Laser Ablation for Cancer: Past, Present and Future
Abstract
:1. Introduction
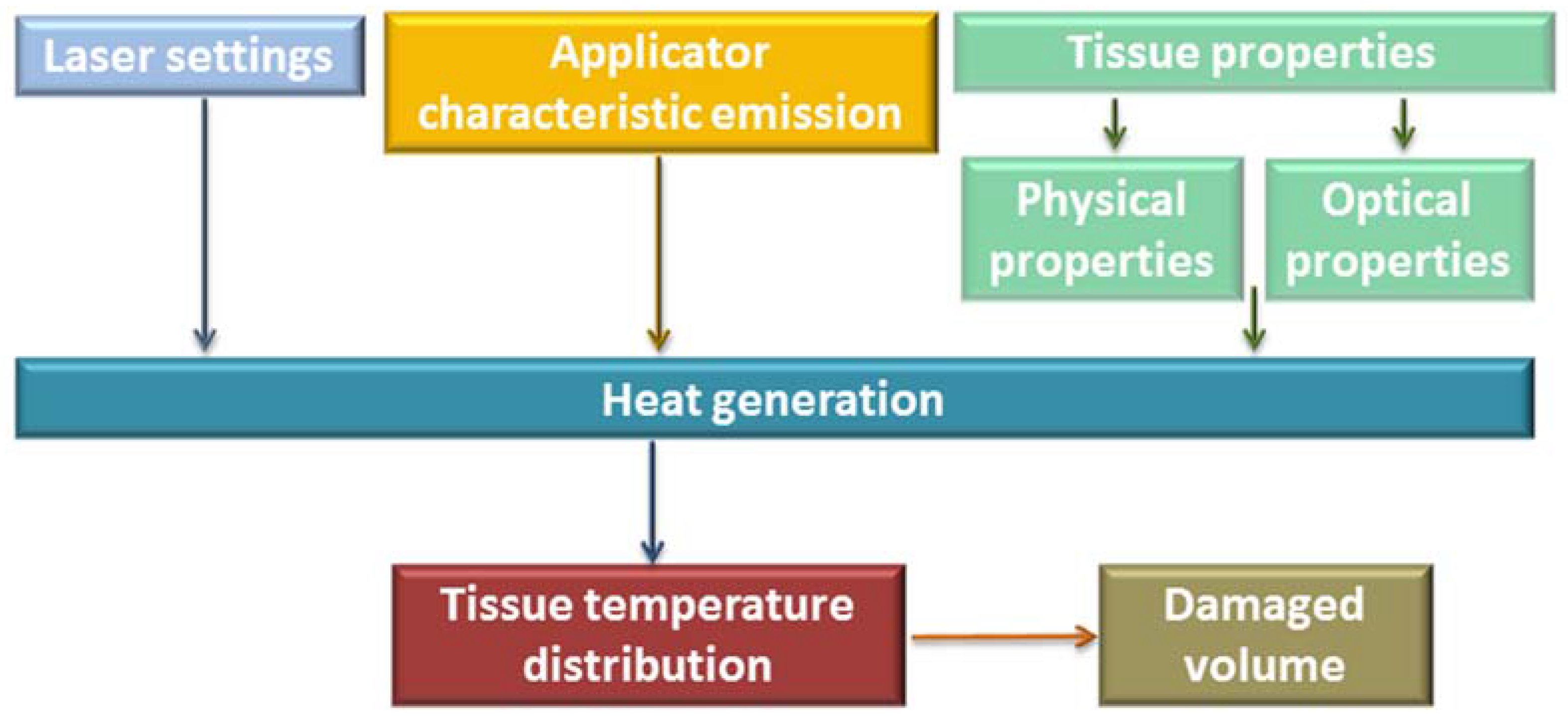
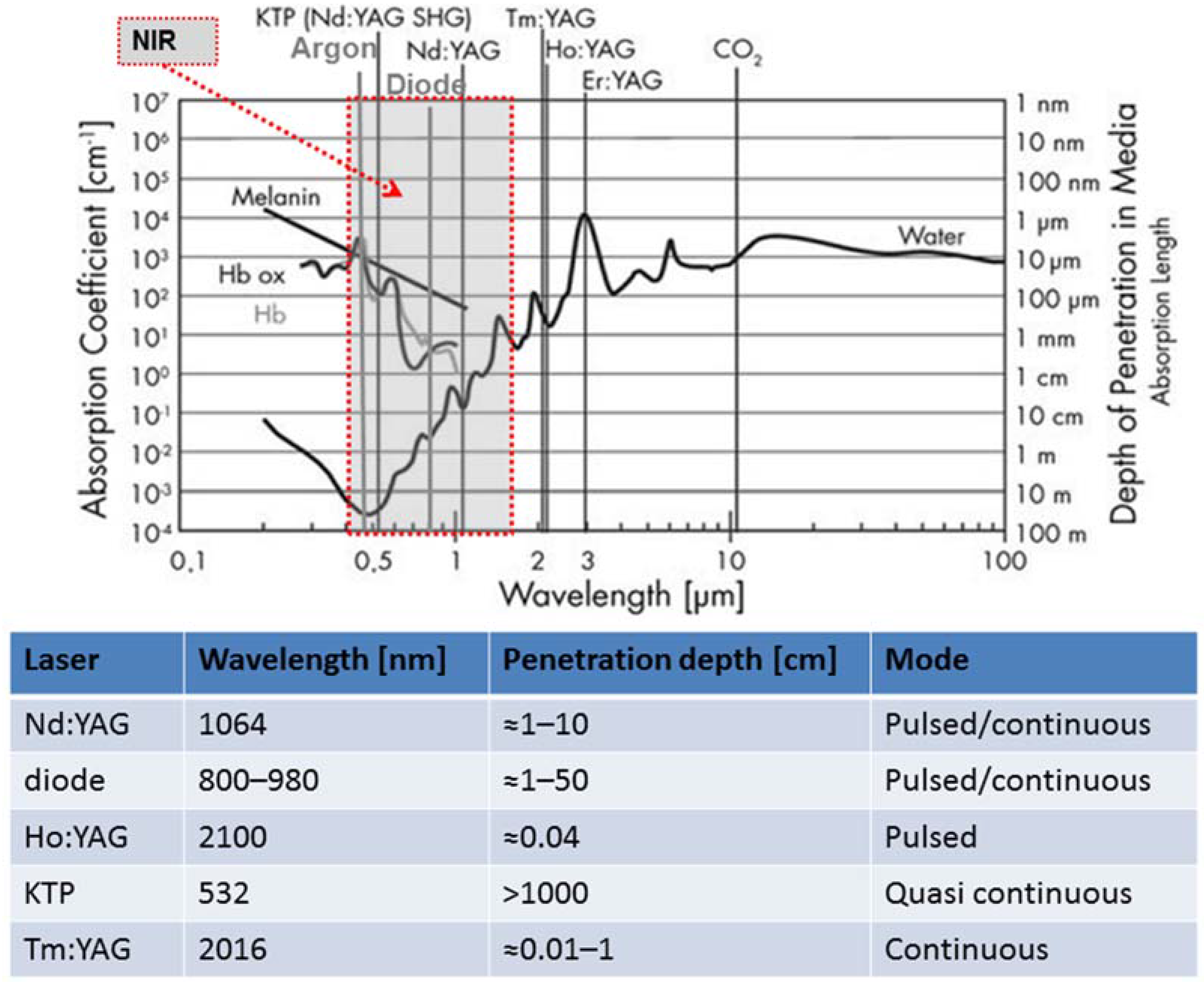
2. Basic Components of a Laser and Factors of Influence on the Laser Effect on Tissue
3. Lasers in Surgery
4. New Solutions to Guide Laser Ablation
5. Discussion
Author Contributions
Conflicts of Interest
References
- Solon, L.R.; Aronson, R.; Gould, G. Physiological implications of laser beams. Science 1961, 134, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Bown, S.G. Phototherapy in tumors. World J. Surg. 1983, 7, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Muschter, R.; Hofstetter, A. Interstitial laser therapy outcomes in benign prostatic hyperplasia. J. endourol. 1995, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F. Other Thermal Ablation Techniques: Microwave and Interstitial Laser Ablation of Liver Tumors. Ann. Surg. Oncol. 2003, 10, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.J.; Fuentes, D.; Elliott, A.A.; Weinberg, J.S.; Ahrar, K. Laser-induced thermal therapy for tumor ablation. Crit. Rev. Biomed. Eng. 2010, 38, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.J.; Roggan, A. Laser-induced interstitial thermotherapy; SPIE Optical Engineering Press: Bellingham, WA, USA, 1995. [Google Scholar]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, M.; Christophi, C. Interstitial laser thermotherapy for liver tumours. Br. J. Surg. 2003, 90, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Schena, E.; Caponero, M.A.; Di Matteo, F.M.; Martino, M.; Pandolfi, M.; Silvestri, S. Theoretical Analysis and Experimental Evaluation of Laser-Induced Interstitial Thermotherapy in ex Vivo Porcine Pancreas. IEEE Trans. Biomed. Eng. 2012, 59, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Schwarzmaier, H.-J.; Goldbach, T.; Ulrich, F.; Schober, R.; Kahn, T.; Kaufmann, R.; Wolbarsht, M.L. Improved laser applicators for interstitial thermotherapy of brain structures. In Proceedings of the Clinical Applications of Modern Imaging Technology II, Los Angeles, CA, USA, 23 January 1994; Cerullo, L.J., Heiferman, K.S., Liu, H., Podbielska, H., Wist, A.O., Zamorano, L.J., Eds.; International Society for Optics and Photonics: Orlando, FL, USA, 1994; pp. 4–12. [Google Scholar]
- Möller, P.H.; Lindberg, L.; Henriksson, P.H.; Persson, B.R.R.; Tranberg, K.-G. Interstitial laser thermotherapy: Comparison between bare fibre and sapphire probe. Lasers Med. Sci. 1995, 10, 193–200. [Google Scholar] [CrossRef]
- Sturesson, C. Interstitial laser-induced thermotherapy: Influence of carbonization on lesion size. Lasers Surg. Med. 1998, 22, 51–57. [Google Scholar] [CrossRef]
- Heisterkamp, J.; van Hillegersberg, R.; Sinofsky, E.; Ijzermans, J.N.M. Heat-resistant cylindrical diffuser for interstitial laser coagulation: Comparison with the bare-tip fiber in a porcine liver model. Lasers Surg. Med. 1997, 20, 304–309. [Google Scholar] [CrossRef]
- Mensel, B.; Weigel, C.; Hosten, N. Laser-Induced Thermotherapy. In Minimally Invasive Tumor Therapies; Springer: Berlin/Heidelberg, Germany, 2006; pp. 69–75. [Google Scholar]
- Germer, C.T.; Albrecht, D.; Roggan, A.; Buhr, H.J. Technology for In Situ Ablation by Laparoscopic and Image-Guided Interstitial Laser Hyperthermia. Surg. Innov. 1998, 5, 195–203. [Google Scholar] [CrossRef]
- Germer, C.T.; Albrecht, D.; Isbert, C.; Ritz, J.; Roggan, A.; Buhr, H.J. Diffusing Fibre Tip for the Minimally Invasive Treatment of Liver Tumours by Interstitial Laser Coagulation (ILC): An Experimental Ex Vivo Study. Lasers Med. Sci. 1999, 14, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Steger, A.C.; Lees, W.R.; Shorvon, P.; Walmsley, K.; Bown, S.G. Multiple-fibre low-power interstitial laser hyperthermia: Studies in the normal liver. Br. J. Surg. 1992, 79, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Schena, E.; Giurazza, F.; Del Vescovo, R.; Caponero, M.A.; Mortato, L.; Panzera, F.; Cazzato, R.L.; Grasso, F.R.; Di Matteo, F.M.; et al. Temperature monitoring and lesion volume estimation during double-applicator laser-induced thermotherapy in ex vivo swine pancreas: A preliminary study. Lasers Med. Sci. 2014, 29, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.; Malcontenti-Wilson, C.; Christophi, C. Interstitial laser hyperthermia for colorectal liver metastases: The effect of thermal sensitization and the use of a cylindrical diffuser tip on tumor necrosis. J. Clin. Laser Med. Surg. 2002, 20, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Pacella, C.M.; Bizzarri, G.; Magnolfi, F.; Cecconi, P.; Caspani, B.; Anelli, V.; Bianchini, A.; Valle, D.; Pacella, S.; Manenti, G.; et al. Laser Thermal Ablation in the Treatment of Small Hepatocellular Carcinoma: Results in 74 Patients. Radiology 2001, 221, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Farshid, P.; Naguib, N.N.N.; Darvishi, A.; Bazrafshan, B.; Mbalisike, E.; Burkhard, T.; Zangos, S. Thermal ablation of liver metastases from colorectal cancer: Radiofrequency, microwave and laser ablation therapies. Radiol. Med. 2014, 119, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Freier, V.; Nour-Eldin, N.-E.A.; Eichler, K.; Zangos, S.; Naguib, N.N.N. Magnetic Resonance–Guided Laser-Induced Interstitial Thermotherapy of Breast Cancer Liver Metastases and Other Noncolorectal Cancer Liver Metastases. Investig. Radiol. 2013, 48, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Dick, E.A.; Joarder, R.; De Jode, M.; Taylor-Robinson, S.D.; Thomas, H.C.; Foster, G.R.; Gedroyc, W.M. MR-guided Laser Thermal Ablation of Primary and Secondary Liver Tumours. Clin. Radiol. 2003, 58, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Pech, M.; Wieners, G.; Freund, T.; Dudeck, O.; Fischbach, F.; Ricke, J.; Seemann, M.D. MR-guided interstitial laser thermotherapy of colorectal liver metastases: Efficiency, safety and patient survival. Eur. J. Med. Res. 2007, 12, 161–168. [Google Scholar] [PubMed]
- Ritz, J.-P.; Lehmann, K.S.; Zurbuchen, U.; Wacker, F.; Brehm, F.; Isbert, C.; Germer, C.T.; Buhr, H.J.; Holmer, C. Improving laser-induced thermotherapy of liver metastases—Effects of arterial microembolization and complete blood flow occlusion. Eur. J. Surg. Oncol. 2007, 33, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Christophi, C.; Nikfarjam, M.; Malcontenti-Wilson, C.; Muralidharan, V. Long-term Survival of Patients with Unresectable Colorectal Liver Metastases treated by Percutaneous Interstitial Laser Thermotherapy. World J. Surg. 2004, 28, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Windahl, T.; Skeppner, E.; Andersson, S.-O.; Fugl-Meyer, K.S. Sexual function and satisfaction in men after laser treatment for penile carcinoma. J. Urol. 2004, 172, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Lont, A.P.; Gallee, M.P.W.; Meinhardt, W.; van Tinteren, H.; Horenblas, S. Penis Conserving Treatment for T1 and T2 Penile Carcinoma: Clinical Implications of a Local Recurrence. J. Urol. 2006, 176, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.P.; Boon, T.A.; van Venrooij, G.E.; Wijburg, C.J. Long-Term Follow-up After Laser Therapy for Penile Carcinoma. Urology 2007, 69, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, B.; Tilki, D.; Seitz, M.; Bader, M.J.; Reich, O.; Schneede, P.; Hungerhuber, E.; Stief, C.G.; Gratzke, C. Organ-preserving neodymium-yttrium-aluminium-garnet laser therapy for penile carcinoma: A long-term follow-up. BJU Int. 2010, 106, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Jocham, D.; Beer, A.; Staehler, G. Adjuvant Laser Treatment of Bladder Cancer: 8 Years’ Experience with the Nd-YAG Laser 1064 nm. BJU Int. 1989, 63, 476–478. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; González, R.; Conde, S.; Morales, V.; Martinez-Molina, E. Jejunal and Bladder Perforation: Complication of Intravesical Nd-YAG Laser Irradiation of Bladder Tumour. Acta Chir. Belg. 2008, 108, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Beisland, H.O.; Sander, S.; Fossberg, E. Neodymium:Yag laser irradiation of urinary bladder tumors Follow-up study of 100 consecutively treated patients. Urology 1985, 25, 559–563. [Google Scholar] [CrossRef]
- Kardos, R.; Magasi, P.; Karsza, A. Nd-Yag laser treatment of bladder tumours. Int. Urol. Nephrol. 1994, 26, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, S.; Foccoli, P.; Toninelli, C.; Feijo, S. Nd:YAG laser therapy in lung cancer: An 11-year experience with 2,253 applications in 1,585 patients. J. Bronchol. Int. Pulmonol. 1994, 1, 105–111. [Google Scholar] [CrossRef]
- Schwarzmaier, H.-J.; Eickmeyer, F.; von Tempelhoff, W.; Fiedler, V.U.; Niehoff, H.; Ulrich, S.D.; Yang, Q.; Ulrich, F. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: Preliminary results in 16 patients. Eur. J. Radiol. 2006, 59, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Streitparth, F.; Gebauer, B.; Melcher, I.; Schaser, K.; Philipp, C.; Rump, J.; Hamm, B.; Teichgräber, U. MR-Guided Laser Ablation of Osteoid Osteoma in an Open High-Field System (1.0 T). Cardiovasc. Intervent. Radiol. 2009, 32, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Dick, E.A.; Joarder, R.; De Jode, M.G.; Wragg, P.; Vale, J.A.; Gedroyc, W.M.W. Magnetic resonance imaging-guided laser thermal ablation of renal tumours. BJU Int. 2002, 90, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, F.; Picconi, F.; Martino, M.; Pandolfi, M.; Pacella, C.; Schena, E.; Costamagna, G. Endoscopic ultrasound-guided Nd:YAG laser ablation of recurrent pancreatic neuroendocrine tumor: A promising revolution? Endoscopy 2014, 46, E380–E381. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Cova, L.; Ierace, T.; Baroli, A.; Di Mauro, E.; Pacella, C.M.; Goldberg, S.N.; Solbiati, L. Treatment of Metastatic Lymph Nodes in the Neck from Papillary Thyroid Carcinoma with Percutaneous Laser Ablation. Cardiovasc. Intervent. Radiol. 2016, 39, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Straub, R.; Eichler, K.; Woitaschek, D.; Mack, M.G. Malignant Liver Tumors Treated with MR Imaging–guided Laser-induced Thermotherapy: Experience with Complications in 899 Patients (2520 lesions). Radiology 2002, 225, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Zukiwskyj, M.; Daly, P.; Chung, E. Penile cancer and phallus preservation strategies: A review of current literature. BJU Int. 2013, 112, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Skeppner, E.; Windahl, T.; Andersson, S.-O.; Fugl-Meyer, K.S. Treatment-Seeking, Aspects of Sexual Activity and Life Satisfaction in Men with Laser-Treated Penile Carcinoma. Eur. Urol. 2008, 54, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, A.G. Application of lasers in bladder cancer. Semin. Surg. Oncol. 1992, 8, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.W.; Bach, T.; Wolters, M.; Imkamp, F.; Gross, A.J.; Kuczyk, M.A.; Merseburger, A.S.; Herrmann, T.R.W. Current evidence for transurethral laser therapy of non-muscle invasive bladder cancer. World J. Urol. 2011, 29, 433–442. [Google Scholar] [CrossRef] [PubMed]
- D’Hallewin, M.A.; Clays, K.; Persoons, A.; Baert, L. Large-bowel perforation. A rare complication of intravesical Nd-YAG laser irradiation of bladder tumors. Urol. Int. 1989, 44, 373–374. [Google Scholar] [PubMed]
- Dohmoto, M.; Hünerbein, M.; Schlag, P.M. Palliative endoscopie therapy of rectal carcinoma. Eur. J. Cancer 1996, 32, 25–29. [Google Scholar] [CrossRef]
- Sherwood, L.A.; Knowles, G.; Wilson, R.G.; Potter, M.A. Retrospective review of laser therapy for palliation of colorectal tumours. Eur. J. Oncol. Nurs. 2006, 10, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Simoff, M.J. Advances in Supportive and Palliative Care for Lung Cancer Patients; Springer: Berlin/Heidelberg, Germany, 2011; pp. 575–593. [Google Scholar]
- Schilling, A.; Böwering, R.; Keiditsch, E. Use of the neodymium-YAG laser in the treatment of ureteral tumors and urethral condylomata acuminata. Clinical experience. Eur. Urol. 1986, 12 (Suppl. 1), 30–33. [Google Scholar] [PubMed]
- Akimov, A.B.; Seregin, V.E.; Rusanov, K.V.; Tyurina, E.G.; Glushko, T.A.; Nevzorov, V.P.; Nevzorova, O.F.; Akimova, E.V. Nd:YAG interstitial laser thermotherapy in the treatment of breast cancer. Lasers Surg. Med. 1998, 22, 257–267. [Google Scholar] [CrossRef]
- Johnson, D.E. Use of the holmium:YAG (Ho:YAG) laser for treatment of superficial bladder carcinoma. Lasers Surg. Med. 1994, 14, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Razvi, H.A.; Chun, S.S.; Denstedt, J.D.; Sales, J.L. Soft-Tissue Applications of the Holmium:YAG Laser in Urology. J. Endourol. 1995, 9, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.W.; Wolters, M.; Cash, H.; Jutzi, S.; Imkamp, F.; Kuczyk, M.A.; Merseburger, A.S.; Herrmann, T.R.W. Current evidence of transurethral Ho:YAG and Tm:YAG treatment of bladder cancer: Update. World J. Urol. 2015, 33, 571–579. [Google Scholar]
- Syed, H.A.; Biyani, C.S.; Bryan, N.; Brough, S.J.S.; Powell, C.S. Holmium:YAG Laser Treatment of Recurrent Superficial Bladder Carcinoma: Initial Clinical Experience. J. Endourol. 2001, 15, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Gilling, P.; Fraundorfer, M. Holmium laser resection of bladder tumors (HoLRBT). Tech. Urol. 1998, 4, 12–14. [Google Scholar] [PubMed]
- Jønler, M.; Lund, L.; Bisballe, S. Holmium:YAG laser vaporization of recurrent papillary tumours of the bladder under local anaesthesia. BJU Int. 2004, 94, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Khan, S.A.; Salam, M.A.; Hossain, S.; Islam, R. Holmium YAG laser treatment of superficial bladder carcinoma. Mymensingh Med. J. 2005, 14, 13–15. [Google Scholar] [PubMed]
- Zhu, Y.; Jiang, X.; Zhang, J.; Chen, W.; Shi, B.; Xu, Z. Safety and Efficacy of Holmium Laser Resection for Primary Nonmuscle-Invasive Bladder Cancer Versus Transurethral Electroresection: Single-Center Experience. Urology 2008, 72, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.A.; Zisengwe, G.; Athanasiou, T.; O’Brien, T.; Thomas, K. Outpatient laser ablation of non-muscle-invasive bladder cancer: Is it safe, tolerable and cost-effective? BJU Int. 2013, 112, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Xishuang, S.; Deyong, Y.; Xiangyu, C.; Tao, J.; Quanlin, L.; Hongwei, G.; Jibin, Y.; Dongjun, W.; Zhongzhou, H.; Jianbo, W.; et al. Comparing the safety and efficiency of conventional monopolar, plasmakinetic, and holmium laser transurethral resection of primary non-muscle invasive bladder cancer. J. Endourol. 2010, 24, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; lida, S.; Tomiyasu, K.; Inoue, M.; Noda, S. Transurethral endoscopic treatment of upper urinary tract tumors using a holmium:YAG laser. Lasers Surg. Med. 2003, 32, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Lindner, U.; Weersink, R.A.; Haider, M.A.; Gertner, M.R.; Davidson, S.R.H.; Atri, M.; Wilson, B.C.; Fenster, A.; Trachtenberg, J. Image Guided Photothermal Focal Therapy for Localized Prostate Cancer: Phase I Trial. J. Urol. 2009, 182, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Amin, Z.; Lees, W.R.; Bown, S.G. Interstitial laser photocoagulation for the treatment of prostatic cancer. Br. J. Radiol. 1993, 66, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Atri, M.; Gertner, M.R.; Haider, M.A.; Weersink, R.A.; Trachtenberg, J. Contrast-enhanced ultrasonography for real-time monitoring of interstitial laser thermal therapy in the focal treatment of prostate cancer. Can. Urol. Assoc. J. 2009, 3, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Alizadeh, H.; Wong, L.; Buy, X.; Dietemann, J.-L.; Roy, C. Osteoid Osteoma: Percutaneous Laser Ablation and Follow-up in 114 Patients. Radiology 2007, 242, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; McNichols, R.J.; Stafford, R.J.; Itzcovitz, J.; Guichard, J.-P.; Reizine, D.; Delaloge, S.; Vicaut, E.; Payen, D.; Gowda, A.; et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery 2008, 63, ONS21–ONS29. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, K.; Francescatti, D.S.; Bloom, K.J. Laser therapy for small breast cancers. Am. J. Surg. 2002, 184, 359–363. [Google Scholar] [CrossRef]
- Haraldsdóttir, K.H.; Ivarsson, K.; Götberg, S.; Ingvar, C.; Stenram, U.; Tranberg, K.-G. Interstitial laser thermotherapy (ILT) of breast cancer. Eur. J. Surg. Oncol. 2008, 34, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Gillams, A.R.; Lees, W.R. Survival after percutaneous, image-guided, thermal ablation of hepatic metastases from colorectal cancer. Dis. Colon Rectum 2000, 43, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Paulides, M.M.; Stauffer, P.R.; Neufeld, E.; Maccarini, P.F.; Kyriakou, A.; Canters, R.A.M.; Diederich, C.J.; Bakker, J.F.; Van Rhoon, G.C. Simulation techniques in hyperthermia treatment planning. Int. J. Hyperth. 2013, 29, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Bruggmoser, G. Some aspects of quality management in deep regional hyperthermia. Int. J. Hyperth. 2012, 28, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Rijnen, Z.; Bakker, J.F.; Canters, R.A.M.; Togni, P.; Verduijn, G.M.; Levendag, P.C.; Van Rhoon, G.C.; Paulides, M.M. Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int. J. Hyperth. 2013, 29, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.P.; Kotte, A.N.; Crezee, J. Planning, optimisation and evaluation of hyperthermia treatments. Int. J. Hyperth. 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gellermann, J.; Wust, P.; Stalling, D.; Seebass, M.; Nadobny, J.; Beck, R.; Hege, H.-C.; Deuflhard, P.; Felix, R. Clinical evaluation and verification of the hyperthermia treatment planning system hyperplan. Int. J. Radiat. Oncol. 2000, 47, 1145–1156. [Google Scholar] [CrossRef]
- Verhaart, R.F.; Verduijn, G.M.; Fortunati, V.; Rijnen, Z.; van Walsum, T.; Veenland, J.F.; Paulides, M.M. Accurate 3D temperature dosimetry during hyperthermia therapy by combining invasive measurements and patient-specific simulations. Int. J. Hyperth. 2015, 31, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasa, G.; Gellermann, J.; Rau, B.; Nadobny, J.; Schlag, P.; Deuflhard, P.; Felix, R.; Wust, P. Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int. J. Radiat. Oncol. 2003, 55, 407–419. [Google Scholar] [CrossRef]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Crezee, H.; van Leeuwen, C.M.; Oei, A.L.; Stalpers, L.J.A.; Bel, A.; Franken, N.A.; Kok, H.P. Thermoradiotherapy planning: Integration in routine clinical practice. Int. J. Hyperth. 2016, 32, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.; Wust, P.; Stauffer, P.; Bardati, F.; van Rhoon, G.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Viglianti, B.L.; Lora-Michiels, M.; Hanson, M.; Hoopes, P.J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperth. 2003, 19, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Schena, E.; Silvestri, S. Techniques for temperature monitoring during laser-induced thermotherapy: An overview. Int. J. Hyperth. 2013, 29, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ukimura, O.; de Castro Abreu, A.L.; Hung, A.J.; Gill, I.S. Cryosurgery for clinical T3 prostate cancer. BJU Int. 2014, 113, 684–685. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Majocchi, L. Assessment of temperature measurement error and its correction during Nd:YAG laser ablation in porcine pancreas. Int. J. Hyperth. 2014, 30, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Bele, C.; Orza, A.I.; Lucan, C.; Stiufiuc, R.; Manaila, I.; Iulia, F.; Dana, I.; et al. Selective ex vivo photothermal ablation of human pancreatic cancer with albumin functionalized multiwalled carbon nanotubes. Int. J. Nanomedicine 2011, 6, 915–928. [Google Scholar] [PubMed]
- Matsumoto, R.; Selig, A.M.; Colucci, V.M.; Jolesz, F.A. Interstitial Nd:YAG laser ablation in normal rabbit liver: Trial to maximize the size of laser-induced lesions. Lasers Surg. Med. 1992, 12, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Haynes, M.; Stang, J.; Moghaddam, M. Real-time Microwave Imaging of Differential Temperature for Thermal Therapy Monitoring. IEEE Trans. Biomed. Eng. 2014, 61, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Marien, A.; Gill, I.; Ukimura, O.; Nacim, B.; Villers, A. Target ablation—Image-guided therapy in prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Manns, F.; Milne, P.J.; Gonzalez-Cirre, X.; Denham, D.B.; Parel, J.M.; Robinson, D.S. In situ temperature measurements with thermocouple probes during laser interstitial thermotherapy (LITT): Quantification and correction of a measurement artifact. Lasers Surg. Med. 1998, 23, 94–103. [Google Scholar] [CrossRef]
- Van Nimwegen, S.A.; L’Eplattenier, H.F.; Rem, A.I.; van der Lugt, J.J.; Kirpensteijn, J. Nd:YAG surgical laser effects in canine prostate tissue: Temperature and damage distribution. Phys. Med. Biol. 2009, 54, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.D.; Gertner, M.R.; Sherar, M.D. Temperature measurement artefacts of thermocouples and fluoroptic probes during laser irradiation at 810 nm. Phys. Med. Biol. 2001, 46, N149–N157. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, F.; Martino, M.; Rea, R.; Pandolfi, M.; Panzera, F.; Stigliano, E.; Schena, E.; Saccomandi, P.; Silvestri, S.; Pacella, C.M.; et al. US-guided application of Nd:YAG laser in porcine pancreatic tissue: An ex vivo study and numerical simulation. Gastrointest. Endosc. 2013, 78, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Tosi, D.; Saccomandi, P.; Lewis, E.; Kim, T. Fiber Optic Sensors for Temperature Monitoring during Thermal Treatments: An Overview. Sensors 2016, 16, 1144. [Google Scholar] [CrossRef] [PubMed]
- Taffoni, F.; Formica, D.; Saccomandi, P.; Di Pino, G.; Schena, E. Optical fiber-based MR-compatible sensors for medical applications: An overview. Sensors 2013, 13, 14105–14120. [Google Scholar] [CrossRef] [PubMed]
- Cavaiola, C.; Saccomandi, P.; Massaroni, C.; Tosi, D.; Giurazza, F.; Frauenfelder, G.; Beomonte Zobel, B.; Di Matteo, F.M.; Caponero, M.A.; Polimadei, A.; et al. Error of a Temperature Probe for Cancer Ablation Monitoring Caused by Respiratory Movements: Ex vivo and in vivo analysis. IEEE Sens. J. 2016, 16, 5934–5941. [Google Scholar] [CrossRef]
- Polito, D.; Caponero, M.A.; Polimadei, A.; Saccomandi, P.; Massaroni, C.; Silvestri, S.; Schena, E. A needle-like probe for temperature monitoring during laser ablation based on FBG: Manufacturing and characterization. J. Med. Device 2015, 9, 041006. [Google Scholar] [CrossRef]
- Cappelli, S.; Saccomandi, P.; Massaroni, C.; Polimadei, A.; Silvestri, S.; Caponero, M.A.; Frauenfelder, G.; Schena, E. Magnetic Resonance-compatible needle-like probe based on Bragg grating technology for measuring temperature during Laser Ablation. In Proceedings of the 2015 37th IEEE Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 1287–1290. [Google Scholar]
- Rieke, V.; Pauly, K.B. MR thermometry. J. Magn. Reson. Imaging 2008, 27, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, G.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Frauenfelder, G.; Di Matteo, F.M.; Beomonte Zobel, B.; Silvestri, S.; Schena, E. Magnetic resonance-based thermometry during laser ablation on ex vivo swine pancreas and liver. Med. Eng. Phys. 2015, 37, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Straub, R.; Zangos, S.; Mack, M.G.; Eichler, K. MR-guided laser-induced thermotherapy (LITT) of liver tumours: Experimental and clinical data. Int. J. Hyperth. 2004, 20, 713–724. [Google Scholar] [CrossRef]
- Vogl, T.J.; Müller, P.K.; Hammerstingl, R.; Weinhold, N.; Mack, M.G.; Philipp, C.; Deimling, M.; Beuthan, J.; Pegios, W.; Riess, H. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: Technique and prospective results. Radiology 1995, 196, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Raman, S.; Priester, A.M.; Garritano, J.; Margolis, D.J.A.; Lieu, P.; Macairan, M.L.; Huang, J.; Grundfest, W.; Marks, L.S. Focal Laser Ablation of Prostate Cancer: Phase I Clinical Trial. J. Urol. 2016, 196, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.; Diakite, M.; Payne, A.; Parker, D.L. In vivo evaluation of multi-echo hybrid PRF/T1 approach for temperature monitoring during breast MR-guided focused ultrasound surgery treatments. Magn. Reson. Med. 2014, 72, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Bydder, G.M.; Kreel, L. The temperature dependence of computed tomography attenuation values. J. Comput. Assist. Tomogr. 1979, 3, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Saccomandi, P.; Giurazza, F.; Del Vescovo, R.; Mortato, L.; Martino, M.; Panzera, F.; Di Matteo, F.M.; Zobel, B.B.; Silvestri, S. Monitoring of temperature increase and tissue vaporization during laser interstitial thermotherapy of ex vivo swine liver by computed tomography. In Proceedings of the IEEE 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 378–381. [Google Scholar]
- Pandeya, G.D.; Klaessens, J.H.; Greuter, M.J.; Schmidt, B.; Flohr, T.; Van Hillegersberg, R.; Oudkerk, M. Feasibility of computed tomography based thermometry during interstitial laser heating in bovine liver. Eur. Radiol. 2011, 21, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Mortato, L.; Di Matteo, F.M.; Panzera, F.; Del Vescovo, R.; Beomonte Zobel, B.; Silvestri, S. Experimental assessment of CT-based thermometry during laser ablation of porcine pancreas. Phys. Med. Biol. 2013, 58, 5705–5716. [Google Scholar] [CrossRef] [PubMed]
- Fani, F.; Schena, E.; Saccomandi, P.; Silvestri, S. CT-based thermometry: An overview. Int. J. Hyperth. 2014, 30, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Frauenfelder, G.; Massaroni, C.; Saccomandi, P.; Giurazza, F.; Pitocco, F.; Marano, R.; Schena, E. Emerging clinical applications of computed tomography. Med. Devices 2015, 8, 265–278. [Google Scholar]
- Weiss, N.; Sosna, J.; Goldberg, S.N.; Azhari, H. Non-invasive temperature monitoring and hyperthermic injury onset detection using X-ray CT during HIFU thermal treatment in ex vivo fatty tissue. Int. J. Hyperth. 2014, 30, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.; Schena, E.; Saccomandi, P.; Zhumkhawala, A.; Aboody, K.; Berlin, J.M. Gold nanorod-mediated near-infrared laser ablation: In vivo experiments on mice and theoretical analysis at different settings. Int. J. Hyperth. 2017, 33, 150–159. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, C.M.; Ding, L.; Jin, C.; Cao, P.; Siddiqui, I.; Hwang, D.M.; Chen, J.; Wilson, B.C.; Zheng, G.; Hedley, D.W. Porphysome nanoparticles for enhanced photothermal therapy in a patient-derived orthotopic pancreas xenograft cancer model: A pilot study. J. Biomed. Opt. 2016, 21, 84002. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yuan, A.; Zhao, X.; Lian, H.; Zhuang, J.; Chen, W.; Zhang, Q.; Liu, G.; Zhang, S.; Chen, W.; et al. Self-assembled tumor-targeting hyaluronic acid nanoparticles for photothermal ablation in orthotopic bladder cancer. Acta Biomater. 2017, 53, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.; Schena, E.; Zhumkhawala, A.; Aboody, K.S.; Berlin, J.M. Internal temperature increase during photothermal tumour ablation in mice using gold nanorods. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 2563–2566. [Google Scholar]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.C.; Palazuelos, M.; Sharma, P.; Powers, K.W.; Roberts, S.M.; Grobmyer, S.R.; Moudgil, B.M. Nanoparticle Characterization for Cancer Nanotechnology and Other Biological Applications. In Cancer Nanotechnology: Methods and Protocols; Humana Press: New York, NY, USA, 2010; pp. 39–65. [Google Scholar]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
| Author | Tumor (Number of Patients) | Diameter of Tumor | Applicator | Laser Settings | Follow Up/Complications |
|---|---|---|---|---|---|
| Pacella et al., 2001 [20] | HCC (74) | 0.8–4 cm | Bare fiber | P = 5 W t = 6–12 min | SR: 99%, 68%, and 15% at 1, 3, and 5 y |
| Vogl et al., 2002 [21] | Malignant liver tumor (899) | --- | Bare or Cooled fiber | P = 4–5 W bare P = 35–45 W cooled fiber t = 3–28 min | 0.1% death 13% of overall complications |
| Vogl et al., 2013 [22] | Malignant liver tumor (401) | <5 cm | Cooled fiber | P mean for each applicator 34 W | SR: 86.5% and 33.4% at 1 and 5 y |
| Dick et al., 2003 [23] | Primary and secondary liver tumor (35) | --- | Cooled fiber | P = 25 W t = 10–30 min | Mean SR: HCC: 14.6 m (for HCC), 15.2 m (for met) Carcinoid (all patients alive from 1 to 47 m post ablation) |
| Pech et al., 2007 [24] | Colorectal liver met (66) | ≤5 cm | Cooled diffuser tip fiber | P = 10 W per cm diffusor length t = 15–37 min | Median of SR 23 m Major complications rate 2.3% |
| Ritz et al., 2007 [25] | Colorectal liver met (56) | ≤5 cm | Cooled diffuser fiber tip | P = 24–30 W t = 20–28 min | After 6 m follow-up, tumor recurrence in 6 patients Morbidity rate 21.4% |
| Christophi et al., 2004 [26] | Colorectal liver met (80) | <10 cm | Bare fiber | P = 2–4 W | Overall complications 16% |
| Windahl et al., 2004 [27] | Penile cancer (67) | <3 cm | --- | --- | Median follow up: 42 m Local recurrence rate 19% |
| Lont et al., 2005 [28] | Penile cancer (257) | <3 cm | --- | --- | Median follow up 106 m Local recurrence rate 37.5% |
| Meijer et al., 2007 [29] | Penile cancer (44) | --- | --- | P = 25–35 W | Follow up 3 m–16 y Local disease: 48% of the patients |
| Schlenker et al., 2010 [30] | Penile cancer (54) | --- | Cooled bare fiber | P = 30–50 W t = 60–150 s | Local recurrence: 42%, mean time to local recurrence 53 m |
| Beer et al., 1989 [31] | Bladder cancer (252) | --- | Bare fiber | P = 40–50 W t per pulse = 3–4 s | Total complications 15% Only 1 bladder perforation |
| J. Ruiz-Tovar et al., 2008 [32] | Bladder cancer (1) | --- | Bare fiber | P = 35 W t per pulse = 2 s | Bladder perforation |
| Beisland et al., 1985 [33] | Bladder cancer (100) | --- | Bare fiber | P = 45–50 W t per pulse < 4 s | 1 bowel perforation, 2 severe bleeding |
| Kardos et al., 1994 [34] | Bladder cancer (116) | 7 mm of average | --- | P = 30–40 W t (per pulse) = 2–3 s | No major complications |
| Cavaliere et al., 1994 [35] | Breast cancer (1585) | P = 20–30 W t (per pulse) = 4–5 s | Major limitation: rapid regrowth of the tumor | ||
| Schwarzmaier et al. 2006 [36] | Glioblastoma (16) | >20 mm | Diffuser tip | P = 6 W | Overall survival longer than those reported from natural history or after chemotherapy |
| Streitparth et al., 2009 [37] | Osteoid osteoma (1) | 5 mm | Bare fiber | P = 2.3 W t = 11 min | --- |
| Dick et al., 2002 [38] | Renal tumor (9) | --- | Cooled Bare fiber | P = 25 W t = 10–30 min | Two minor and one major complications |
| Di Matteo et al. 2013 [39] | Neuroendocrine Pancreatic tumor (1) | --- | Bare fiber | P = 4 W t = 5 min | --- |
| Mauri et al., 2016 [40] | cervical lymph node met (24) | --- | --- | 1 or 2 fibers, P = 3–4 W t = 5–10 min | No major complications; 2 minor complications (8.3%). |
| Author | Tumor (Number of Patients) | Diameter of Tumor | Applicator | Laser Settings | Follow Up/Complications |
|---|---|---|---|---|---|
| Syed et al., 2001 [55] | Bladder (41) | <1 cm | Bare fiber | E = 0.5–1.0 J f = 5–10 Hz | No complications |
| Razvi et al., 1995 [53] | Bladder (25) | <1 cm | Bare fiber | E = 0.5–1.0 J P = 4–7.2 W f = 8–14 Hz | No complications |
| Das et al., 1998 [56] | Bladder (23) | --- | Bare fiber | --- | 1× recatheterization |
| Johnson, 1994 [52] | Bladder (15) | 2–15 mm | Bare fiber | E = 1 J P = 10 W f = 10 Hz | No complications |
| Jonler et al., 2004 [57] | Bladder (52) | 2–30 mm | Bare fiber | E = 1 J P = 40 W f = 40 Hz | Recurrence |
| Hossain et al., 005 [58] | Bladder (30) | <40 mm | Bare fiber | E = 0.5–1.2 J f = 10–12 Hz | Recurrence |
| Zhu et al., 2008 [59] | Bladder (101) | --- | Bare fiber | E = 1.5–2.2 J P = 20–40 W f = 15–20 Hz | --- |
| Xishuang et al., 008 [61] | Bladder (64) | --- | Bare fiber | E = 1.5 J P = 30 W f = 20 Hz | --- |
| Wong et al., 2013 [60] | Bladder (54) | <30 mm | Bare fiber | E = 0.6–0.8 J f = 10–15 Hz | Recurrence |
| Matsuoka et al., 2003 [62] | Upper urinary tract (30) | 5–30 mm | Bare fiber | E = 0.5–1 J P = 15–40 W f = 5–20 Hz | Recurrence |
| Author | Tumor (Number of Patients) | Diameter of Tumor | Applicator | Laser Settings | Follow Up/Complications |
|---|---|---|---|---|---|
| Atri et al., 2009 [65] | Prostatic carcinoma (1) | 16 mm | 1 bare fiber | Two lasers at P = 15–2 W, t = 12 min | Necrotic tissue in targeted area |
| Amin et al., 1993 [64] | Prostatic carcinoma (1) | --- | 3 applicators | P = 2 W t = 500 s | Biopsies confirmed the presence of necrosis |
| Linder et al., 2009[63] | Prostatic carcinoma (12) | --- | 1 or 2 applicators | --- | 67% of patients free of tumor in the target at 6 m |
| Gangi et al., 2007 [66] | Osteoid osteoma (114) | <24 mm | Bare fiber | P = 2 W t ≤ 600 s | 6 recurrence, 1 unsuccessful treatment |
| Carpentier et al., 2008 [67] | Metastatic intracranial tumor (4) | <3 cm | Light-diffusing tip | P = 15 W | No tumor recurrence |
| Dowlatshahi et al., 2002 [68] | Breast tumor (54) | 5–23 mm | --- | P = 5 W | Complete destruction of 93% of the tumors |
| Haraldsdóttir et al., 2008 [69] | Breast tumor (54) | --- | Bare fiber | P = 3 W | Small skin necrosis in two patients |
| Gillams et al., 2000 [70] | Hepatic met (69) | --- | Bare fiber | P = 2–2.7 W for each fiber t = 440 s | LA improves survival in patients with inoperable but limited liver met. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schena, E.; Saccomandi, P.; Fong, Y. Laser Ablation for Cancer: Past, Present and Future. J. Funct. Biomater. 2017, 8, 19. https://doi.org/10.3390/jfb8020019
Schena E, Saccomandi P, Fong Y. Laser Ablation for Cancer: Past, Present and Future. Journal of Functional Biomaterials. 2017; 8(2):19. https://doi.org/10.3390/jfb8020019
Chicago/Turabian StyleSchena, Emiliano, Paola Saccomandi, and Yuman Fong. 2017. "Laser Ablation for Cancer: Past, Present and Future" Journal of Functional Biomaterials 8, no. 2: 19. https://doi.org/10.3390/jfb8020019






