Optimization of Polymer-ECM Composite Scaffolds for Tissue Engineering: Effect of Cells and Culture Conditions on Polymeric Nanofiber Mats
Abstract
:1. Introduction
2. Results and Discussion
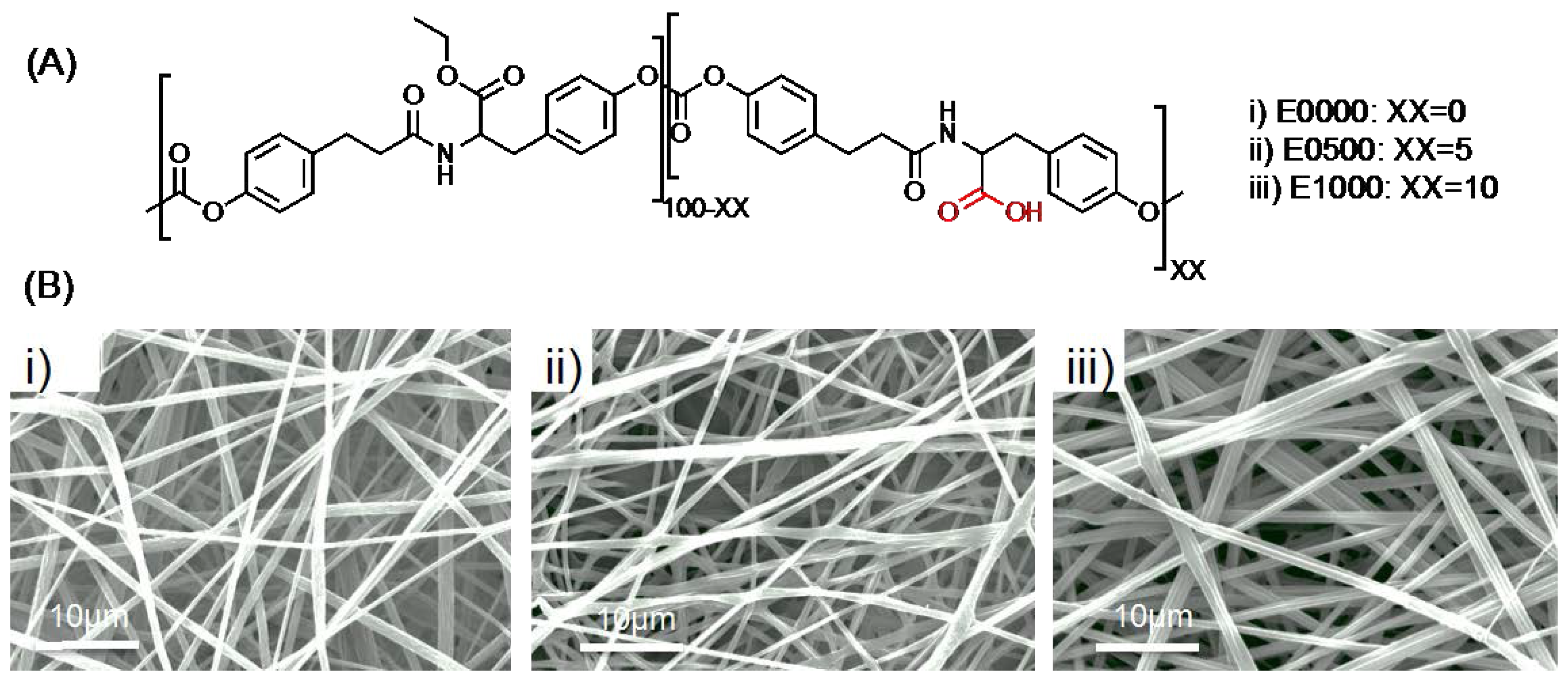
2.1. Fabrication of Fibrous Scaffolds
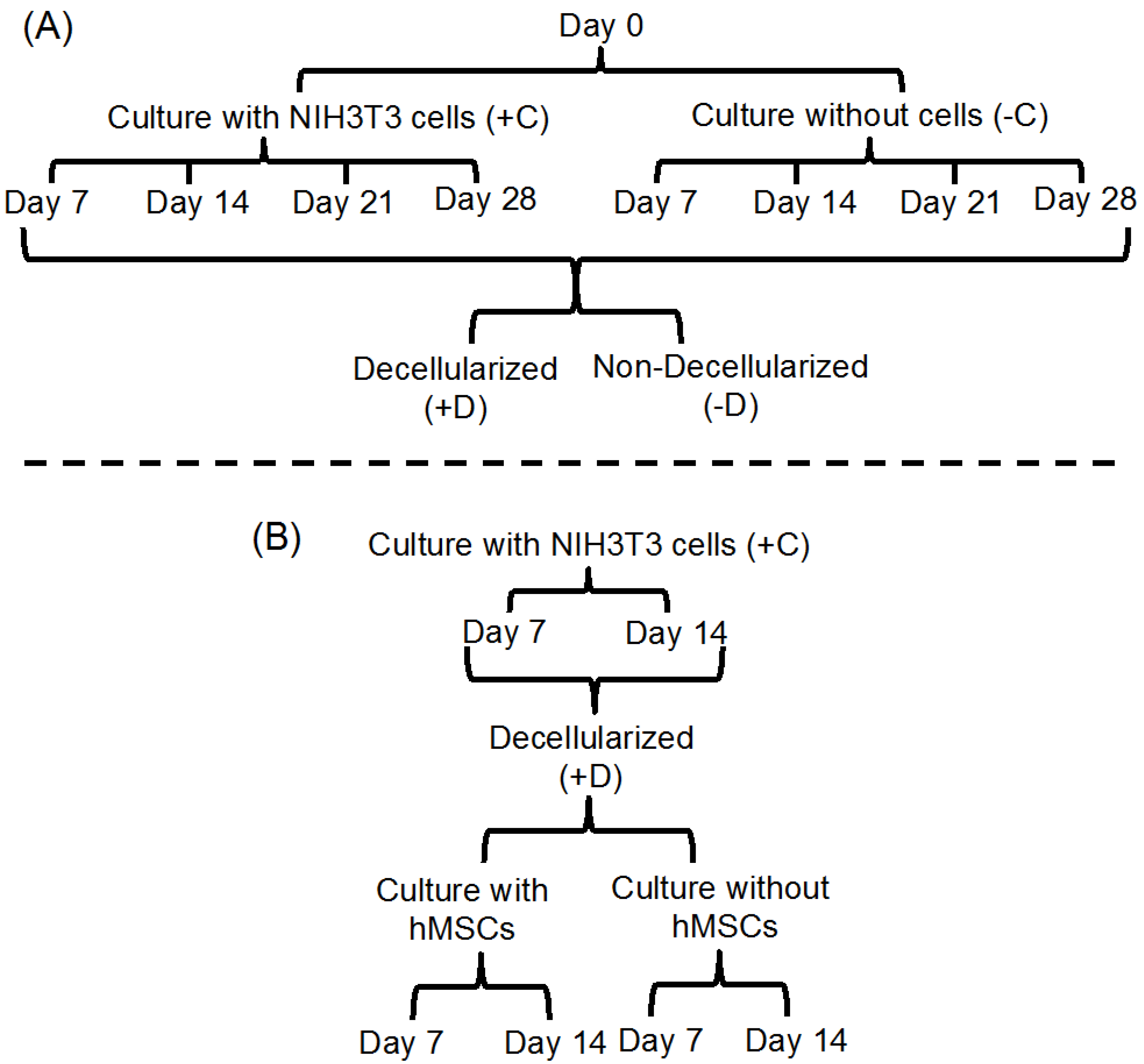
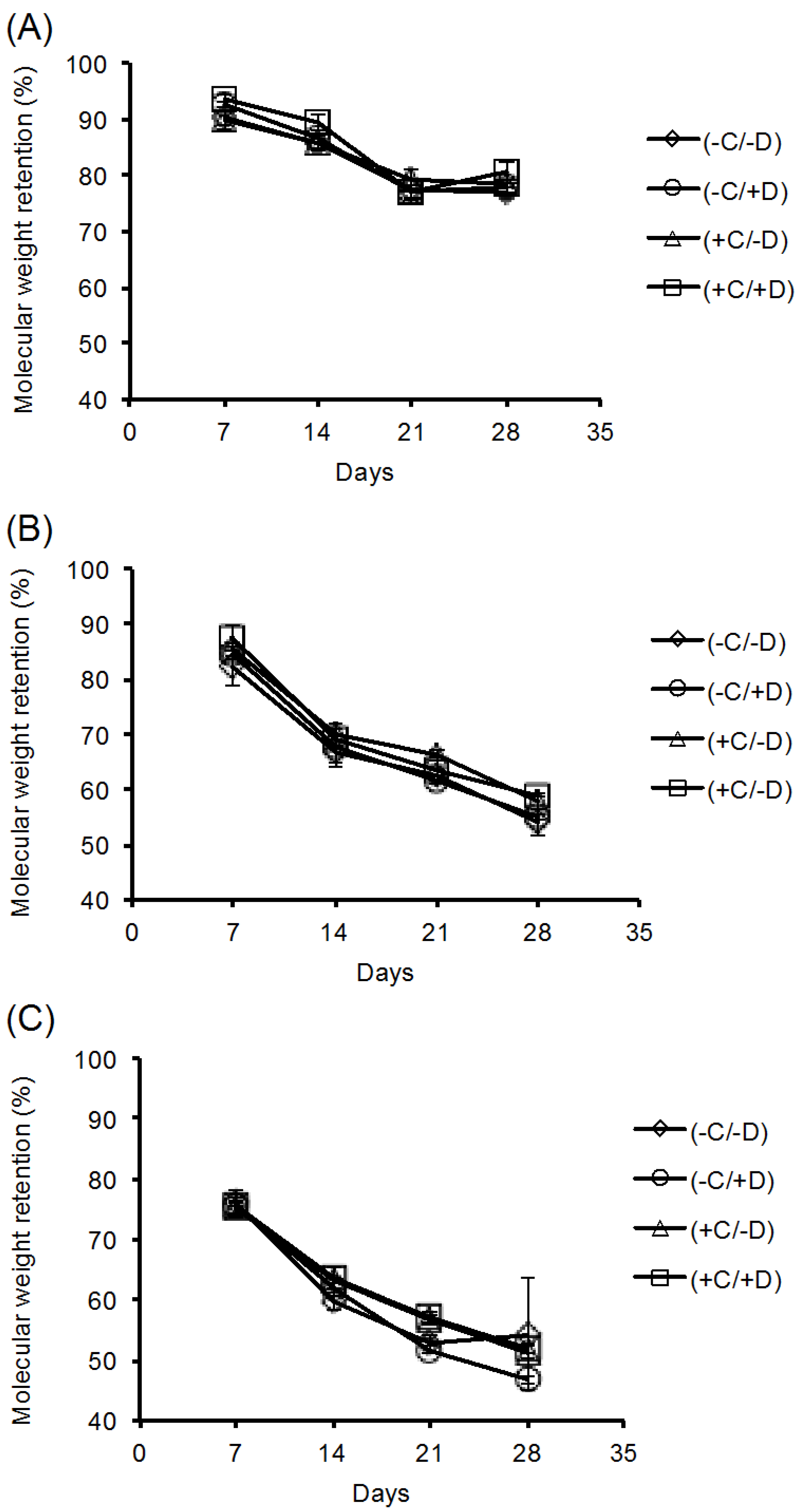
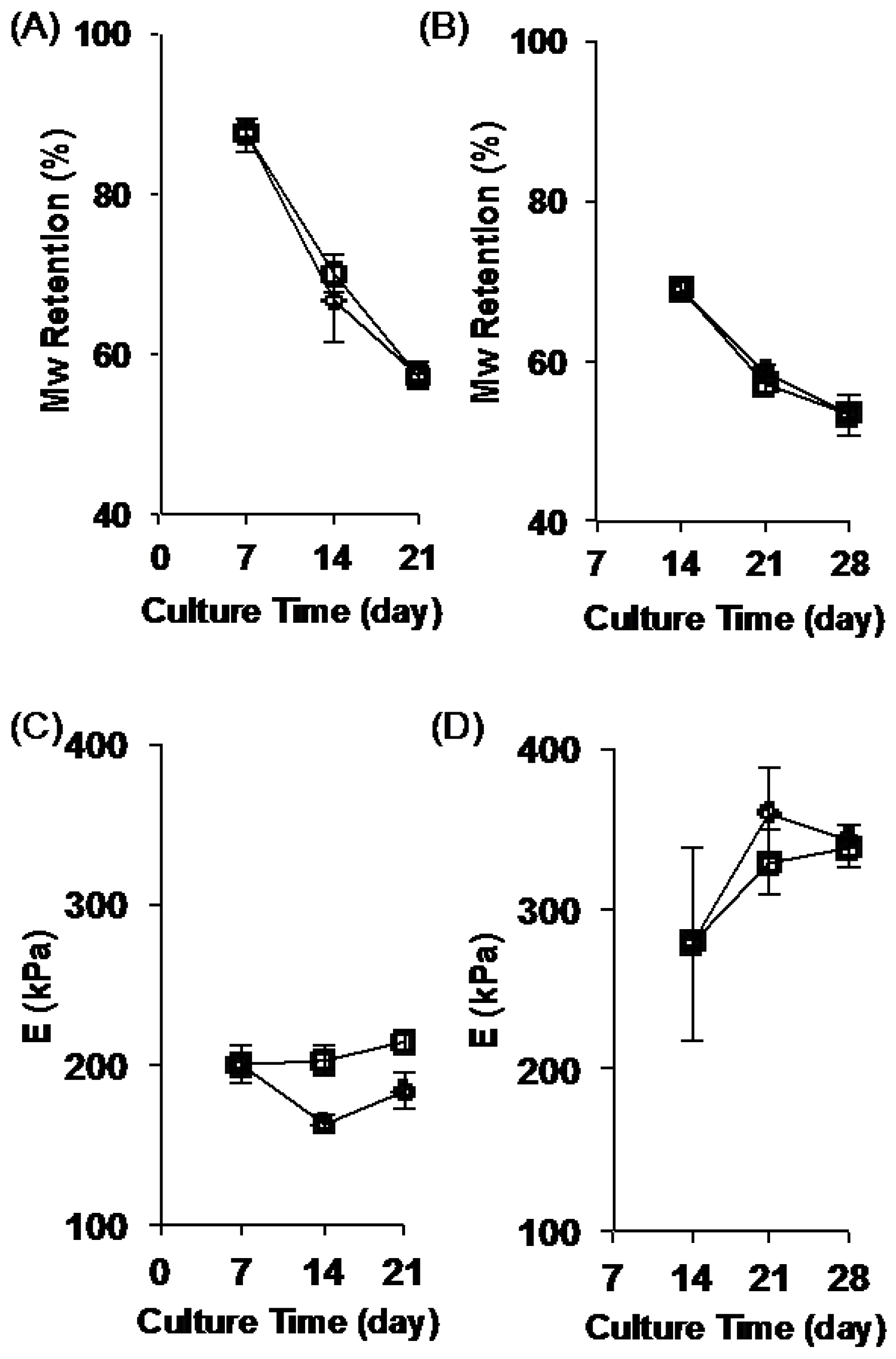
2.2. Stability of the Fibrous Scaffolds
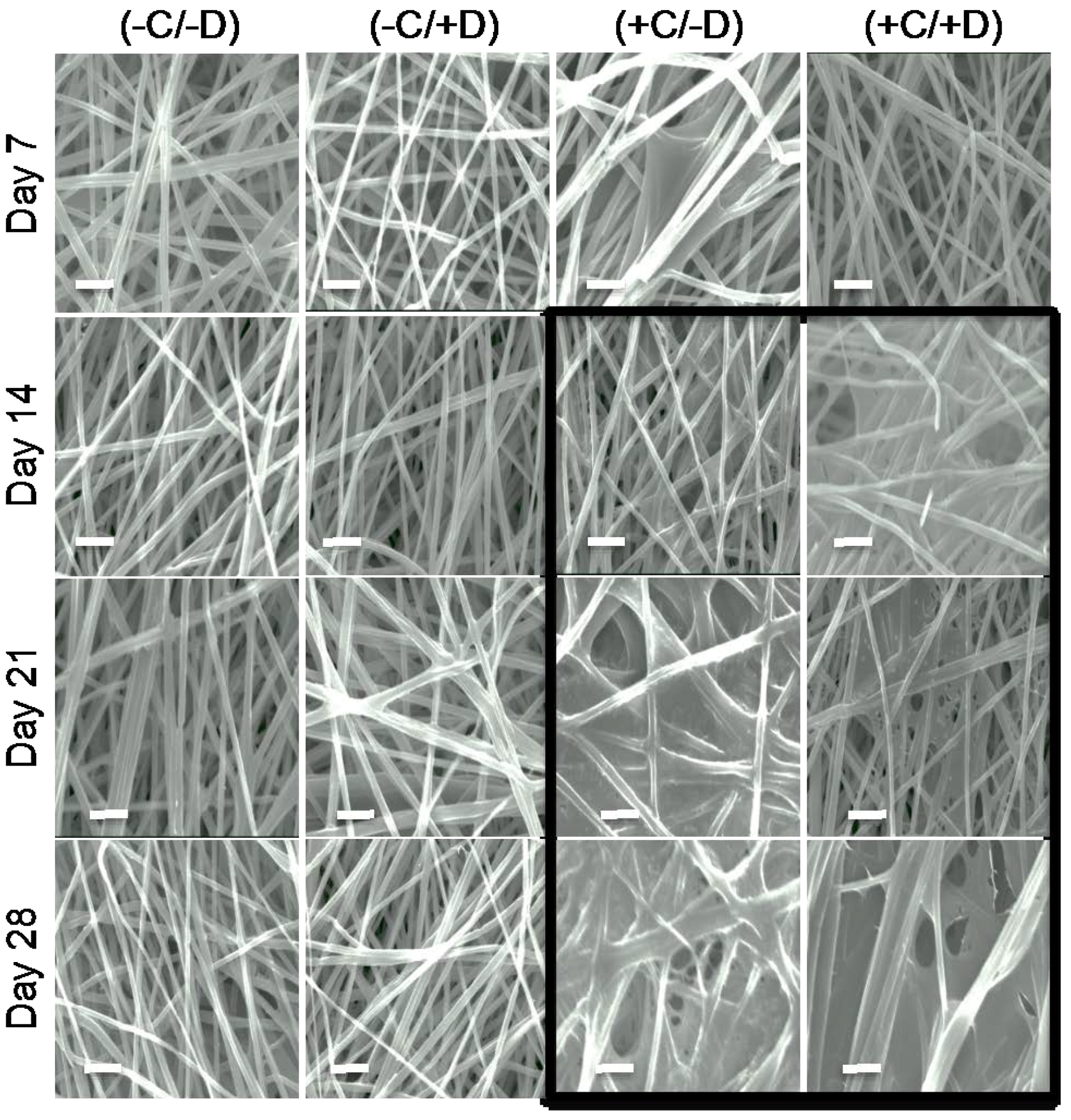
2.3. Deposition of ECM on Scaffolds Cultured in the Presence of Cells
2.4. Change in Scaffold Stiffness as Function of Time
2.5. Testing the Full Cellularization-Decell-Recell Cycle of ECM-Polymer Composite Scaffolds
3. Materials and Methods
3.1. Fabrication of Fibrous Scaffolds
3.2. Electrospinning
3.3. Fiber Mat Characterization
3.4. Cell Culture
3.5. Decellularization
3.6. Cell Culture on ECM-Polymer Composite Scaffolds
3.7. Immunostaining
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.J.; Burdick, J.A. Engineering ecm signals into biomaterials. Mater. Today 2012, 15, 454–459. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Grinnell, F. Cellular adhesiveness and extracellular substrata. Int. Rev. Cytol. 1978, 53, 65–144. [Google Scholar] [PubMed]
- Tian, F.; Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Yokoyama, Y.; Estrada, G.G.; Kobayashi, H. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J. Biomed. Mater. Res. Part A 2008, 84, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. Part A 2003, 67, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Ponery, A.S.; Nur-E-Kamal, A.; Kamal, J.; Meshel, A.S.; Sheetz, M.P.; Schindler, M.; Meiners, S. Morphology, cytoskeletal organization, and myosin dynamics of mouse embryonic fibroblasts cultured on nanofibrillar surfaces. Mol. Cell. Biochem. 2007, 301, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Jiang, Y.J.; Tuan, R.S. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006, 12, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Wang, F.W.; Matsumoto, K.; Yamada, K.M. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 2009, 184, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Florek, C.A.; Kim, J.J.; Neubauer, T.; Moore, J.C.; Cohen, R.I.; Kohn, J.; Grumet, M.; Moghe, P.V. Microfibrous substrate geometry as a critical trigger for organization, self-renewal, and differentiation of human embryonic stem cells within synthetic 3-dimensional microenvironments. FASEB J. 2012, 26, 3240–3251. [Google Scholar] [CrossRef] [PubMed]
- Nur-E-Kamal, A.; Ahmed, I.; Kamal, J.; Schindler, M.; Meiners, S. Three dimensional nanofibrillar surfaces induce activation of RAC. Biochem. Biophys. Res. Commun. 2005, 331, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Dhaliwal, A.; Arvind, V.; Patel, P.J.; Beijer, N.R.M.; de Boer, J.; Murthy, N.S.; Kohn, J.; Moghe, P.V. Organizational metrics of interchromatin speckle factor domains: Integrative classifier for stem cell adhesion & lineage signaling. Integr. Biol. 2015, 7, 435–446. [Google Scholar]
- Xie, J.; Willerth, S.M.; Li, X.; Macewan, M.R.; Rader, A.; Sakiyama-Elbert, S.E.; Xia, Y. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 2009, 30, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Mauck, R.L.; Cooper, J.A.; Yuan, X.; Tuan, R.S. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J. Biomech. 2007, 40, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Baker, B.M.; Sen, S.; Wible, E.E.; Elliott, D.M.; Mauck, R.L. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat. Mater. 2009, 8, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Mauck, R.L.; Elliott, D.M. Issls prize winner: Integrating theoretical and experimental methods for functional tissue engineering of the annulus fibrosus. Spine 2008, 33, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.A.; Florek, C.A.; Kohn, J.; Michniak, B.B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int. J. Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Dis. Today 2014, 19, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 2013, 34, 5571–5580. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.L.; Mauck, R.L.; Burdick, J.A. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011, 32, 8771–8782. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Vepari, C.; Jin, H.J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef]
- Mu, Y.; Wu, F.; Lu, Y.; Wei, L.; Yuan, W. Progress of electrospun fibers as nerve conduits for neural tissue repair. Nanomedicine 2014, 9, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Macri, L.K.; Sheihet, L.; Singer, A.J.; Kohn, J.; Clark, R.A.F. Ultrafast and fast bioerodible electrospun fiber mats for topical delivery of a hydrophilic peptide. J. Controll. Release 2012, 161, 813–820. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Marklein, R.A.; Burdick, J.A. Controlling stem cell fate with material design. Adv. Mater. 2010, 22, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Burdick, J.A. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 2013, 24, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.; Holtorf, H.L.; Sikavitsas, V.I.; Jansen, J.A.; Mikos, A.G. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials 2005, 26, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Levorson, E.J.; Mountziaris, P.M.; Hu, O.; Kasper, F.K.; Mikos, A.G. Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, part 1: Investigation of cocultures and seeding densities for improved extracellular matrix deposition. Tissue Eng. Part C Methods 2014, 20, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, K.; Cheng, Q.; Pryzhkova, M.; Harris, G.M.; Jabbarzadeh, E. Enhanced differentiation of human embryonic stem cells on extracellular matrix-containing osteomimetic scaffolds for bone tissue engineering. Tissue Eng. Part C Methods 2014, 20, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.A.; Mikos, A.G.; Kasper, F.K. Scaffold/extracellular matrix hybrid constructs for bone-tissue engineering. Adv. Healthc. Mater. 2013, 2, 13–24. [Google Scholar] [CrossRef]
- Brocchini, S.; James, K.; Tangpasuthadol, V.; Kohn, J. A combinatorial approach for polymer design. J. Am. Chem. Soc. 1997, 119, 4553–4554. [Google Scholar] [CrossRef]
- Brocchini, S.; James, K.; Tangpasuthadol, V.; Kohn, J. Structure-property correlations in a combinatorial library of degradable biomaterials. J. Biomed. Mater. Res. 1998, 42, 66–75. [Google Scholar] [CrossRef]
- Kohn, J. New approaches to biomaterials design. Nat. Mater. 2004, 3, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.; Welsh, W.J.; Knight, D. A new approach to the rationale discovery of polymeric biomaterials. Biomaterials 2007, 28, 4171–4177. [Google Scholar] [CrossRef] [PubMed]
- Bourke, S.L.; Kohn, J. Polymers derived from the amino acid l-tyrosine: Polycarbonates, polyarylates and copolymers with poly(ethylene glycol). Adv. Drug Deliv. Rev. 2003, 55, 447–466. [Google Scholar] [CrossRef]
- Costache, A.D.; Ghosh, J.; Knight, D.D.; Kohn, J. Computational methods for the development of polymeric biomaterials. Adv. Eng. Mater. 2010, 12, B3–B17. [Google Scholar] [CrossRef]
- James, K.; Levene, H.; Parsons, J.R.; Kohn, J. Small changes in polymer chemistry have a large effect on the bone-implant interface: Evaluation of a series of degradable tyrosine-derived polycarbonates in bone defects. Biomaterials 1999, 20, 2203–2212. [Google Scholar] [CrossRef]
- Tangpasuthadol, V.; Pendharkar, S.M.; Kohn, J. Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part I: Study of model compounds. Biomaterials 2000, 21, 2371–2378. [Google Scholar] [CrossRef]
- Lewitus, D.; Smith, K.L.; Shain, W.; Kohn, J. Ultrafast resorbing polymers for use as carriers for cortical neural probes. Acta Biomater. 2011, 7, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Tangpasuthadol, V. Thermo-Mechanical Properties and Hydrolytic Degradation of Tyrosine-Derived Polymers for Use in Biomedical Applications; Rutgers University: New Brunswick, NJ, USA, 1999. [Google Scholar]
- Abramson, S.D. Selected Bulk and Surface Properties and Biocompatibility of a New Class of Tyrosine-Derived Polycarbonates; Rutgers University: Piscataway, NJ, USA, 2002. [Google Scholar]
- Mao, Y.; Schwarzbauer, J.E. Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. J. Cell Sci. 2005, 118, 4427–4436. [Google Scholar] [CrossRef] [PubMed]
- Magno, M.H.R.; Kim, J.; Srinivasan, A.; McBride, S.; Bolikal, D.; Darr, A.; Hollinger, J.O.; Kohn, J. Synthesis, degradation and biocompatibility of tyrosine-derived polycarbonate scaffolds. J. Mater. Chem. 2010, 20, 8885–8893. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, R.; Guvendiren, M.; Freeman, O.; Mao, Y.; Kohn, J. Optimization of Polymer-ECM Composite Scaffolds for Tissue Engineering: Effect of Cells and Culture Conditions on Polymeric Nanofiber Mats. J. Funct. Biomater. 2017, 8, 1. https://doi.org/10.3390/jfb8010001
Goyal R, Guvendiren M, Freeman O, Mao Y, Kohn J. Optimization of Polymer-ECM Composite Scaffolds for Tissue Engineering: Effect of Cells and Culture Conditions on Polymeric Nanofiber Mats. Journal of Functional Biomaterials. 2017; 8(1):1. https://doi.org/10.3390/jfb8010001
Chicago/Turabian StyleGoyal, Ritu, Murat Guvendiren, Onyi Freeman, Yong Mao, and Joachim Kohn. 2017. "Optimization of Polymer-ECM Composite Scaffolds for Tissue Engineering: Effect of Cells and Culture Conditions on Polymeric Nanofiber Mats" Journal of Functional Biomaterials 8, no. 1: 1. https://doi.org/10.3390/jfb8010001





