Fe Core–Carbon Shell Nanoparticles as Advanced MRI Contrast Enhancer
Abstract
:1. Introduction
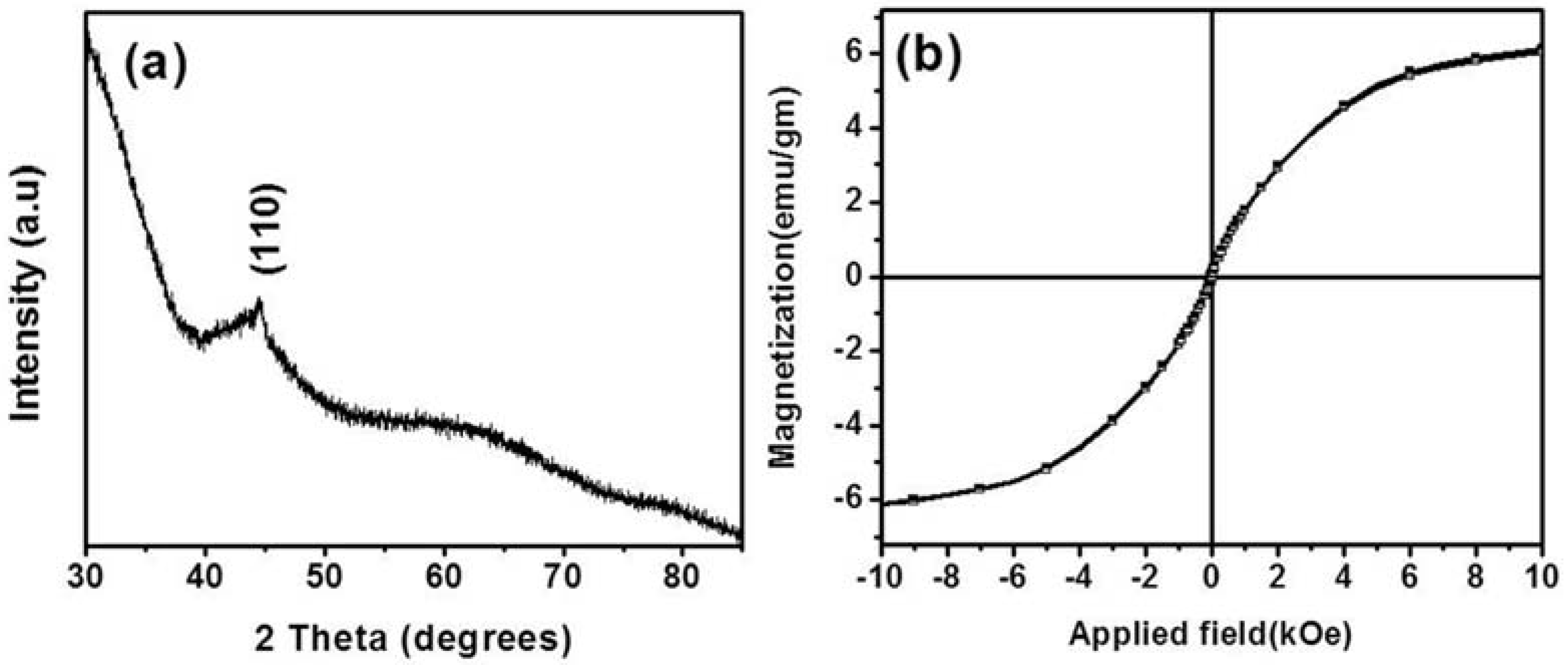
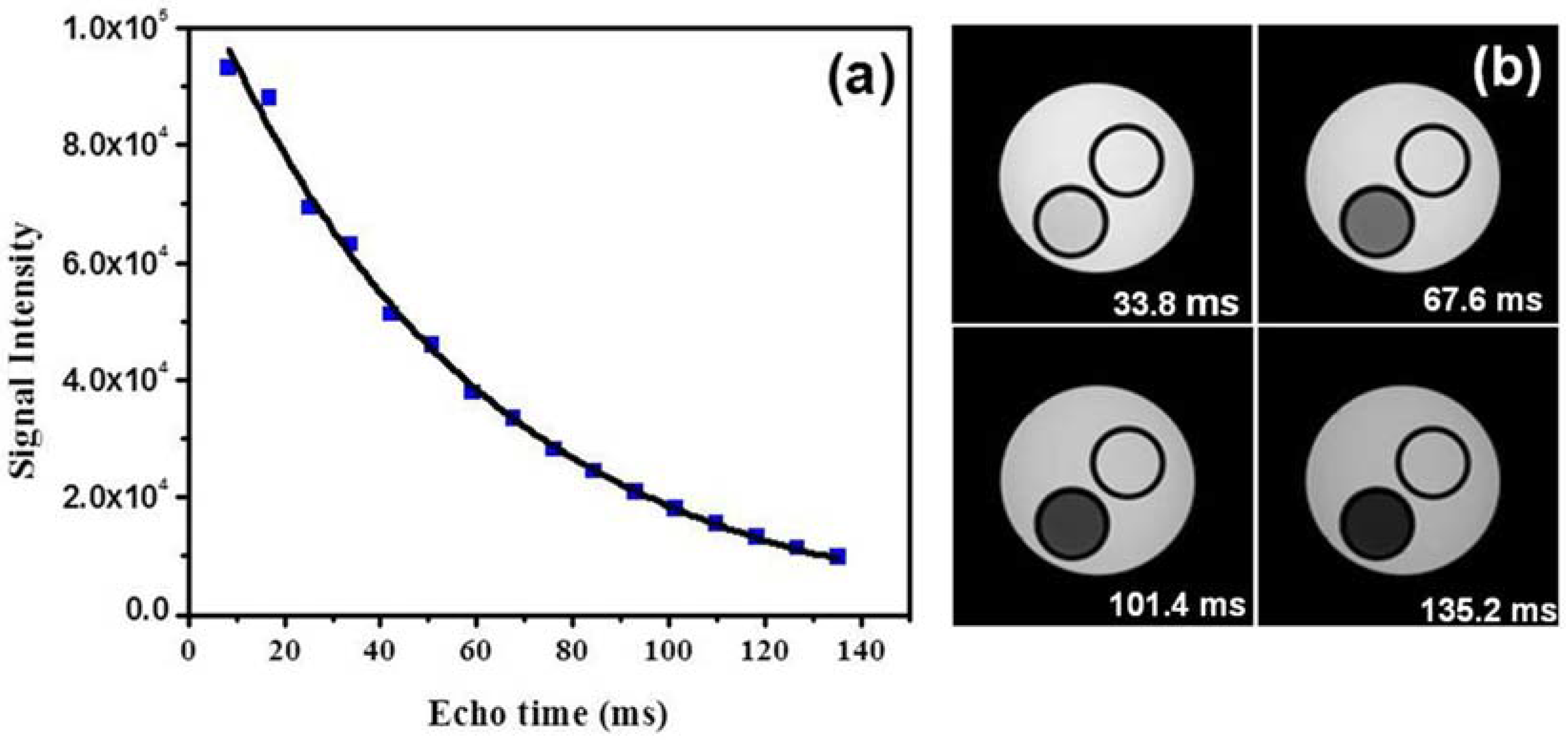
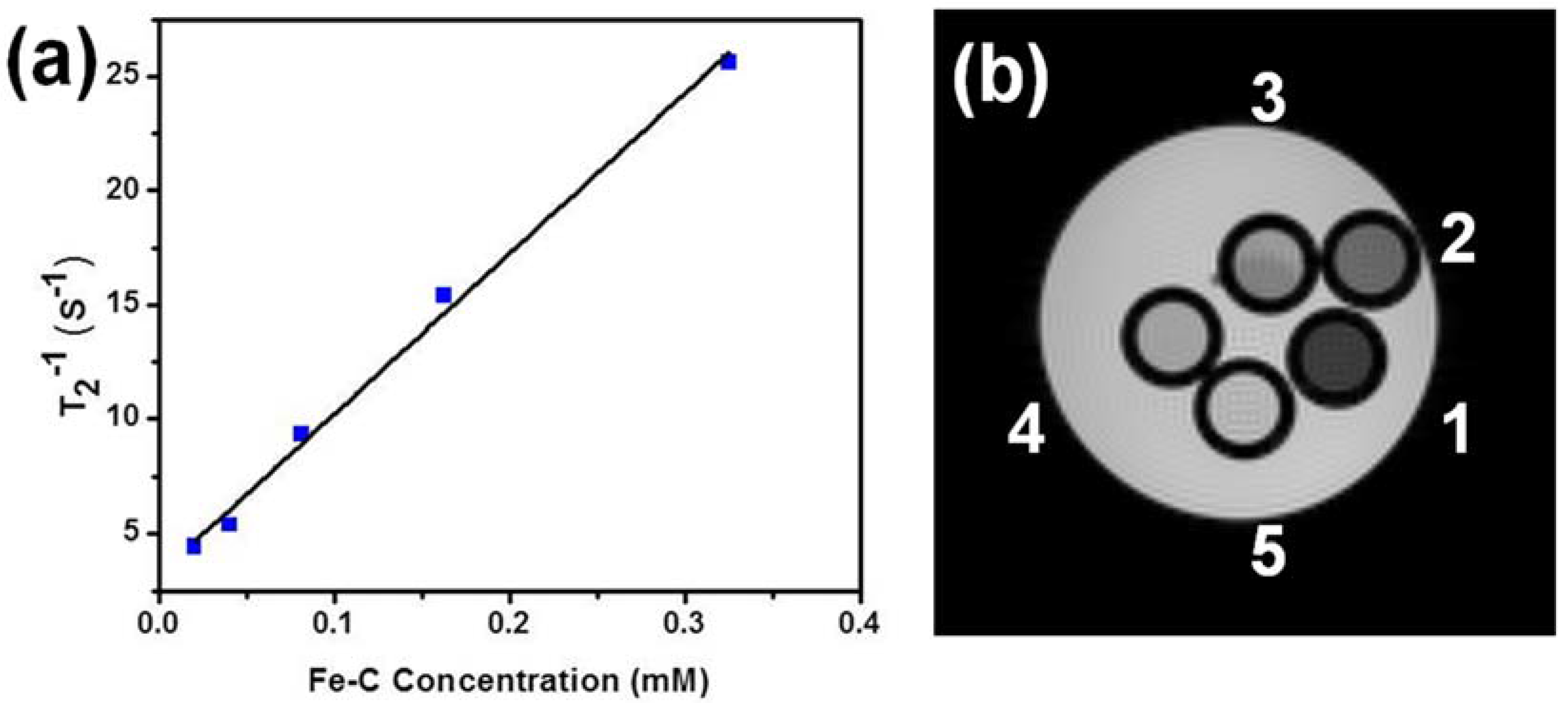
2. Results and Discussion
3. Materials and Methods
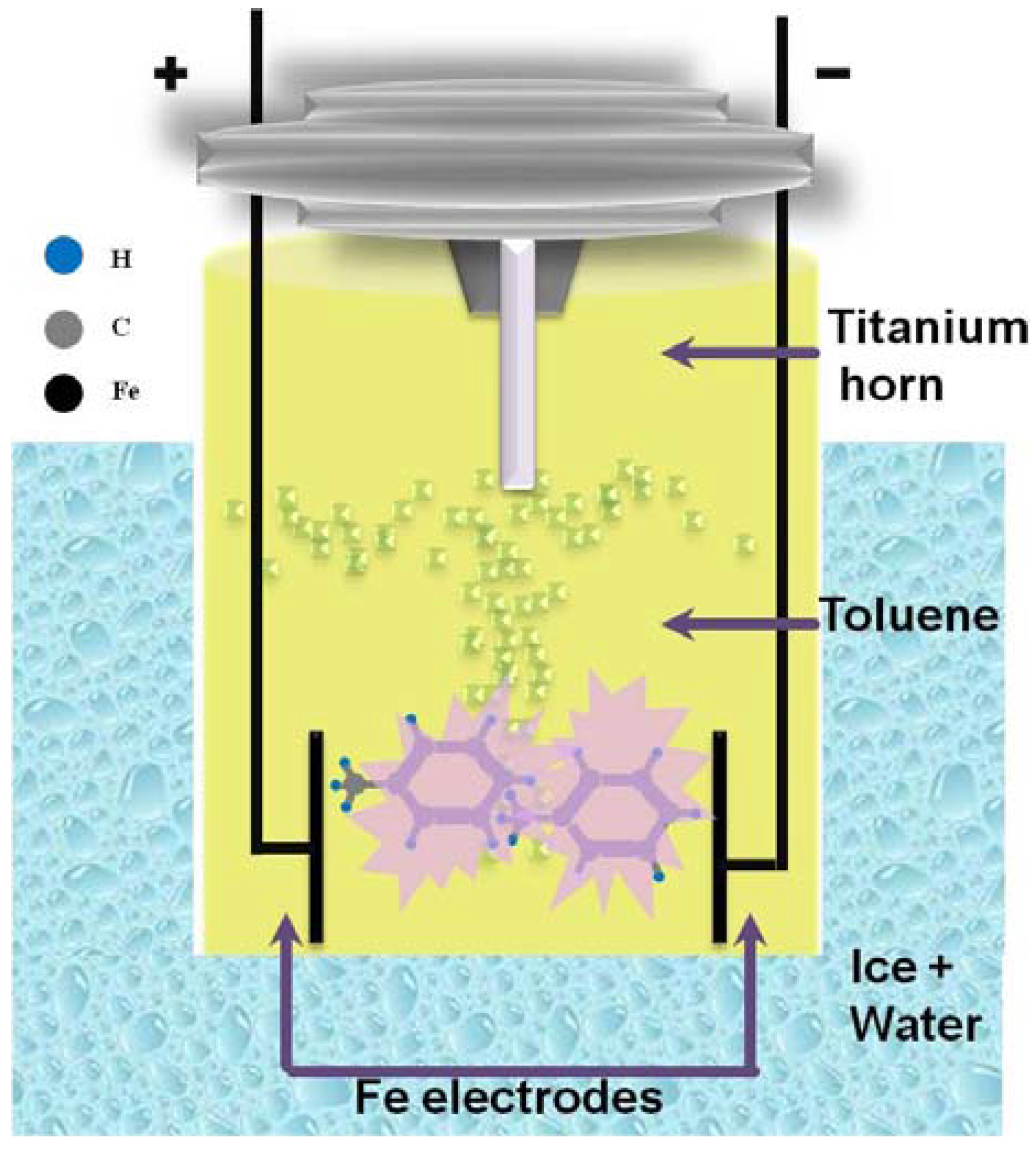
3.1. Synthesis of Core–Shell Nanoparticles
3.2. Characterization of Core–Shell Nanoparticles
3.3. MRI Relaxometry
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Del. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, Functionalization, and Biomedical Applications of Multifunctional Magnetic Nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Del. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.-M.; Jun, Y.-W.; Song, H.-T.; Kim, S.; Choi, J.-S.; Lee, J.-H.; Yoon, S.; Kim, K.-S.; Suh, J.-S.; Cheon, J. In Vivo Magnetic Resonance Detection of Cancer by Using Multifunctional Magnetic Nanocrystals. J. Am. Chem. Soc. 2005, 127, 12387–12391. [Google Scholar] [CrossRef] [PubMed]
- Bomatí-Miguel, O.; Morales, M.P.; Tartaj, P.; Ruiz-Cabello, J.; Bonville, P.; Santos, M.; Zhao, X.; Veintemillas-Verdaguer, S. Fe-based nanoparticulate metallic alloys as contrast agents for magnetic resonance imaging. Biomaterials 2005, 26, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Várallyay, P.; Bagó, A.G.; Muldoon, L.L.; Nesbit, G.; Nixon, R. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumours. Neuropathol. Appl. Neurobiol. 2004, 30, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.M.; Skrtic, S.; Sharer, K.; Hill, J.M.; Dunbar, C.E.; Koretsky, A.P. MRI detection of single particles for cellular imaging. Proc. Natl. Acad. Sci. USA 2004, 101, 10901–10906. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, J.R.; Cahill, K.S.; Tharin, S.A.; Shapiro, E.M. Cellular magnetic resonance imaging: Nanometer and micrometer size particles for noninvasive cell localization. Neurotherapeutics 2007, 4, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.J.; Yoon, H.G.; Suh, J.S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R.; Kelly, K.A.; Sun, E.Y.; Weissleder, R. Targeted delivery of multifunctional magnetic nanoparticles. Nanomedicine 2007, 2, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.; Carlesso, N.; Tung, C.-H.; Tang, X.-W.; Cory, D.; Scadden, D.T.; Weissleder, R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotech. 2000, 18, 410–414. [Google Scholar]
- Moore, A.; Marecos, E.; Alexei Bogdanov, J.; Weissleder, R. Tumoral Distribution of Long-circulating Dextran-coated Iron Oxide Nanoparticles in a Rodent Model. Radiology 2000, 214, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Gandha, K.; Elkins, K.; Poudyal, N.; Liu, X.; Liu, J.P. High Energy Product Developed from Cobalt Nanowires. Sci. Rep. 2014, 4, 5345. [Google Scholar] [CrossRef] [PubMed]
- Gandha, K.; Mohapatra, J.; Hossain, M.K.; Elkins, K.; Poudyal, N.; Rajeshwar, K.; Liu, J.P. Mesoporous iron oxide nanowires: Synthesis, magnetic and photocatalytic properties. RSC Adv. 2016, 6, 90537–90546. [Google Scholar] [CrossRef]
- Ohno, K.; Mori, C.; Akashi, T.; Yoshida, S.; Tago, Y.; Tsujii, Y.; Tabata, Y. Fabrication of Contrast Agents for Magnetic Resonance Imaging from Polymer-Brush-Afforded Iron Oxide Magnetic Nanoparticles Prepared by Surface-Initiated Living Radical Polymerization. Biomacromolecules 2013, 14, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhaohui, W.; Taekyung, Y.; Changzhong, J.; Woo-Sik, K. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Tech. Adv. Mater. 2015, 16, 023501. [Google Scholar]
- Karagoz, B.; Yeow, J.; Esser, L.; Prakash, S.M.; Kuchel, R.P.; Davis, T.P.; Boyer, C. An Efficient and Highly Versatile Synthetic Route to Prepare Iron Oxide Nanoparticles/Nanocomposites with Tunable Morphologies. Langmuir 2014, 30, 10493–10502. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Ahmad, T.; Rhee, I.; Chang, Y.; Jin, S.-U.; Hong, S. Carbon-coated iron oxide nanoparticles as contrast agents in magnetic resonance imaging. Nanoscale Res. Lett. 2012, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Bonder, M.J.; Balakrishnan, S.; Wang, X.; Mao, H.; Hadjipanayis, G.C. Metallic Iron Nanoparticles for MRI Contrast Enhancement and Local Hyperthermia. Small 2008, 4, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Akbar, A.; Naseem, S. Controlled Nanostructuring of Multiphase Core-Shell Iron Oxide Nanoparticles. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Huo, J.; Song, H.; Chen, X. Preparation of carbon-encapsulated iron nanoparticles by co-carbonization of aromatic heavy oil and ferrocene. Carbon 2004, 42, 3177–3182. [Google Scholar] [CrossRef]
- Gu, L.; Koymen, A.R.; Mohanty, S.K. Crystalline magnetic carbon nanoparticle assisted photothermal delivery into cells using CW near-infrared laser beam. Sci. Rep. 2014, 4, 5106. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, S.; Yoo, D.; Shin, T.; Kim, H.; Gomes, M.D.; Kim, S.H.; Pines, A.; heon, J.C. Distance-dependent magnetic resonance tuning as a versatile MRI sensing platform for biological targets. Nat. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K. Handbook of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Chaudhary, R.P.; Koymen, A.R. Synthesis of magnetic GdC2 nanoparticles using cavitation plasma. Mater. Lett. 2015, 158, 194–197. [Google Scholar] [CrossRef]
- Chaudhary, R.P.; Mohanty, S.K.; Koymen, A.R. Novel method for synthesis of Fe core and C shell magnetic nanoparticles. Carbon 2014, 79, 67–73. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, R.P.; Kangasniemi, K.; Takahashi, M.; Mohanty, S.K.; Koymen, A.R. Fe Core–Carbon Shell Nanoparticles as Advanced MRI Contrast Enhancer. J. Funct. Biomater. 2017, 8, 46. https://doi.org/10.3390/jfb8040046
Chaudhary RP, Kangasniemi K, Takahashi M, Mohanty SK, Koymen AR. Fe Core–Carbon Shell Nanoparticles as Advanced MRI Contrast Enhancer. Journal of Functional Biomaterials. 2017; 8(4):46. https://doi.org/10.3390/jfb8040046
Chicago/Turabian StyleChaudhary, Rakesh P., Kim Kangasniemi, Masaya Takahashi, Samarendra K. Mohanty, and Ali R. Koymen. 2017. "Fe Core–Carbon Shell Nanoparticles as Advanced MRI Contrast Enhancer" Journal of Functional Biomaterials 8, no. 4: 46. https://doi.org/10.3390/jfb8040046




