Mn2+-ZnSe/ZnS@SiO2 Nanoparticles for Turn-on Luminescence Thiol Detection
Abstract
:1. Introduction
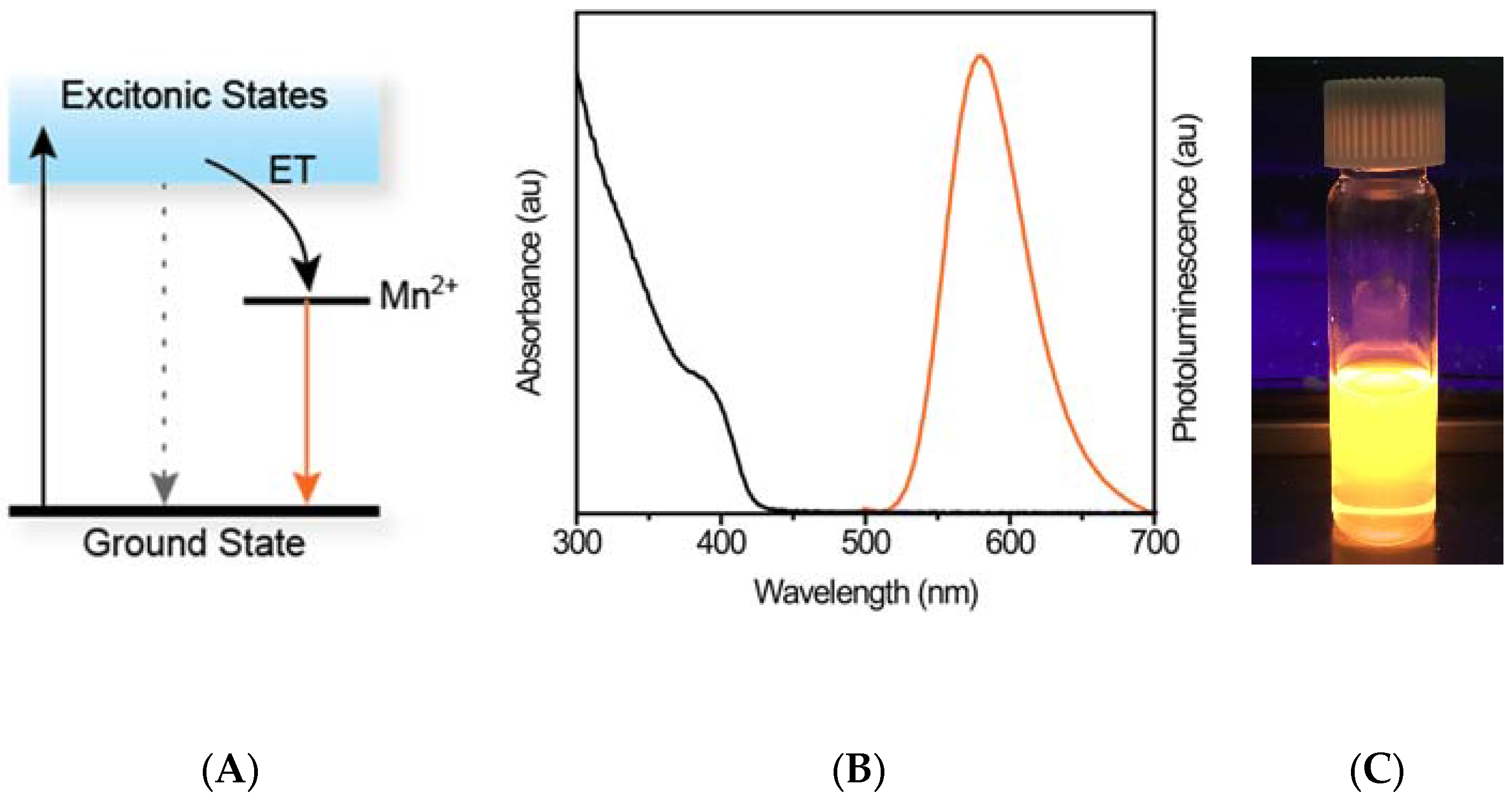
2. Results and Discussion
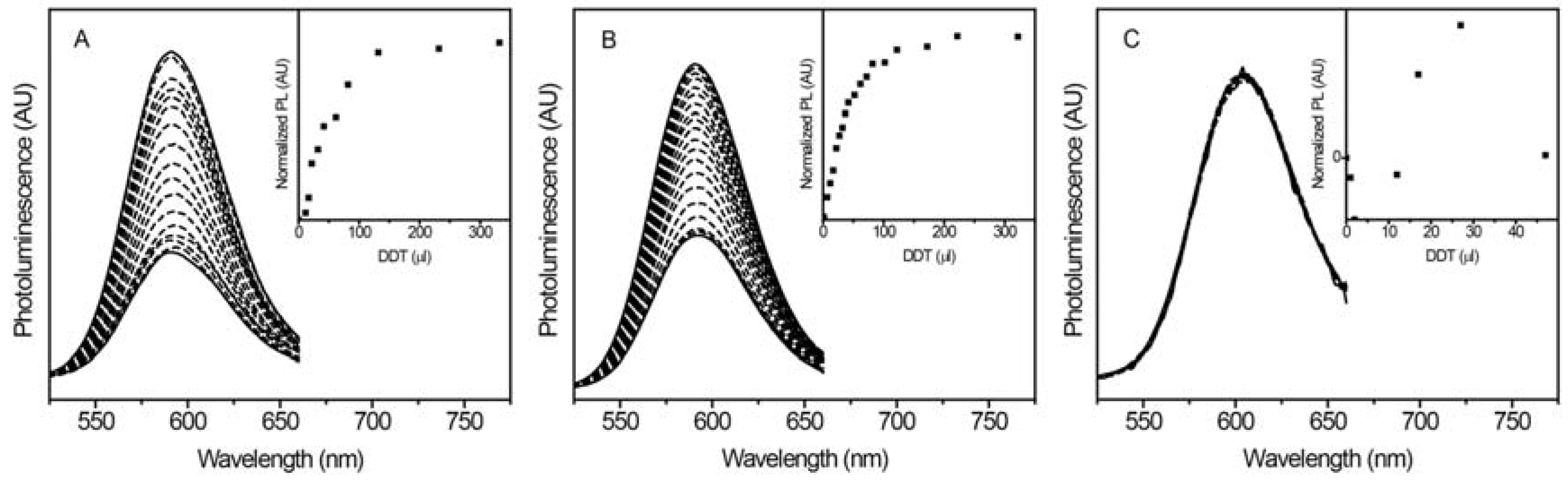
2.1. Shell-Thickness Dependent Thiol Detection in Organic Solvent
2.2. Thiol Sensing in Aqeuous Solution
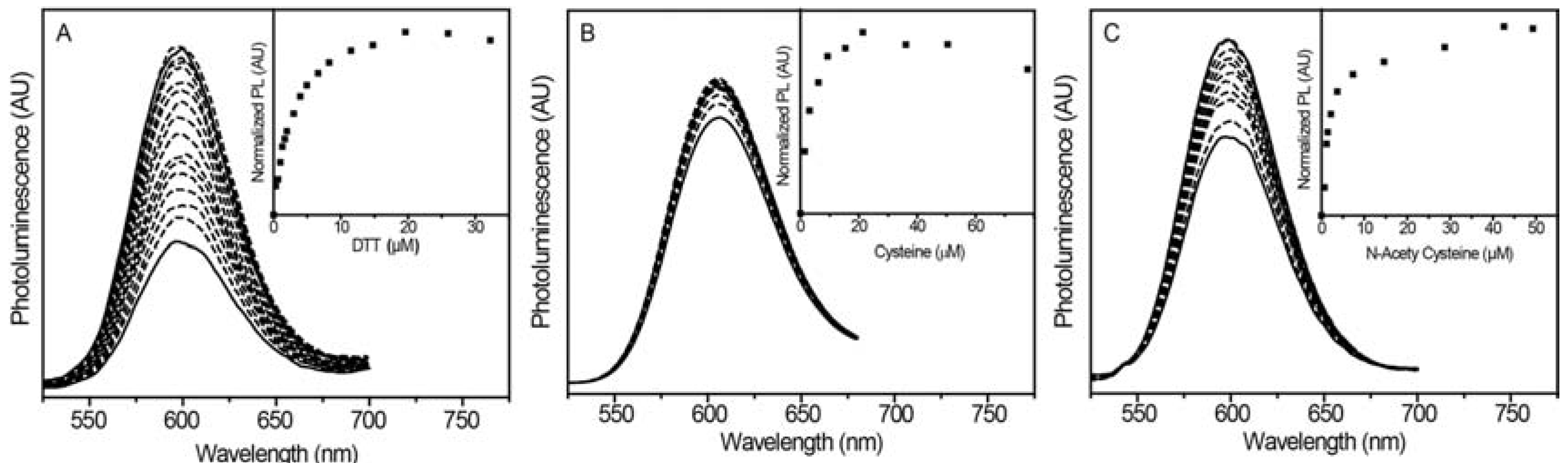
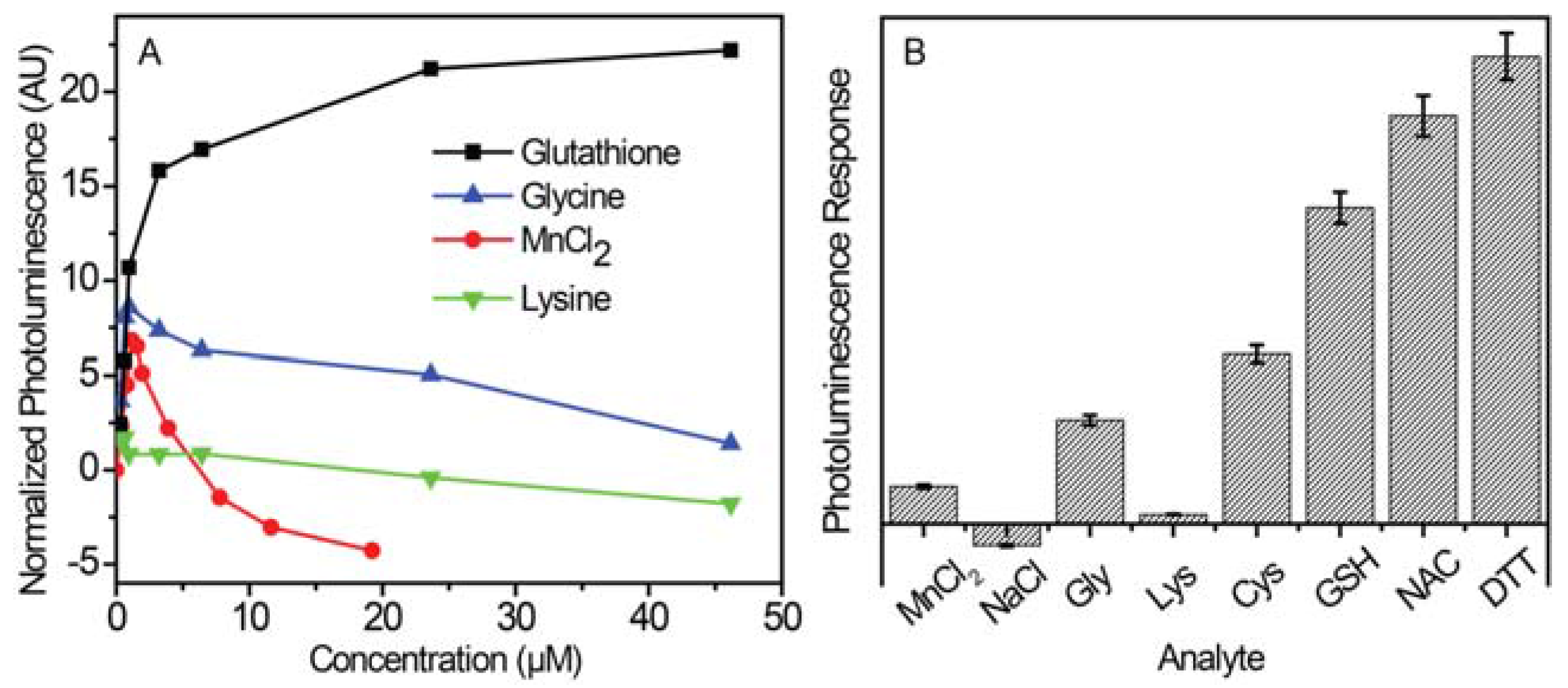
2.2.1. Luminescence Enhancement with Different Thiols
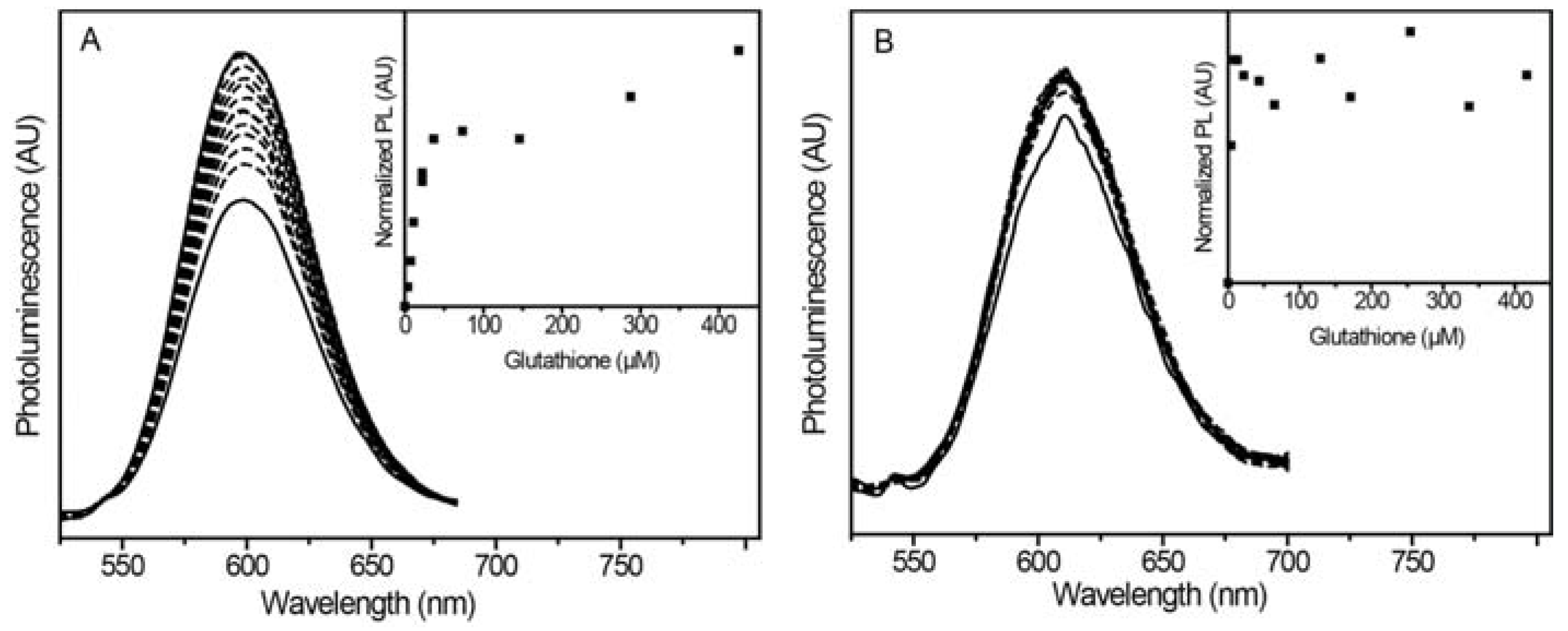
2.2.2. Glutathione Sensing with Different Shell Thicknesses
2.2.3. Thiol Selectivity
2.3. Prospects for Multimodal Imaging and Sensing
3. Conclusions
4. Materials and Methods
4.1. [NMe4]2[Zn4(SePh)10]
4.2. Mn2+:ZnSe and Mn2+:ZnCdSe Nanocrystals
4.3. ZnS Passivation Shell Growth
4.4. Water-Soluble Mn2+:ZnSe Nanoparticles
4.5. Agarose Gel Protocol
4.6. Physical Measurements
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Blasberg, R.; Piwnica-Worms, D. Imaging: Strategies, Controversies, and Opportunities. Clin. Cancer Res. 2012, 18, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Li, C. A targeted approach to cancer imaging and therapy. Nat. Mater. 2014, 13, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.M.; Modo, M.M.J. Introduction: The Emergence of Nanoparticles as Imaging Platform in Biomedicine. In Nanoparticles in Biomedical Imaging; Fundamental Biomedical Technologies; Bulte, J.W.M., Modo, M.M.J., Eds.; Springer: New York, NY, USA, 2008; pp. 1–5. ISBN 978-0-387-72026-5. [Google Scholar]
- Louie, A. Multimodality Imaging Probes: Design and Challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Tang, T.; Louie, A.Y. Nanoparticle-based multimodal PET/MRI probes. NanoMed 2015, 10, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Wui Wong, T. Nanotechnology-Enabled Drug Delivery for Cancer Therapy A2. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, UK, 2015; Chapter 11; pp. 173–193. ISBN 978-0-323-32889-0. [Google Scholar]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer US: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Cumberland, S.L.; Hanif, K.M.; Javier, A.; Khitrov, G.A.; Strouse, G.F.; Woessner, S.M.; Yun, C.S. Inorganic Clusters as Single-Source Precursors for Preparation of CdSe, ZnSe, and CdSe/ZnS Nanomaterials. Chem. Mater. 2002, 14, 1576–1584. [Google Scholar] [CrossRef]
- Wang, S.; Jarrett, B.R.; Kauzlarich, S.M.; Louie, A.Y. Core/Shell Quantum Dots with High Relaxivity and Photoluminescence for Multimodality Imaging. J. Am. Chem. Soc. 2007, 129, 3848–3856. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Ma, X.; Pantazis, P.; Kauzlarich, S.M.; Louie, A.Y. Paramagnetic, Silicon Quantum Dots for Magnetic Resonance and Two-Photon Imaging of Macrophages. J. Am. Chem. Soc. 2010, 132, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Vacchino, J.F.; Beeson, C.; McConnell, H.M. Potentiometric Measurement of Intracellular Redox Activity. J. Am. Chem. Soc. 1998, 120, 2464–2473. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Rama, M.J.; Walters, J.D.; Orte, A.; Hall, E.A.H. Fluorescent nanoparticles for intracellular sensing: A review. Anal. Chim. Acta 2012, 751, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.A.; de la Rica, R. Photoluminescent nanosensors for intracellular detection. Anal. Methods 2015, 7, 7067–7075. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.J.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Chew, O.; Whelan, J.; Millar, A.H. Molecular Definition of the Ascorbate-Glutathione Cycle in Arabidopsis Mitochondria Reveals Dual Targeting of Antioxidant Defenses in Plants. J. Biol. Chem. 2003, 278, 46869–46877. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.M.; Metzler, M.; Lehmann, L. Mutagenicity of the mycotoxin patulin in cultured Chinese hamster V79 cells, and its modulation by intracellular glutathione. Arch. Toxicol. 2005, 79, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized Redox State of Glutathione in the Endoplasmic Reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Xu, R.; Vandiver, M.S.; Cha, J.Y.; Snowman, A.M.; Snyder, S.H. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature 2014, 509, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Van ’t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Tachibana, C.; Rahr Winther, J.; Appenzeller-Herzog, C. Intracellular glutathione pools are heterogeneously concentrated. Redox Biol. 2013, 1, 508–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jüstel, T. Phosphors for Plasma Display Panels. In Luminescence; Ronda, C., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 61–73. ISBN 978-3-527-62106-4. [Google Scholar]
- Blasse, G.; Grabmaier, B.C. Lamp Phosphors. In Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994; pp. 108–133. ISBN 978-3-540-58019-5. [Google Scholar]
- Beaulac, R.; Ochsenbein, S.T.; Gamelin, D.R. Colloidal Transition-Metal-Doped Quantum Dots. In Nanocrystal Quantum Dots, Second Edition; Klimov, V.I., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 397–453. ISBN 978-1-4200-7926-5. [Google Scholar]
- Thakar, R.; Chen, Y.; Snee, P.T. Efficient Emission from Core/(Doped) Shell Nanoparticles: Applications for Chemical Sensing. Nano Lett. 2007, 7, 3429–3432. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Dutta, D. Quantum Dots for Cancer Imaging. In Nanoparticles in Biomedical Imaging; Bulte, J.W.M., Modo, M.M.J., Eds.; Fundamental Biomedical Technologies; Springer: New York, NY, USA, 2008; pp. 463–485. ISBN 978-0-387-72026-5. [Google Scholar]
- Wu, P.; Yan, X.-P. Doped quantum dots for chemo/biosensing and bioimaging. Chem. Soc. Rev. 2013, 42, 5489–5521. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Santra, S.; Holloway, P.H. Syntheses and Applications of Mn-Doped II-VI Semiconductor Nanocrystals. J. Nanosci. Nanotechnol. 2005, 5, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Beaulac, R.; Archer, P.I.; Gamelin, D.R. Luminescence in colloidal Mn2+-doped semiconductor nanocrystals. J. Solid State Chem. 2008, 181, 1582–1589. [Google Scholar] [CrossRef]
- Jahanbin, T.; Gaceur, M.; Gros-Dagnac, H.; Benderbous, S.; Merah, S.A. High potential of Mn-doped ZnS nanoparticles with different dopant concentrations as novel MRI contrast agents: synthesis and in vitro relaxivity studies. J. Nanopart. Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Yang, C.; Liu, M.; Sum, T.C.; Yong, K.-T. Synthesis and Characterization of Mn:ZnSe/ZnS/ZnMnS Sandwiched QDs for Multimodal Imaging and Theranostic Applications. Small 2016, 12, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Hofmann, A.; Ackermann, T.; Boeglin, C.; Viswanatha, R.; Peng, X.; Rodríguez, A.F.; Nolting, F.; Rühl, E. Magnetic and Structural Investigation of ZnSe Semiconductor Nanoparticles Doped With Isolated and Core-Concentrated Mn2+ Ions. Adv. Funct. Mater. 2009, 19, 2501–2510. [Google Scholar] [CrossRef]
- Banerjee, S.; Kar, S.; Perez, J.M.; Santra, S. Quantum Dot-Based OFF/ON Probe for Detection of Glutathione. J. Phys. Chem. C 2009, 113, 9659–9663. [Google Scholar] [CrossRef]
- Deng, R.; Xie, X.; Vendrell, M.; Chang, Y.-T.; Liu, X. Intracellular Glutathione Detection Using MnO2-Nanosheet-Modified Upconversion Nanoparticles. J. Am. Chem. Soc. 2011, 133, 20168–20171. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; An, X.; Su, H.; Shen, W.; Zhu, L.; Ma, X.; Chen, Z.; Wang, X. Rhodamine 6G conjugated-quantum dots used for highly sensitive and selective ratiometric fluorescence sensor of glutathione. Talanta 2012, 94, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, C.; Zhong, X.; Zhao, C.; Zhu, L. Highly selective detection of glutathione using a quantum-dot-based OFF-ON fluorescent probe. Chem. Commun. 2010, 46, 2971–2973. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, F.; Yan, X.; Wang, L.; Yan, J.; Ding, H.; Ding, L. Sensitive detection of biothiols and histidine based on the recovered fluorescence of the carbon quantum dots-Hg(II) system. Anal. Chim. Acta 2015, 859, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Silvi, S.; Credi, A. Luminescent sensors based on quantum dot-molecule conjugates. Chem. Soc. Rev. 2015, 44, 4275–4289. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhny, G.; Murray, R.W. Ligand Effects on Optical Properties of CdSe Nanocrystals. J. Phys. Chem. B 2005, 109, 7012–7021. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Battaglia, D.M.; Liu, Y.; Peng, X. Efficient, Stable, Small, and Water-Soluble Doped ZnSe Nanocrystal Emitters as Non-Cadmium Biomedical Labels. Nano Lett. 2007, 7, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Archer, P.I.; Santangelo, S.A.; Gamelin, D.R. Inorganic Cluster Syntheses of TM2+-Doped Quantum Dots (CdSe, CdS, CdSe/CdS): Physical Property Dependence on Dopant Locale. J. Am. Chem. Soc. 2007, 129, 9808–9818. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive Ion Layer Adsorption and Reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, E.J.; Vlaskin, V.A.; Gamelin, D.R. Water-Soluble Dual-Emitting Nanocrystals for Ratiometric Optical Thermometry. J. Am. Chem. Soc. 2011, 133, 14978–14980. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Xu, Y.; Lin, M.; Chan, Y. Aqueous-Phase Reactions on Hollow Silica-Encapsulated Semiconductor Nanoheterostructures. J. Am. Chem. Soc. 2012, 134, 8754–8757. [Google Scholar] [CrossRef] [PubMed]
- Mirzahosseini, A.; Noszál, B. Species-Specific Standard Redox Potential of Thiol-Disulfide Systems: A Key Parameter to Develop Agents against Oxidative Stress. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Patra, B.K.; Guria, A.K.; Pradhan, N. The Redox Chemistry at the Interface for Retrieving and Brightening the Emission of Doped Semiconductor Nanocrystals. J. Phys. Chem. Lett. 2013, 4, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Li, Y.; Wu, Y.-Y.; He, Y.; Yan, X.-P. Ascorbic Acid Induced Enhancement of Room Temperature Phosphorescence of Sodium Tripolyphosphate-Capped Mn-Doped ZnS Quantum Dots: Mechanism and Bioprobe Applications. Chem. Eur. J. 2010, 16, 12988–12994. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Peng, X. Efficient and Color-Tunable Mn-Doped ZnSe Nanocrystal Emitters: Control of Optical Performance via Greener Synthetic Chemistry. J. Am. Chem. Soc. 2007, 129, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- McPhail, M.R.; Weiss, E.A. Role of Organosulfur Compounds in the Growth and Final Surface Chemistry of PbS Quantum Dots. Chem. Mater. 2014, 26, 3377–3384. [Google Scholar] [CrossRef]
- Bullen, C.; Mulvaney, P. The Effects of Chemisorption on the Luminescence of CdSe Quantum Dots. Langmuir 2006, 22, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.L.; Gamelin, D.R. Photoluminescence Brightening via Electrochemical Trap Passivation in ZnSe and Mn2+-Doped ZnSe Quantum Dots. J. Am. Chem. Soc. 2012, 134, 6819–6825. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Weaver, A.L.; Gamelin, D.R. Redox Brightening of Colloidal Semiconductor Nanocrystals Using Molecular Reductants. J. Am. Chem. Soc. 2012, 134, 16175–16177. [Google Scholar] [CrossRef] [PubMed]
- Dance, I.G.; Choy, A.; Scudder, M.L. Syntheses, properties, and molecular and crystal structures of (Me4N)4[E4M10(SPh)16] (E = sulfur or selenium; M = zinc or cadmium): molecular supertetrahedral fragments of the cubic metal chalcogenide lattice. J. Am. Chem. Soc. 1984, 106, 6285–6295. [Google Scholar] [CrossRef]
- Vlaskin, V.A.; Beaulac, R.; Gamelin, D.R. Dopant-Carrier Magnetic Exchange Coupling in Colloidal Inverted Core/Shell Semiconductor Nanocrystals. Nano Lett. 2009, 9, 4376–4382. [Google Scholar] [CrossRef] [PubMed]
- Yazdanparast, M.S.; Webb, M.T.; McLaurin, E.J. Single-step synthesis of hyperbranched, luminescent Mn2+-doped ZnSe1-xSx nanocrystals using dichalcogenide precursors. J. Mater. Chem. C 2016, 4, 6907–6913. [Google Scholar] [CrossRef]
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent Dependence of the Fluorescence Lifetimes of Xanthene Dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence Quantum Yields and Their Relation to Lifetimes of Rhodamine 6G and Fluorescein in Nine Solvents: Improved Absolute Standards for Quantum Yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef]
| Run | NCs (s) | +DTT (s) | Water (s) |
|---|---|---|---|
| 1 | 2.54888 | 2.63426 | 2.60044 |
| 2 | 2.53549 | 2.71390 | 2.69495 |
| 3 | 2.59246 | 2.64803 | 2.59054 |
| 4 | 2.63351 | 2.64220 | 2.61833 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdanparast, M.S.; Jeffries, W.R.; Gray, E.R.; McLaurin, E.J. Mn2+-ZnSe/ZnS@SiO2 Nanoparticles for Turn-on Luminescence Thiol Detection. J. Funct. Biomater. 2017, 8, 36. https://doi.org/10.3390/jfb8030036
Yazdanparast MS, Jeffries WR, Gray ER, McLaurin EJ. Mn2+-ZnSe/ZnS@SiO2 Nanoparticles for Turn-on Luminescence Thiol Detection. Journal of Functional Biomaterials. 2017; 8(3):36. https://doi.org/10.3390/jfb8030036
Chicago/Turabian StyleYazdanparast, Mohammad S., William R. Jeffries, Eric R. Gray, and Emily J. McLaurin. 2017. "Mn2+-ZnSe/ZnS@SiO2 Nanoparticles for Turn-on Luminescence Thiol Detection" Journal of Functional Biomaterials 8, no. 3: 36. https://doi.org/10.3390/jfb8030036





