Induction of Low-Level Hydrogen Peroxide Generation by Unbleached Cotton Nonwovens as Potential Wound Dressing Materials
Abstract
:1. Introduction
1.1. Wound Healing
1.2. Greige Cotton
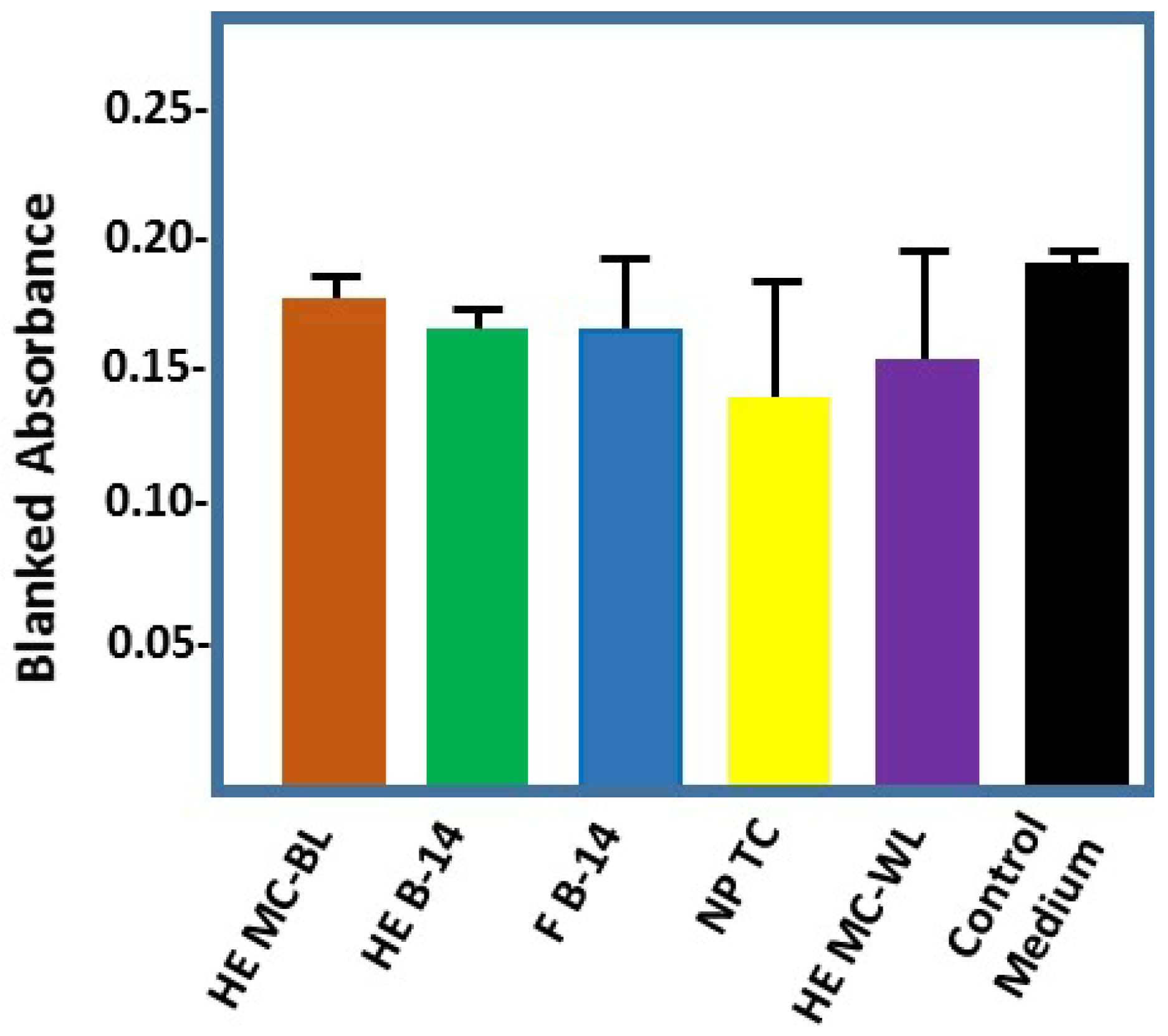
2. Results
2.1. Pectin Levels in Greige Cotton
2.2. Generation of Hydrogen Peroxide
2.3. Elements Present in Pectin
2.4. Effect of Cotton-Based Hydrogen Peroxide on Dermal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.3. Enzymatic Pectin Hydrolysis
4.4. Galacturonic Acid Assay
4.5. Hydrogen Peroxide Determination Using Amplex Red Assay
4.6. Superoxide Dismutase (SOD) Activity
4.7. Total Phenolic Content via Fast Blue RR (FBRR) Method
4.8. Optical Microscope: Sample Preparation and Imaging
4.9. ICP/MS
4.10. Fibroblast Cytotoxicity Assay
Author Contributions
Conflicts of Interest
References
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Elliott, I.M.Z.E.J.R. A Short History of Surgical Dressings; Pharmaceutical Press: London, UK, 1964. [Google Scholar]
- Winter, G.D. Formation of the Scab and the Rate of Epithelization of Superficial Wounds in the Skin of the Young Domestic Pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.A. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. Sci. World J. 2011, 11, 766–787. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.H.; Bode, A.P.; Demcheva, M.; Vournakis, J.N. Hemostatic properties of glucosamine-based materials. J. Biomed. Mater. Res. Part A 2007, 80A, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; Spazierer, D.; Urban, M.D.; Lin, L.; Redl, H.; Goppelt, A. Comparison of regenerated and non-regenerated oxidized cellulose hemostatic agents. Eur. Surg. 2013, 45, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Yao, J.; Liu, K. Study on hemostatic mechanism of fully soluble hemostatic fiber. Blood Coagul Fibrinolysis 2007, 18, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Wakelyn, P.J. Cotton Fiber Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- French, A. Cellulose. In Encyclopedia of Biophysics; Roberts, G.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 248–253. [Google Scholar]
- Condon, B.; Gary, L.; Sawhney, P.; Reynolds, M.; Slopek, R.P.; Delhom, C.; Hui, D. Properties of nonwoven fabrics made with UltraCleanTM cotton. World J. Eng. 2010, 7, 180–184. [Google Scholar]
- Edwards, J.; Prevost, N.; Yager, D. Hydrogen peroxide production from fibrous pectic cellulose analogs and effect on dermal fibroblasts. In Proceedings of the 28th Annual Meeting of the Wound Healing Society, SAWC-Spring/WHS Joint Meeting, Wound Repair Regen, 2016; Volume 24, pp. A1–A30.
- Sawhney, A.P.S.; Condon, B.; Reynolds, M.; Slopek, R.; Hui, D. Advent of Greige Cotton Non-Wovens Made using a Hydro-Entanglement Process. Text. Res. J. 2010, 80, 1540–1549. [Google Scholar] [CrossRef]
- Sawhney, P.; Allen, C.; Reynolds, M.; Condon, B.; Slopek, R. Effect of water pressure on absorbency of hydroentangled greige cotton non-woven fabrics. Text. Res. J. 2012, 82, 21–26. [Google Scholar] [CrossRef]
- Clark, R.F. Overview and general considerations of wound repair. In The Molecular and Cellular Biology of Wound Repair; Clark, R.A.F., Henson, P.M., Eds.; Springer: New York, NY, USA, 1988; pp. 3–33. [Google Scholar]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic. Biol. Med. 2012, 53, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. The general case for redox control of wound repair. Wound Rep. Regen. 2003, 11, 431–438. [Google Scholar] [CrossRef]
- Chan, E.C.; Jiang, F.; Peshavariya, H.M.; Dusting, G.J. Regulation of cell proliferation by NADPH oxidase-mediated signaling: Potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol. Ther. 2009, 122, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. Biophys. Acta 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Braccini, I.; Pérez, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Carpin, S.; Crèvecoeur, M.; de Meyer, M.; Simon, P.; Greppin, H.; Penel, C. Identification of a Ca2+-Pectate Binding Site on an Apoplastic Peroxidase. Plant Cell 2001, 13, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Schweikert, C.; Liszkay, A.; Schopfer, P. Scission of polysaccharides by peroxidase-generated hydroxyl radicals. Phytochemistry 2000, 53, 565–570. [Google Scholar] [CrossRef]
- Fry, S.C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 1998, 332, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Pristov, J.B.; Jovanović, S.V.; Mitrović, A.; Spasojević, I. UV-irradiation provokes generation of superoxide on cell wall polygalacturonic acid. Physiol. Plant 2013, 148, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Zegota, H. Some quantitative aspects of hydroxyl radical induced reactions in γ-irradiated aqueous solutions of pectins. Food Hydrocoll. 2002, 16, 353–361. [Google Scholar] [CrossRef]
- Kim, H.J.; Triplett, B. Involvement of extracellular Cu/Zn superoxide dismutase in cotton fiber primary and secondary cell wall biosynthesis. Plant Signal. Behav. 2008, 3, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kato, N.; Kim, S.; Triplett, B. Cu/Zn superoxide dismutases in developing cotton fibers: Evidence for an extracellular form. Planta 2008, 228, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.Y.; Schmidt, R.J.; Andrews, A.M.; Turner, T.D. A study of hydrogen peroxide generation by, and antioxidant activity of, Granuflex™ (DuoDERM™) Hydrocolloid Granules and some other hydrogel/hydrocolloid wound management materials. Br. J. Dermatol. 1993, 129, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Chung, L.Y.; Turner, T.D. Quantification of hydrogen peroxide generation by Granuflex™ (DuoDERM™) Hydrocolloid Granules and its constituents (gelatin, sodium carboxymethylcellulose, and pectin). Br. J. Dermatol. 1993, 129, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Uluata, S.; McClements, D.J.; Decker, E.A. How the multiple antioxidant properties of ascorbic acid affect lipid oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2015, 63, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Clapp, P.A.; Du, N.; Evans, D.F. Thermal and photochemical production of hydrogen peroxide from dioxygen and tannic acid, gallic acid and other related compounds in aqueous solution. J. Chem. Soc. Faraday Trans. 1990, 86, 2587–2592. [Google Scholar] [CrossRef]
- Razzell, W.; Evans, I.R.; Martin, P.; Wood, W. Calcium Flashes Orchestrate the Wound Inflammatory Response through DUOX Activation and Hydrogen Peroxide Release. Curr. Biol. 2013, 23, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, V.; Belardinelli, M.; Rinaldi, C. Polysaccharides Depolymerization Via Hydroxyl Radicals Attack in Dilute Aqueous Solution. J. Carbohydr. Chem. 1997, 16, 561–572. [Google Scholar] [CrossRef]
- Gan, Q.; Jia, B.; Liu, X.; Zhang, Y.; Liu, M. Studies on Calcium Release and HO Level Produced by the Elicitor Induced Plant Cell by Fluorescence Probing. J. Fluoresc. 2012, 22, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K. Calcium: A Central Regulator of Plant Growth and Development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Role of Free Radicals and Catalytic Metal Ions in Human Disease: An Overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 26. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Zanardi, I.; Belmonte, G.; Gervellati, F.; Bocci, V.; Travagli, V. Ozone mediators effect on “in vitro” scratch wound closure. Free Radic. Res. 2016, 50, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Kim, H.J.; Condon, B.D.; Hinchliffe, D.J.; Chang, S.; McCarty, J.C.; Madison, C.A. High resistance to thermal decomposition in brown cotton is linked to tannins and sodium content. Cellulose 2016, 23, 1137–1152. [Google Scholar] [CrossRef]
- Pan, Q.; Qiu, W.-Y.; Huo, Y.-N.; Yao, Y.-F.; Lou, M.F. Low Levels of Hydrogen Peroxide Stimulate Corneal Epithelial Cell Adhesion, Migration, and Wound Healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Qin, Y.-M.; Pan, Y.; Song, W.-Q.; Mei, W.-Q.; Zhu, Y.-X. A cotton ascorbate peroxidase is involved in hydrogen peroxide homeostasis during fibre cell development. New Phytol. 2007, 175, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Barrett, D.M. Combined enzymatic and colorimetric method for determining the uronic acid and methylester content of pectin: Application to tomato products. Food Chem. 2008, 110, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Boylston, E.K.; Hinojosa, O.; Hebert, J.J. A Quick Embedding Method for Light and Electron Microscopy of Textile Fibers. Biotech. Histochem. 1991, 66, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, D.P.; Evans, J.P. Cotton Fiber Maturity by Image Analysis. Text. Res. J. 1986, 56, 130–139. [Google Scholar] [CrossRef]
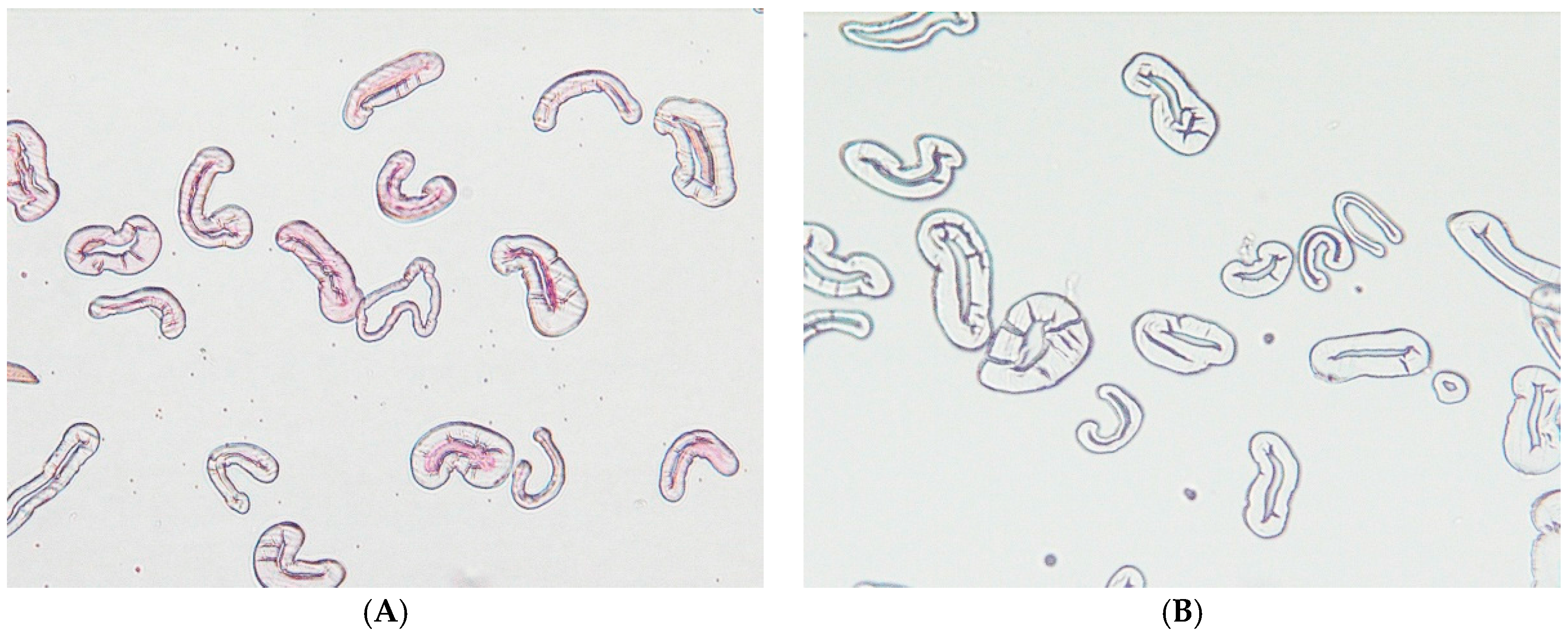
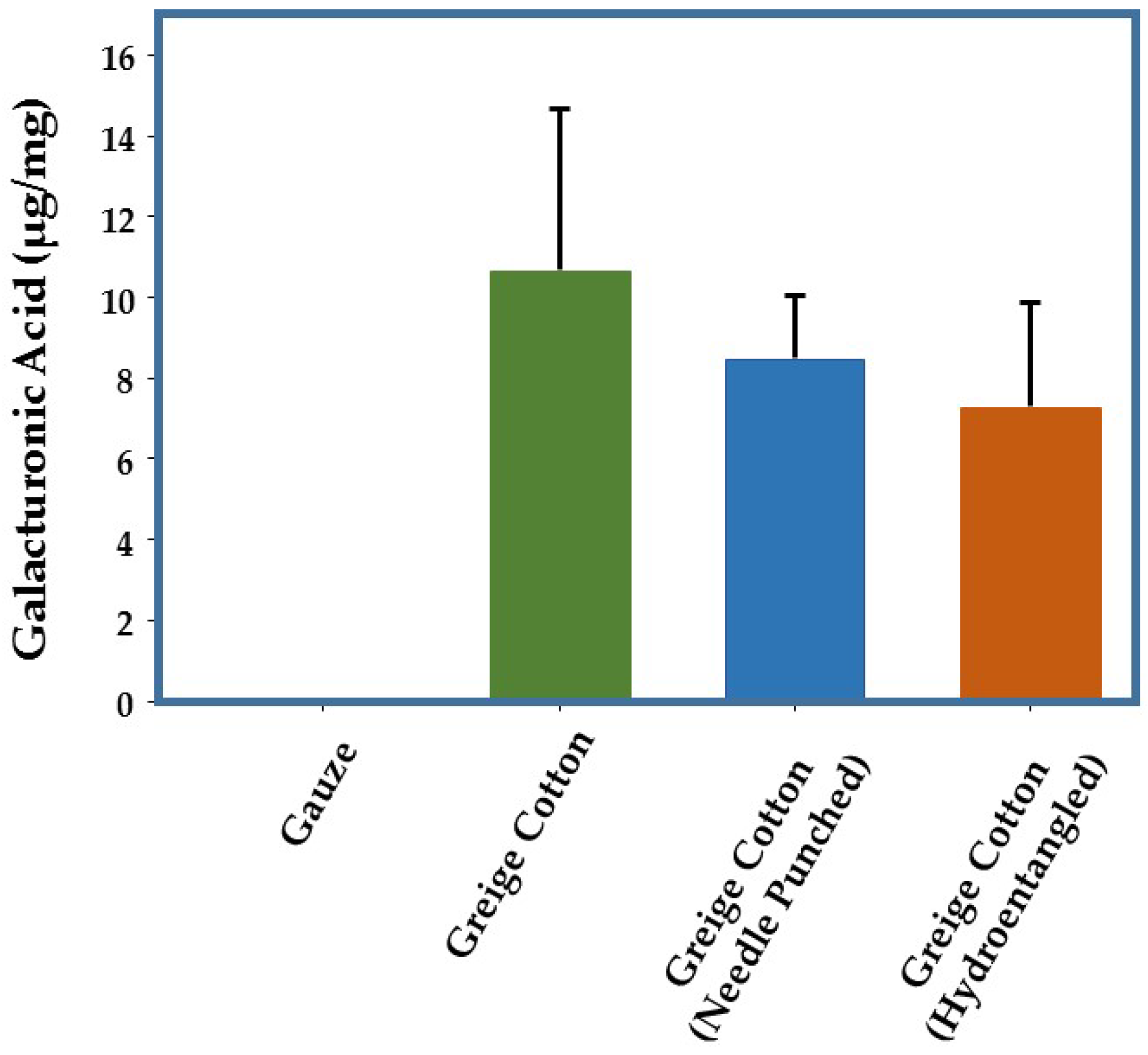
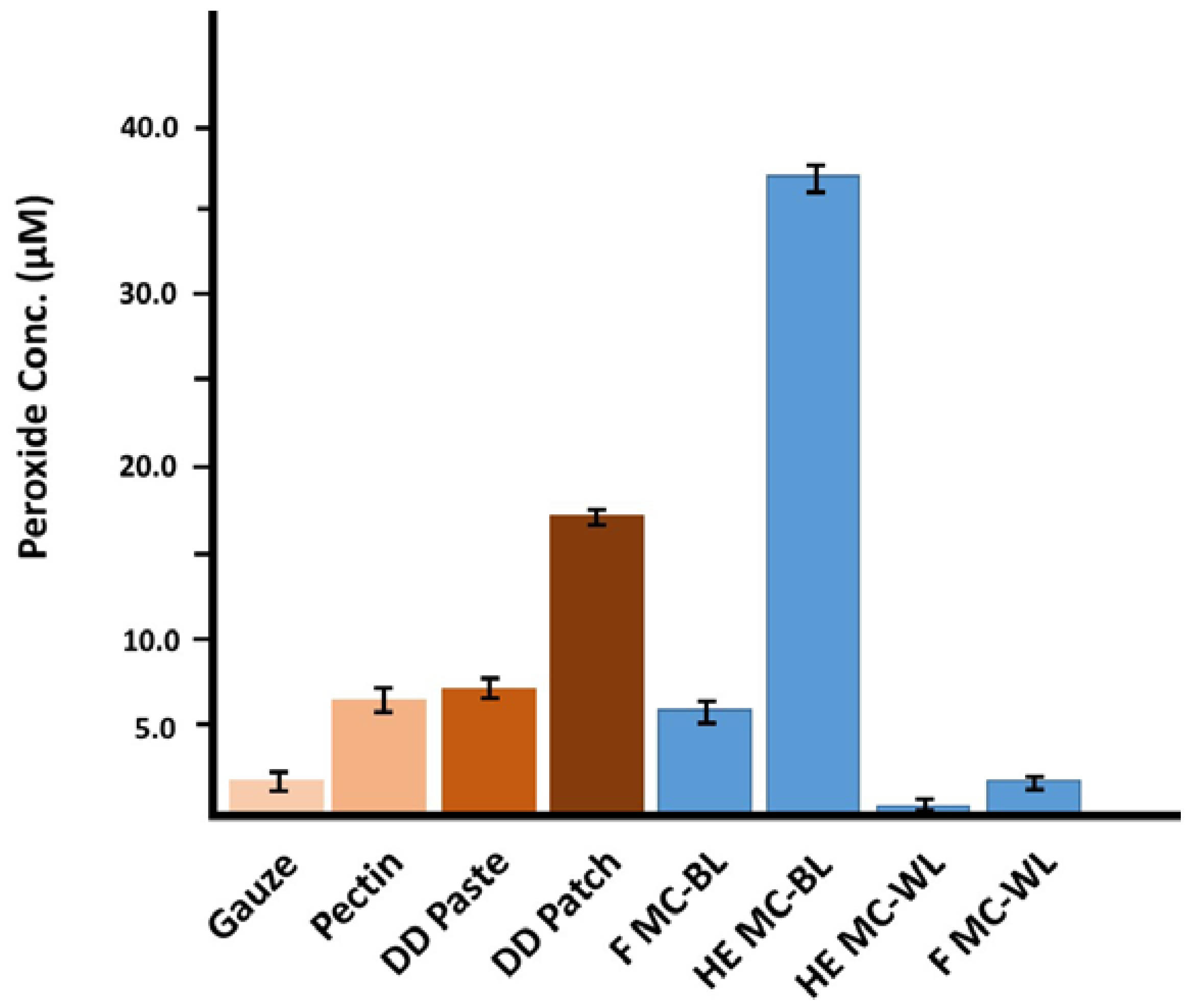
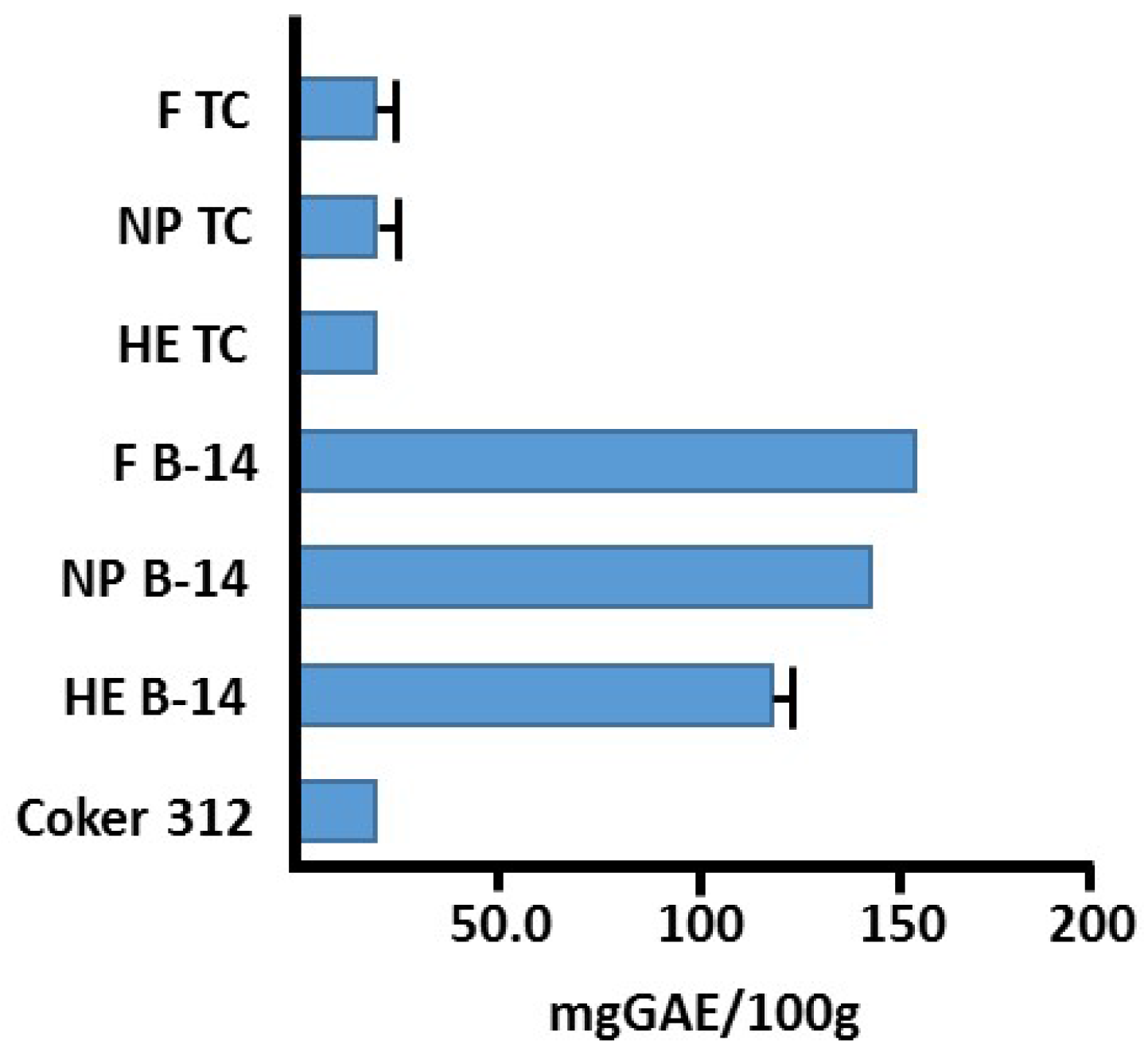
| Sample | Alone | +0.2 mM Cu (II) | +0.2 mM Ascorbate |
|---|---|---|---|
| F-TC (white) | 1.0 | 0.4 | 15.5 |
| NP-TC | 0.8 | 0.4 | 14.5 |
| HE-TC | 0.6 | ND | 12.5 |
| F-B14 (brown) | 2.5 | 3.0 | 17.2 |
| NP-B14 | 2.2 | 2.8 | 17.0 |
| HE-B14 | 2.8 | 3.0 | 15.2 |
| F-Coker312 (white) | 0.8 | 1.2 | 17.4 |
| Sample Name | Inhibition Rate% | SOD Activity (U/mL) |
|---|---|---|
| F TC (White) | 12.43 | 0.09 |
| NP TC© | 10.36 | 0.08 |
| HE TC© | 3.45 | 0.05 |
| F B-14 (Brown) | 63.15 | 1.86 |
| NP B-14 | 72.79 | 3.20 |
| HE B-14 | 61.32 | 1.64 |
| F Coker 312 (White) | 7.68 | 0.07 |
| Sample | 24Mg | 44Ca | 55Mn | 56Fe | 63Cu | 66Zn |
|---|---|---|---|---|---|---|
| F Coker 312 | 1281.4 | 347.7 | 3.7 | 8.7 | 0.8 | 2.3 |
| F TC | 544.2 | 411.0 | 3.7 | 379.9 | 2.1 | 5.4 |
| F B-14 | 614.8 | 601.1 | 7.3 | 360.4 | 2.4 | 59.7 |
| NP TC© | 670.5 | 542.5 | 4.5 | 24.1 | 1.9 | 7.0 |
| NP B-14 | 1069.7 | 955.0 | 5.1 | 23.9 | 2.2 | 8.7 |
| HE TC | 297.0 | 857.4 | 7.4 | 14.5 | 0.8 | 3.5 |
| HE B-14 | 846.8 | 1457.3 | 5.0 | 18.1 | 1.8 | 7.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, J.V.; Prevost, N.T.; Nam, S.; Hinchliffe, D.; Condon, B.; Yager, D. Induction of Low-Level Hydrogen Peroxide Generation by Unbleached Cotton Nonwovens as Potential Wound Dressing Materials. J. Funct. Biomater. 2017, 8, 9. https://doi.org/10.3390/jfb8010009
Edwards JV, Prevost NT, Nam S, Hinchliffe D, Condon B, Yager D. Induction of Low-Level Hydrogen Peroxide Generation by Unbleached Cotton Nonwovens as Potential Wound Dressing Materials. Journal of Functional Biomaterials. 2017; 8(1):9. https://doi.org/10.3390/jfb8010009
Chicago/Turabian StyleEdwards, J. Vincent, Nicolette T. Prevost, Sunghyun Nam, Doug Hinchliffe, Brian Condon, and Dorne Yager. 2017. "Induction of Low-Level Hydrogen Peroxide Generation by Unbleached Cotton Nonwovens as Potential Wound Dressing Materials" Journal of Functional Biomaterials 8, no. 1: 9. https://doi.org/10.3390/jfb8010009






