Enhanced Osseointegration of a Modified Titanium Implant with Bound Phospho-Threonine: A Preliminary In Vivo Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Evaluation of Surface Roughness
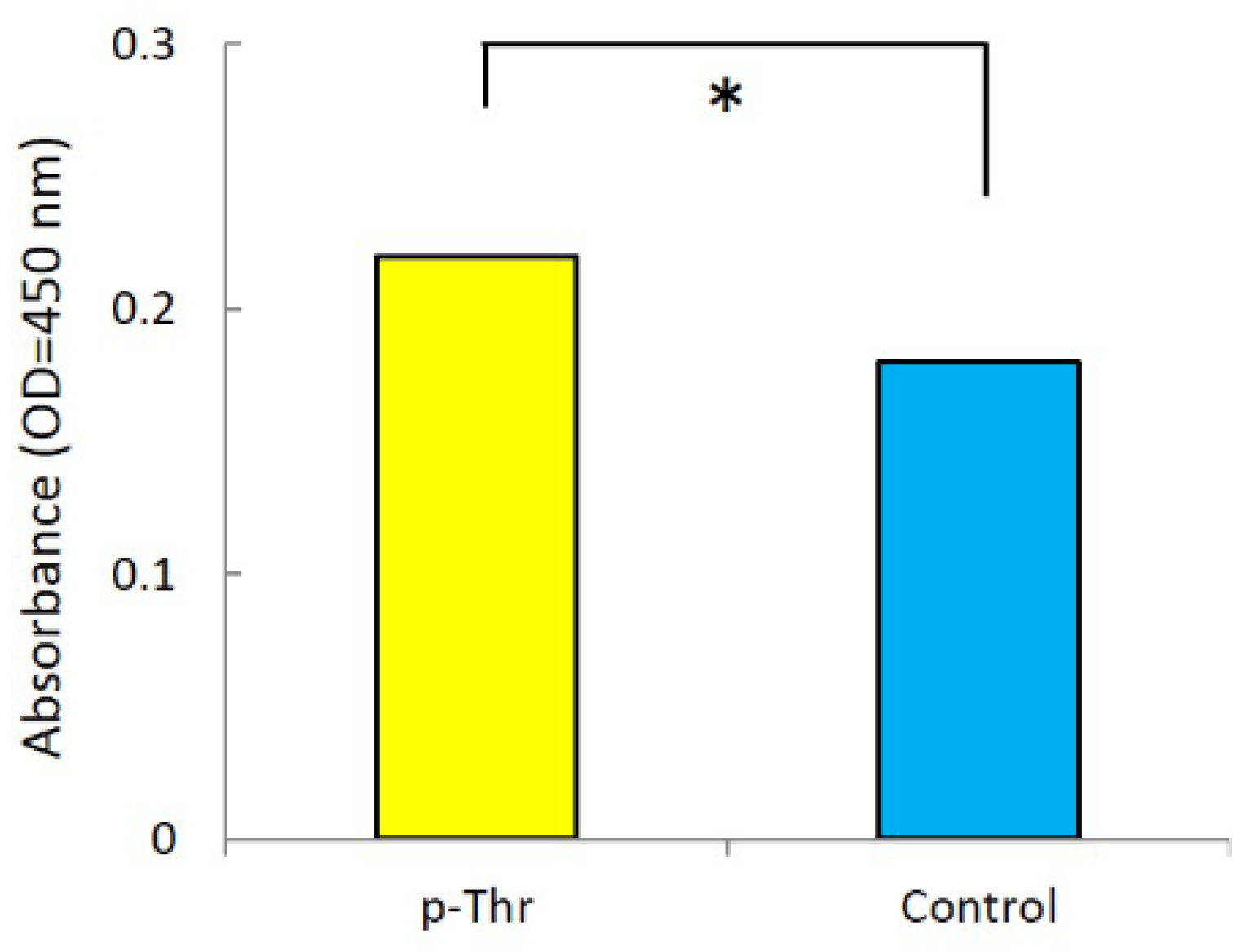
2.1.2. Measurement of Initial Cell Attachment
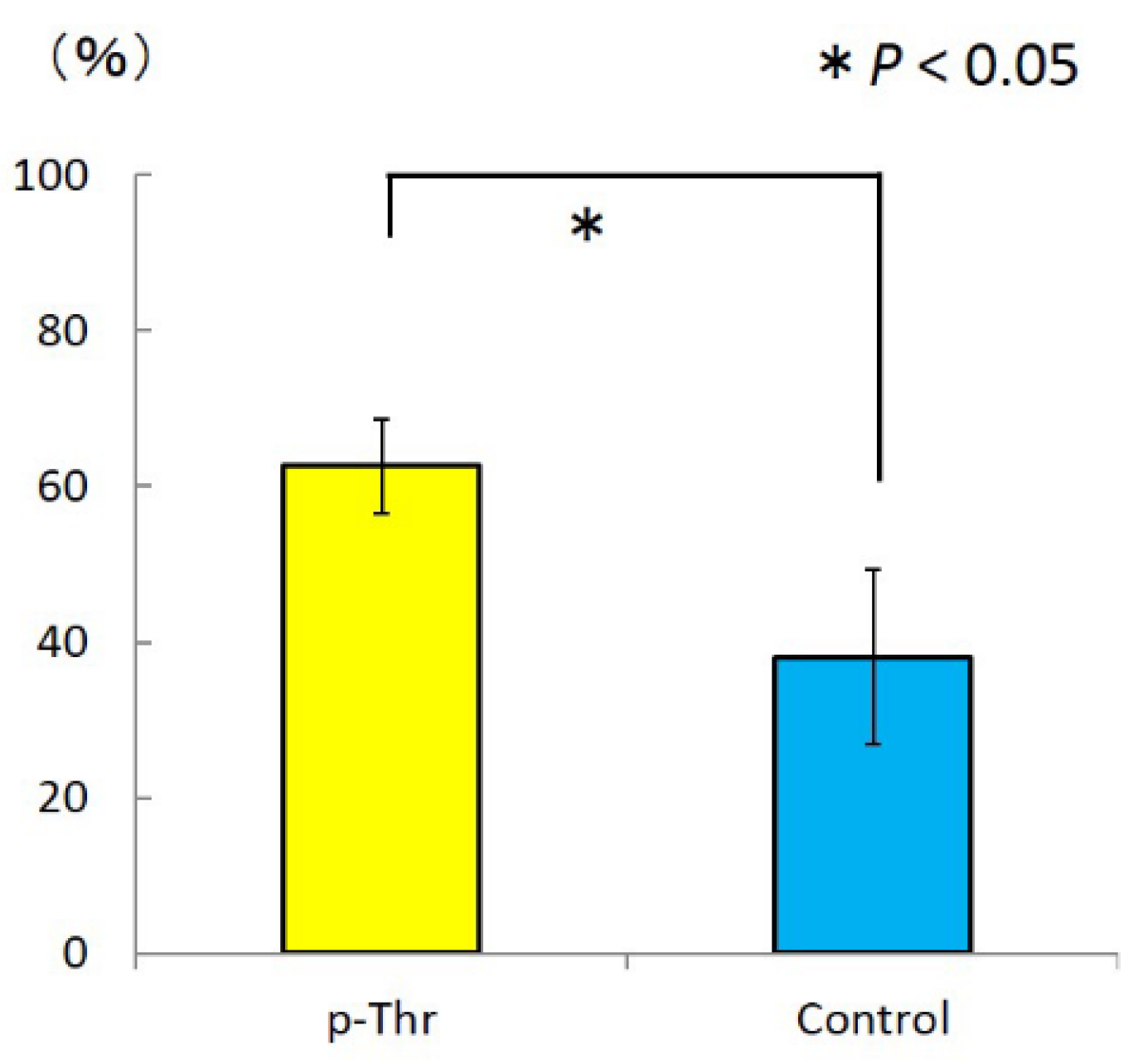
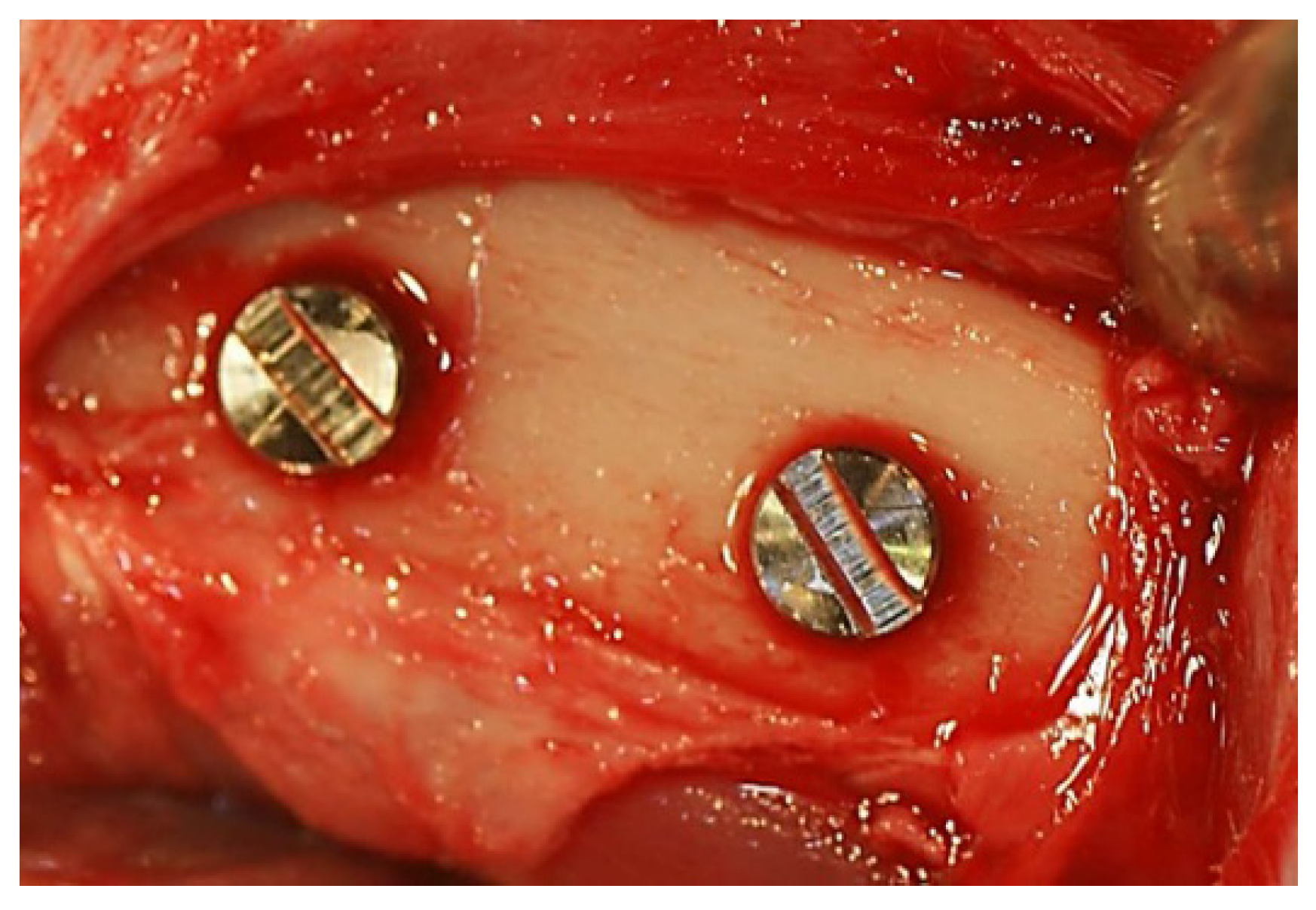
2.1.3. Measurement of Removal Torque and Bone-Implant Contact
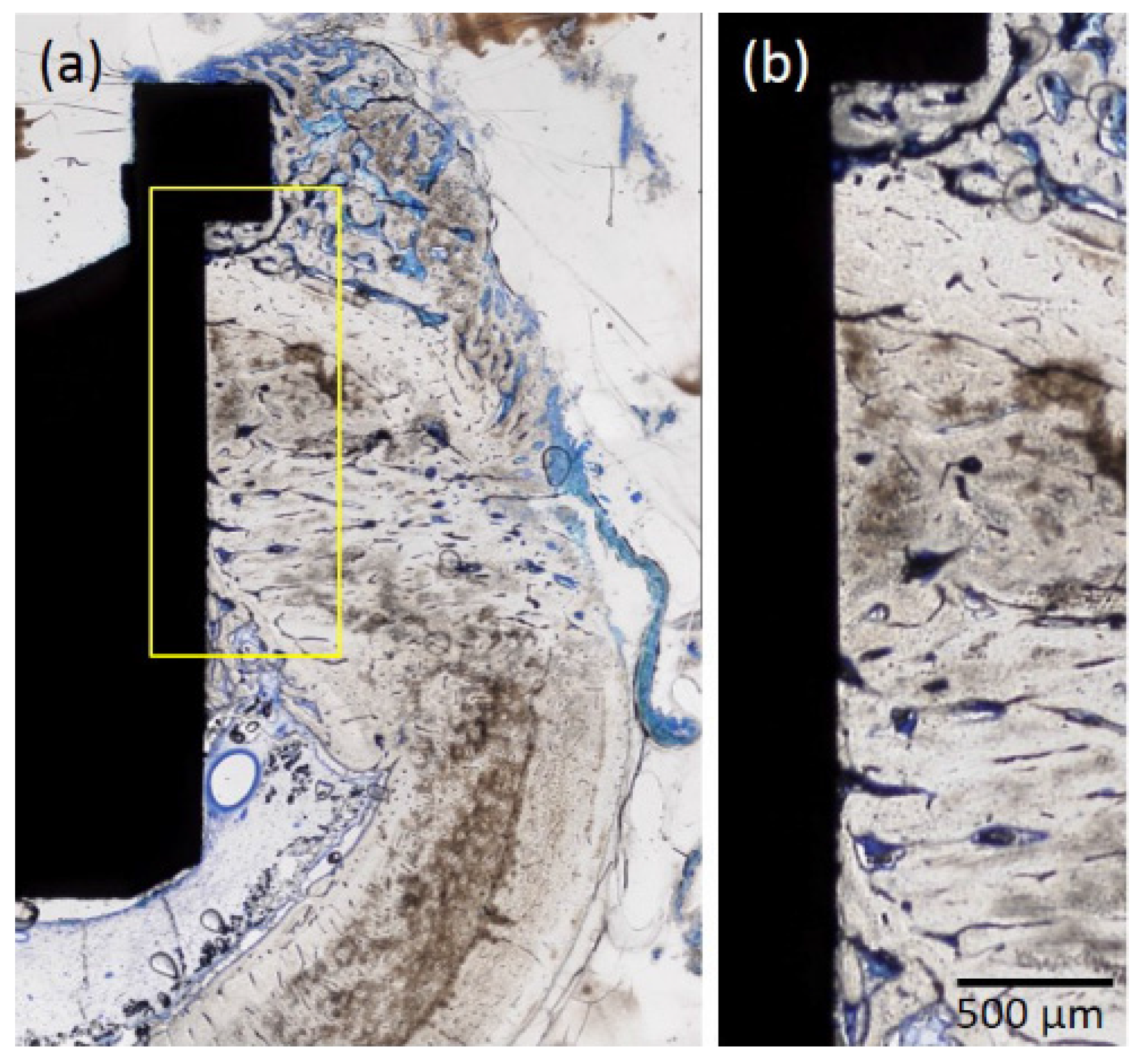
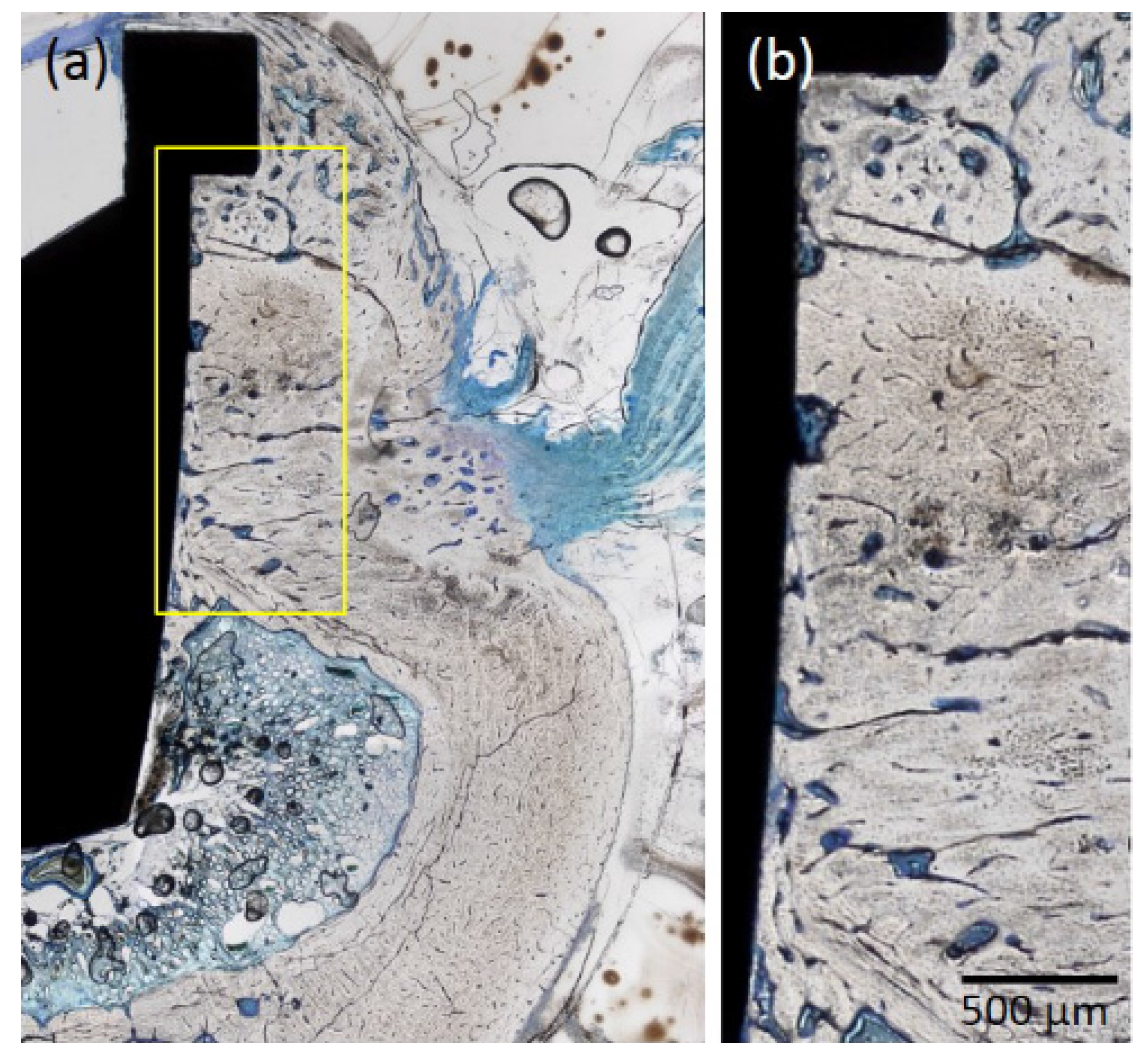
2.1.4. Histological Observations
2.2. Discussion
3. Materials and Methods
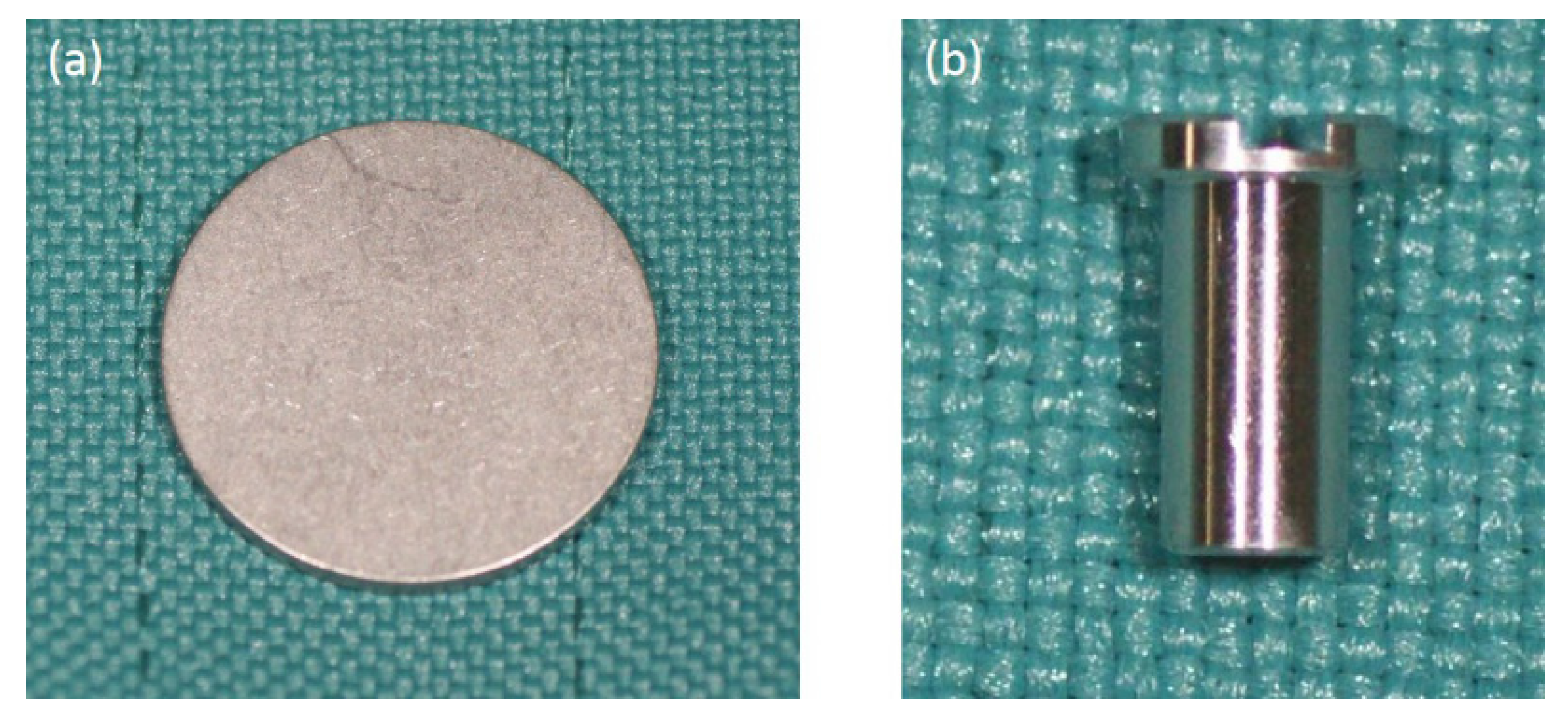
3.1. Fabrication of p-Thr-Binding Surface
3.2. Scanning Electron Microscopy and Surface Roughness Measurement
3.3. Initial Cell Attachment
3.4. In Vivo Assessment of Osseointegration
3.5. Histological and Histomorphometric Evaluation
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Albrektsson, T.; Johansson, C.; Lundgren, A.K.; Sul, Y.; Gottlow, J. Experimental studies on oxidezed implants. A histomorphometrical and biomechanical analysis. Appl. Osseointegr. Res. 2000, 1, 21–24. [Google Scholar]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Lohmann, C.H.; Oefinger, J.; Bonewald, L.F.; Dean, D.D.; Boyan, B.D. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Masaki, C.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C. Effects of implant surface microtopography on osteoblast gene expression. Clin. Oral Implant. Res. 2005, 16, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zinger, O.; Schwartz, Z.; Wieland, M.; Landolt, D.; Boyan, B.D. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin. Oral Implant. Res. 2006, 17, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.P. Acidic and basic properties of hydroxylated metal oxide surfaces. Discuss. Faraday Soc. 1971, 52, 264–275. [Google Scholar] [CrossRef]
- Oue, H.; Doi, K.; Oki, Y.; Makihara, Y.; Kubo, T.; Perrotti, V.; Piattelli, A.; Akagawa, Y.; Tsuga, K. Influence of implant surface topography on primary stability in a standardized osteoporosis rabbit model study. J. Funct. Biomater. 2015, 6, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants, (II). Etiopathogenesis. Eur. J. Oral Sci. 1998, 106, 721–764. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Abe, Y.; Hiasa, K.; Takeuchi, M.; Yoshida, Y.; Suzuki, K.; Akagawa, Y. New surface modification of titanium implant with phospho-amino acid. Dent. Mater. J. 2005, 24, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Glauser, R.; Portmann, M.; Ruhstaller, P.; Lundgren, A.K.; Hämmerle, C.; Gottlow, J. Stability measurements of immediately loaded machined and oxidized implants in the posterior maxilla. A comparative clinical study using resonance frequency analysis. Appl. Osseontegration Res. 2001, 2, 27–29. [Google Scholar]
- Albouy, J.P.; Abrahamsson, I.; Berglundh, T. Spontaneous progression of experimental peri-implantitis at implants with different surface characteristics: an experimental study in dogs. J. Clin. Periodontol. 2012, 39, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bächle, M.; Hannig, C.; Han, J.S.; Wolkewitz, M.; Kohal, R. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.E.; Ducheyne, P. The mechanisms of passive dissolution of titanium in a model physiological environment. J. Biomed. Mater. Res. 1992, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Viornery, C.; Guenther, H.L.; Aronsson, B.O.; Péchy, P.; Descouts, P.; Grätzel, M. Osteoblast culture on polished titanium disks modified with phosphonic acids. J. Biomed. Mater. Res. 2002, 62, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zarb, G.A.; Schmitt, A. Osseointegration and the edentulous predicament. The 10-year-old Toronto study. Br. Dent. J. 1991, 70, 439–444. [Google Scholar] [CrossRef]
- Meredith, N.; Alleyne, D.; Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implant. Res. 1996, 7, 261–267. [Google Scholar] [CrossRef]
- Friberg, B.; Sennerby, L.; Linden, B.; Gröndahl, K.; Lekholm, U. Stability measurements of one-stage Brånemark implants during healing in mandibles. A clinical resonance frequency analysis study. Int. J. Oral Maxillofac. Surg. 1999, 28, 266–272. [Google Scholar] [CrossRef]
- Huang, H.M.; Pan, L.C.; Lee, S.Y.; Chiu, C.L.; Fan, K.H.; Ho, K.N. Assessing the implant/bone interface by using natural frequency analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2000, 90, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Alvarez, E.; Muñoz, F.; Liñares, A.; Cantalapiedra, A. Influence on early osseointegration of dental implants installed with two different drilling protocols: A histomorphometric study in rabbit. Clin. Oral Implant. Res. 2011, 21, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Röstlund, T.; Albrektsson, B.; Albrektsson, T. Removal torques for polished and rough titanium implants. Int. J. Oral Maxillofac. Implant. 1988, 3, 21–24. [Google Scholar]
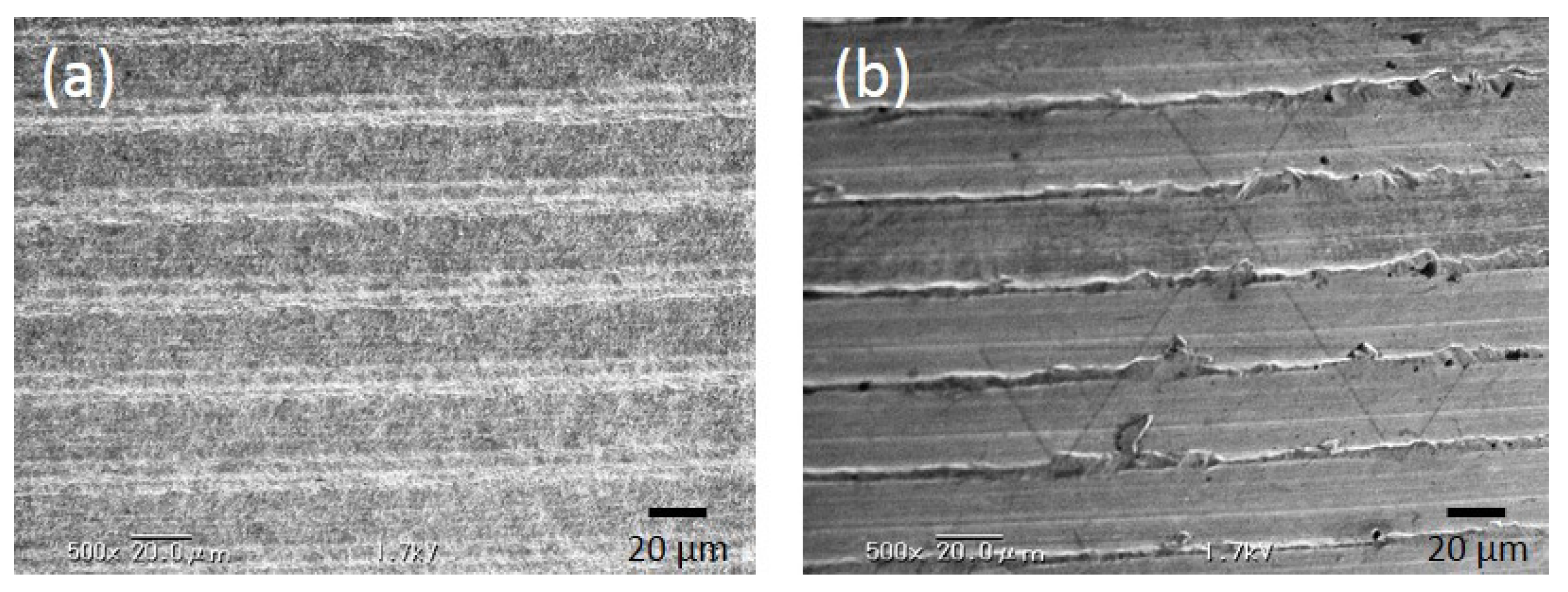

| Group | Ra µm (SD) |
|---|---|
| p-Thr | 0.41 (0.01) * |
| Control | 0.24 (0.02) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okazaki, Y.; Doi, K.; Oki, Y.; Kobatake, R.; Abe, Y.; Tsuga, K. Enhanced Osseointegration of a Modified Titanium Implant with Bound Phospho-Threonine: A Preliminary In Vivo Study. J. Funct. Biomater. 2017, 8, 16. https://doi.org/10.3390/jfb8020016
Okazaki Y, Doi K, Oki Y, Kobatake R, Abe Y, Tsuga K. Enhanced Osseointegration of a Modified Titanium Implant with Bound Phospho-Threonine: A Preliminary In Vivo Study. Journal of Functional Biomaterials. 2017; 8(2):16. https://doi.org/10.3390/jfb8020016
Chicago/Turabian StyleOkazaki, Yohei, Kazuya Doi, Yoshifumi Oki, Reiko Kobatake, Yasuhiko Abe, and Kazuhiro Tsuga. 2017. "Enhanced Osseointegration of a Modified Titanium Implant with Bound Phospho-Threonine: A Preliminary In Vivo Study" Journal of Functional Biomaterials 8, no. 2: 16. https://doi.org/10.3390/jfb8020016





