
3.4. Morphology and Coverage Percentage of Endothelial Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Heart Association. Heart Disease and Stroke Statistics—2010 Update; American Heart Association: Dallas, TX, USA, 2010. [Google Scholar]
- Thom, T.; Haase, N.; Rosamond, W.; Howard, V.J.; Rumsfeld, J.; Manolio, T.; Zheng, Z.; Flegal, K.; O’Donnell, C.; Kittner, S.; et al. Heart disease and stroke statistics—2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113, e85–e151. [Google Scholar] [CrossRef] [PubMed]
- Mantese, V.A.; Timaran, C.H.; Chiu, D.; Begg, R.J.; Brott, T.G.; CREST Investigators. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): Stenting versus carotid endarterectomy for carotid disease. Stroke 2010, 41 (Suppl. 10), S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Ferraro, P.; Corcione, N.; Messina, S.; Maresca, G.; Giordano, G.; Mancusi, R.; Avellino, R.; Peruzzi, M.; Biondi-Zoccai, G. Safety and Efficacy of A Single Embolic Protection Device-Stent Combo for Carotid Revascularization. J. Cardiol. Ther. 2016, 3, 560–565. [Google Scholar] [CrossRef]
- Eltchaninoff, H. The Pros and Cons of Cerebral Embolic Protection during Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2016, 9, 2134–2136. [Google Scholar] [CrossRef] [PubMed]
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S.M.; Ho, M.; Howard, V.; Kissela, B.; et al. Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008, 117, 25–146. [Google Scholar]
- Castagno, C.; Varetto, G.; Sperti, F.; Rossato, D.; Faletti, R.; Rispoli, P. Preoperative and Postoperative Evaluation of New Double Mesh Carotid Stents with Contrast-Enhanced Ultrasound and Diffusion-Weighted Imaging. Ann. Vasc. Surg. 2016, 33, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, M.A.; Faletti, R.; Bergamasco, L.; Fonio, P.; Righi, D. Incidence of New Ischaemic Brain Lesions after Carotid Artery Stenting with the Micromesh Roadsaver Carotid Artery Stent: A Prospective Single-Centre Study. Cardiovasc. Intervent. Radiol. 2016, 39, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Mudra, H.; Staubach, S.; Hein-Rothweiler, R.; Segerer, M.; Strohm, H.; Weber, H.; Ledwoch, J. Long-Term Outcomes of Carotid Artery Stenting in Clinical Practice. Circ. Cardiovasc. Interv. 2016, 9, e003940. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Kulcsár, Z.; Carrera, E.; Cremonesi, A. Carotid artery stenting. Heart 2016, 102, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- De Donato, G.; Setacci, C.; Umemoto, T.; Reimers, B. Commentary: Inside of the Interaction between the Plaque and the Stent Optical Coherence Tomography During Carotid Artery Stenting. J. Endovasc. Ther. 2015, 22, 950–951. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, A.; Castriota, F.; Secco, G.G.; Macdonald, S.; Roffi, M. Carotid artery stenting: An update. Eur. Heart J. 2015, 36, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Shayan, M.; Chun, Y. An overview of thin film nitinol endovascular devices. Acta Biomater. 2015, 21, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Sadideen, H.; Taylor, P.R.; Padayachee, T.S. Restenosis after carotid endarterectomy. Int. J. Clin. Pract. 2006, 60, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Levi, D.; Mohanchandra, K.; Fishbein, M.; Carman, G. Novel micro-patterning processes for thin film NiTi vascular devices. Smart Mater. Struct. 2010, 19, 105021. [Google Scholar] [CrossRef]
- Mohanchandra, K.; Ho, K.K.; Carman, G.P. Electrical characterization of NiTi film on silicon substrate. J. Intell. Mater. Syst. Struct. 2004, 15, 387–392. [Google Scholar] [CrossRef]
- Krejza, J.; Arkuszewski, M.; Kasner, S.E.; Weigele, J.; Ustymowicz, A.; Hurst, R.W.; Cucchiara, B.L.; Messe, S.R. Carotid artery diameter in men and women and the relation to body and neck size. Stroke 2006, 37, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Babiker, M.H.; Gonzalez, L.F.; Albuquerque, F.; Collins, D.; Elvikis, A.; Frakes, D.H. Quantitative effects of coil packing density on cerebral aneurysm fluid dynamics: An in vitro steady flow study. Ann. Biomed. Eng. 2010, 38, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Oktar, S.; Yücel, C.; Karaosmanoglu, D.; Akkan, K.; Ozdemir, H.; Tokgoz, N.; Tali, T. Blood-flow volume quantification in internal carotid and vertebral arteries: Comparison of 3 different ultrasound techniques with phase-contrast MR imaging. Am. J. Neuroradiol. 2006, 27, 363–369. [Google Scholar] [PubMed]
- Hopf-Jensen, S.; Marques, L.; Preiß, M.; Müller-Hülsbeck, S. Initial clinical experience with the micromesh Roadsaver carotid artery stent for the treatment of patients with symptomatic carotid artery disease. J. Endovasc. Ther. 2015, 22, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Bosiers, M.; Deloose, K.; Torsello, G.; Scheinert, D.; Maene, L.; Peeters, P.; Müller-Hülsbeck, S.; Sievert, H.; Langhoff, R.; Bosiers, M.; et al. The CLEAR-ROAD study: Evaluation of a new dual layer micromesh stent system for the carotid artery. EuroIntervention 2015, 12, e671–e676. [Google Scholar] [CrossRef] [PubMed]
- Nerla, R.; Castriota, F.; Micari, A.; Sbarzaglia, P.; Secco, G.G.; Ruffino, M.A.; de Donato, G.; Setacci, C.; Cremonesi, A. Carotid artery stenting with a new-generation double-mesh stent in three high-volume Italian centres: Clinical results of a multidisciplinary approach. EuroIntervention 2016, 12, e677–e683. [Google Scholar] [CrossRef] [PubMed]
- Kedev, S.; Petkoska, D.; Zafirovska, B.; Vasilev, I.; Bertrand, O.F. Safety of slender 5Fr transradial approach for carotid artery stenting with a novel nitinol double-layer micromesh stent. Am. J. Cardiol. 2015, 116, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Kealey, C.P.; Chun, Y.J.; Viñuela, F.E.; Mohanchandra, K.P.; Carman, G.P.; Vinuela, F.; Levi, D.S. In vitro and in vivo testing of a novel, hyperelastic thin film nitinol flow diversion stent. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dai, D.; Kallmes, D.F.; Schroeder, D.; Kealey, C.P.; Gupta, V.; Johnson, A.D.; Kadirvel, R. Preclinical Testing of a Novel Thin Film Nitinol Flow-Diversion Stent in a Rabbit Elastase Aneurysm Model. Am. J. Neuroradiol. 2016, 37, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.; Rockson, S.G. Coronary restenosis: A review of mechanisms and management. Am. J. Med. 2003, 115, 547–553. [Google Scholar] [CrossRef]
- Scott, N.A. Restenosis following implantation of bare metal coronary stents: Pathophysiology and pathways involved in the vascular response to injury. Adv. Drug Deliv. Rev. 2006, 58, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, W.S. The pathophysiology and burden of restenosis. Am. J. Cardiol. 2007, 100, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chan, P. Review: Developments in restenosis. J. Renin Angiotensin Aldosterone Syst. 2002, 3, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.G.; Sharp, D.; Chuang, H.Y.; Mohammad, S.F. The endothelium: Roles in thrombosis and hemostasis. Arch. Pathol. Lab. Med. 1977, 101, 61–64. [Google Scholar] [PubMed]
- Van Hinsbergh, V.W.M. (Ed.) Endothelium—Role in Regulation of Coagulation and Inflammation; Springer: Manila, Philippines, 2012.
- Biela, S.A.; Su, Y.; Spatz, J.P.; Kemkemer, R. Different sensitivity of human endothelial cells, smooth muscle cells and fibroblasts to topography in the nano–micro range. Acta Biomater. 2009, 5, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Vandrangi, P.; Gott, S.C.; Kozaka, R.; Rodgers, V.G.J.; Rao, M.P. Comparative endothelial cell response on topographically patterned titanium and silicon substrates with micrometer to sub-micrometer feature sizes. PLoS ONE 2014, 9, 111465. [Google Scholar] [CrossRef] [PubMed]
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, D.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, G.; Chen, L.; Li, H.; Yu, P.; Bai, M.; Zhang, Q.; Lee, J.; Yu, Q. Investigation of surface endothelialization on biomedical nitinol (NiTi) alloy: Effects of surface micropatterning combined with plasma nanocoatings. Acta Biomater. 2009, 5, 3593–3604. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, W.; Thostrup, P.; Yam, P.T.; Juzwik, C.A.; Ruthazer, E.S.; Dhaunchak, A.S.; Colman, D.R. Cellular response to micropatterned growth promoting and inhibitory substrates. BMC Biotechnol. 2013, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, K.B.; Kirkpatrick, S.J.; Hanson, S.R.; Hinds, M.T. Endothelial cell cytoskeletal alignment independent of fluid shear stress on micropatterned surfaces. Biochem. Biophys. Res. Commun. 2008, 371, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Dike, L.E.; Chen, C.S.; Mrksich, M.; Tien, J.; Whitesides, G.M.; Ingber, D.E. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell. Dev. Biol. Anim. 1999, 35, 441–448. [Google Scholar] [CrossRef] [PubMed]

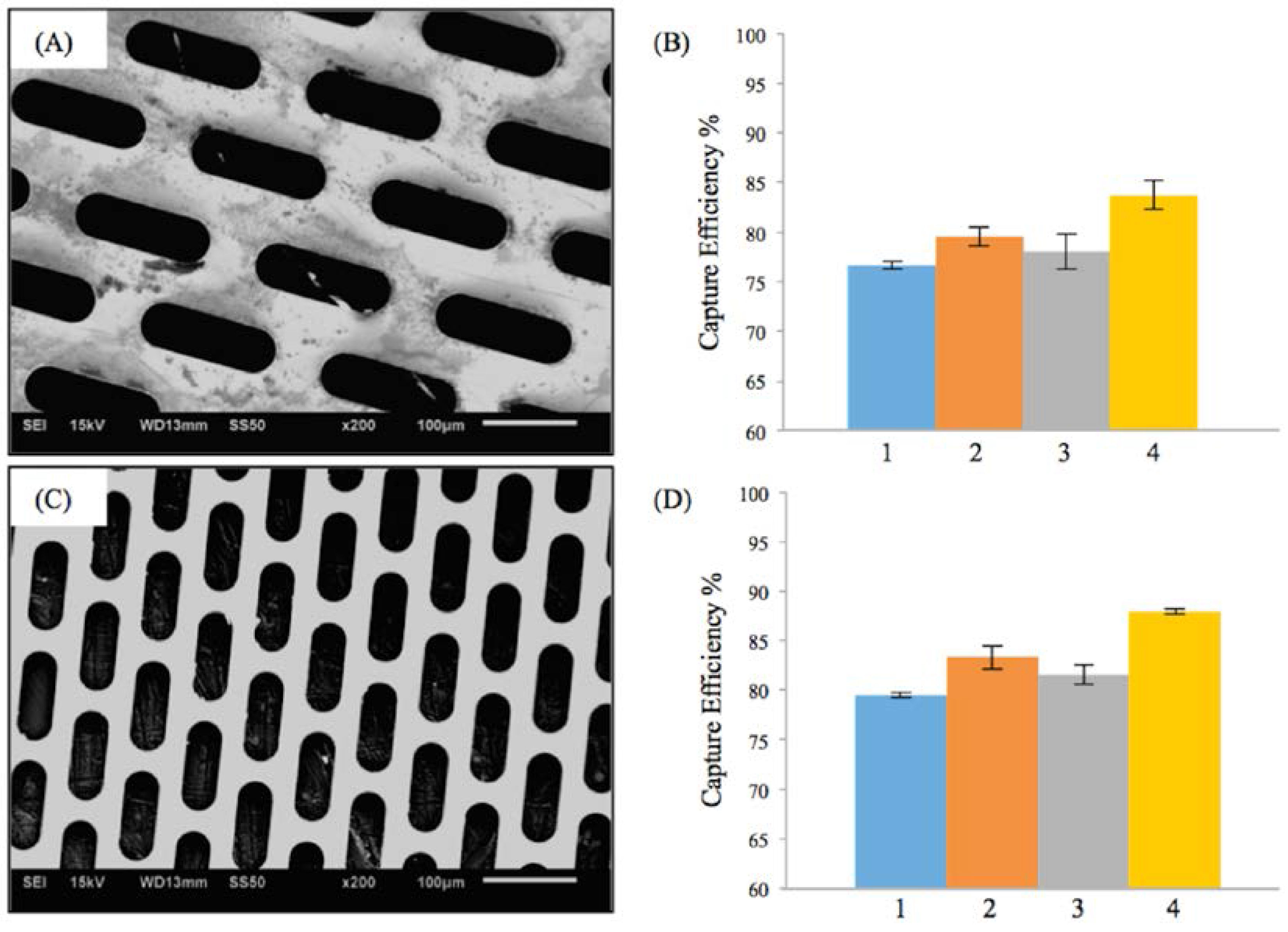
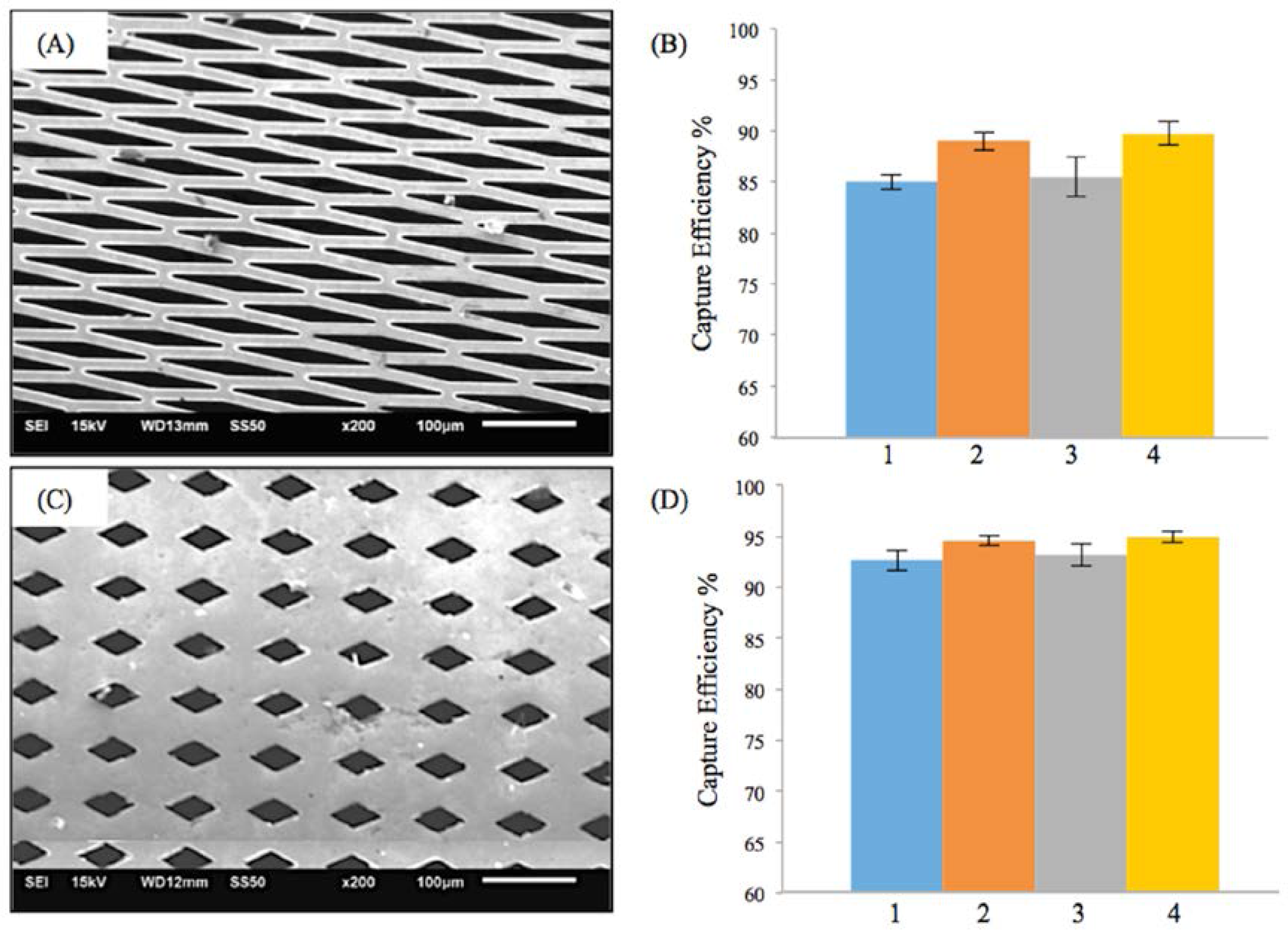
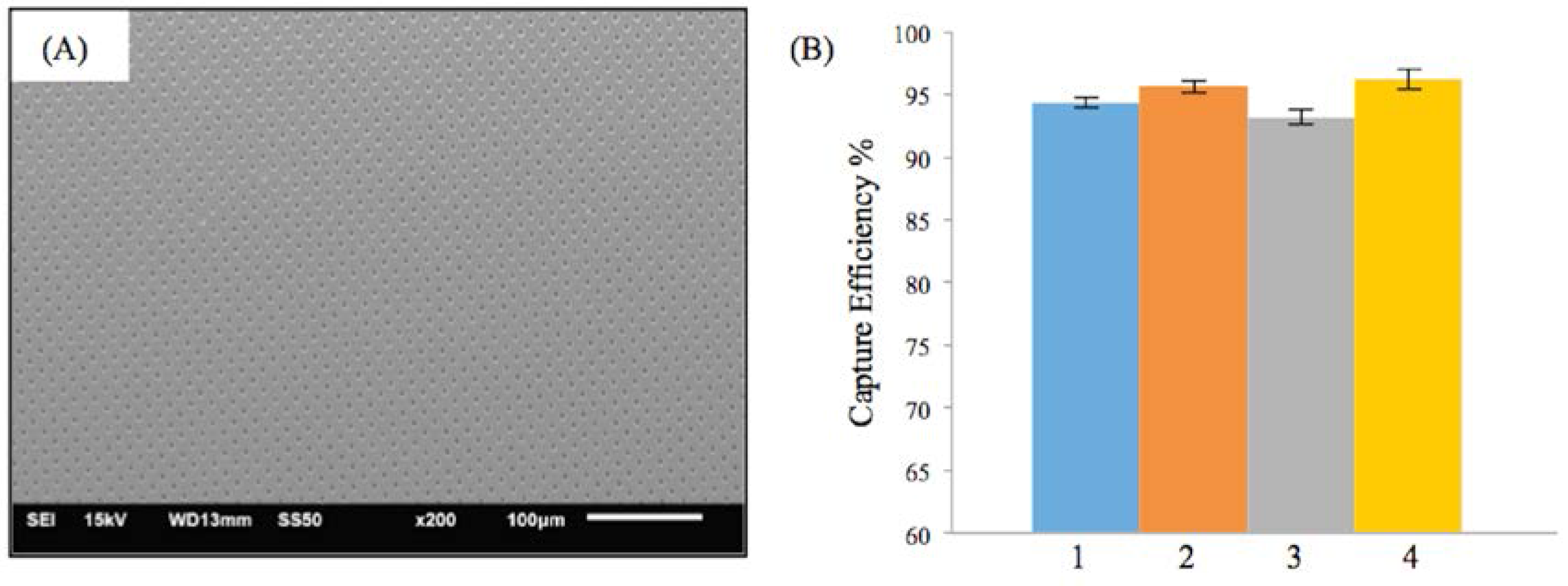
| Test Condition | Flow Rate (mL/min) | Pulsatile Rate (Beats per min) | Weight of Microspheres (mg) | PVA Solution (mg) | Particle Size (μm) |
|---|---|---|---|---|---|
| 1 | 510 | 85 | 30 | 500 | 53–63 |
| 2 | 402 | 65 | 30 | 500 | 53–63 |
| 3 | 510 | 85 | 10 | 500 | 53–63 |
| 4 | 402 | 65 | 10 | 500 | 53–63 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shayan, M.; Jankowitz, B.T.; Shridhar, P.; Chun, Y. Use of Micropatterned Thin Film Nitinol in Carotid Stents to Augment Embolic Protection. J. Funct. Biomater. 2016, 7, 34. https://doi.org/10.3390/jfb7040034
Shayan M, Jankowitz BT, Shridhar P, Chun Y. Use of Micropatterned Thin Film Nitinol in Carotid Stents to Augment Embolic Protection. Journal of Functional Biomaterials. 2016; 7(4):34. https://doi.org/10.3390/jfb7040034
Chicago/Turabian StyleShayan, Mahdis, Brian T. Jankowitz, Puneeth Shridhar, and Youngjae Chun. 2016. "Use of Micropatterned Thin Film Nitinol in Carotid Stents to Augment Embolic Protection" Journal of Functional Biomaterials 7, no. 4: 34. https://doi.org/10.3390/jfb7040034






