Dicationic Imidazolium-Based Ionic Liquid Coatings on Zirconia Surfaces: Physico-Chemical and Biological Characterization
Abstract
:1. Introduction
2. Results and Discussion
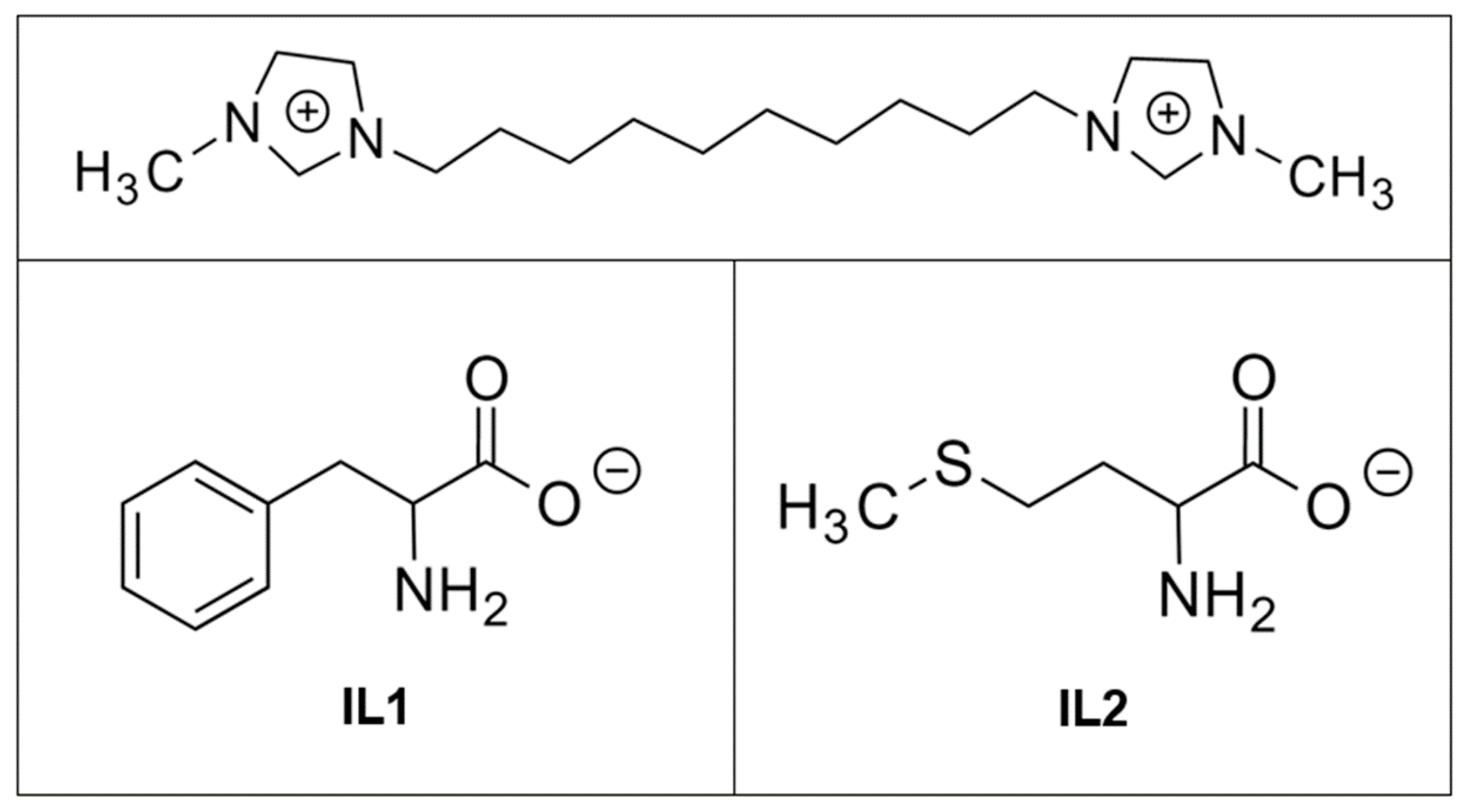
2.1. Coating Characterization
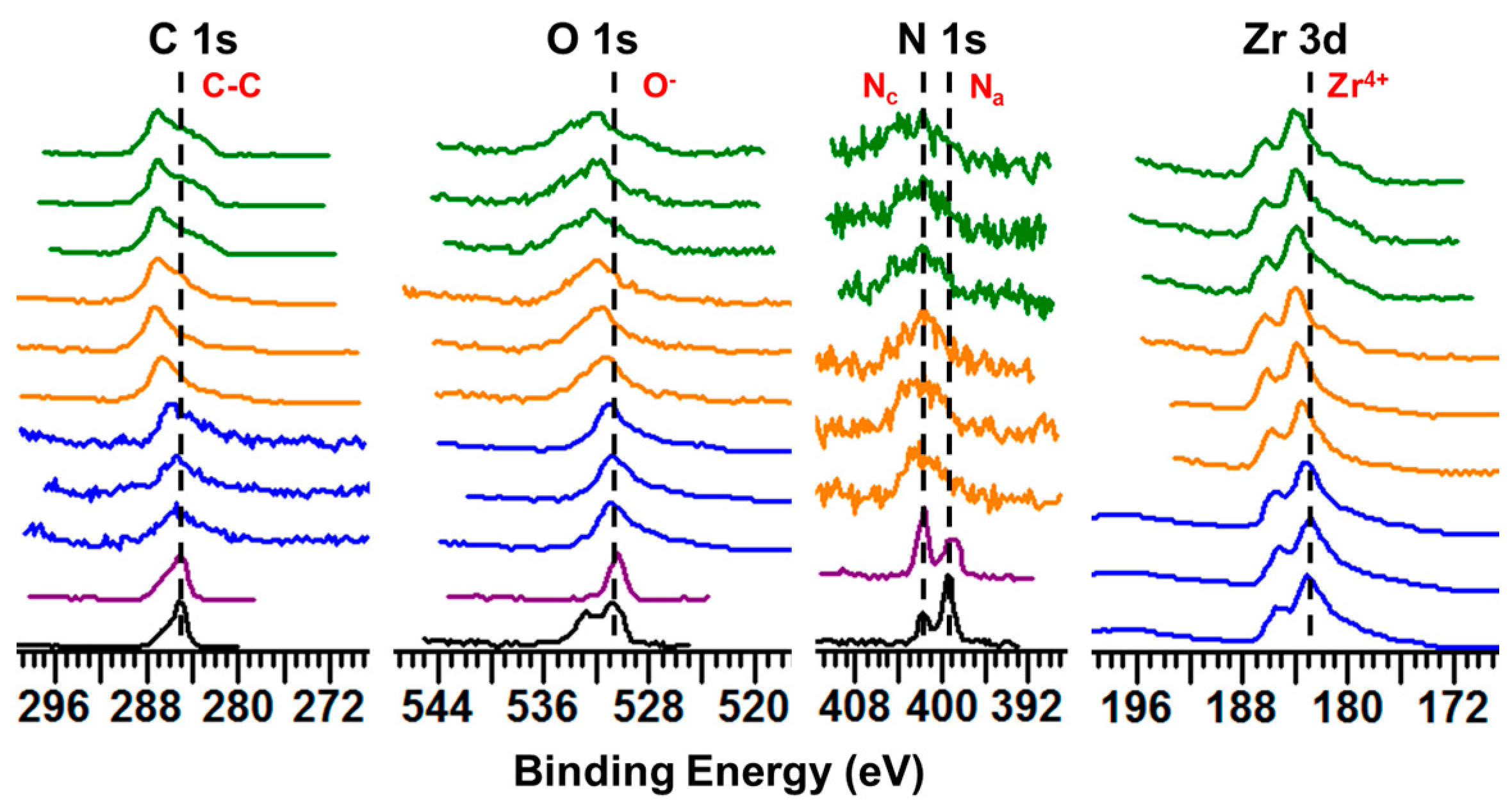
2.1.1. XPS Studies
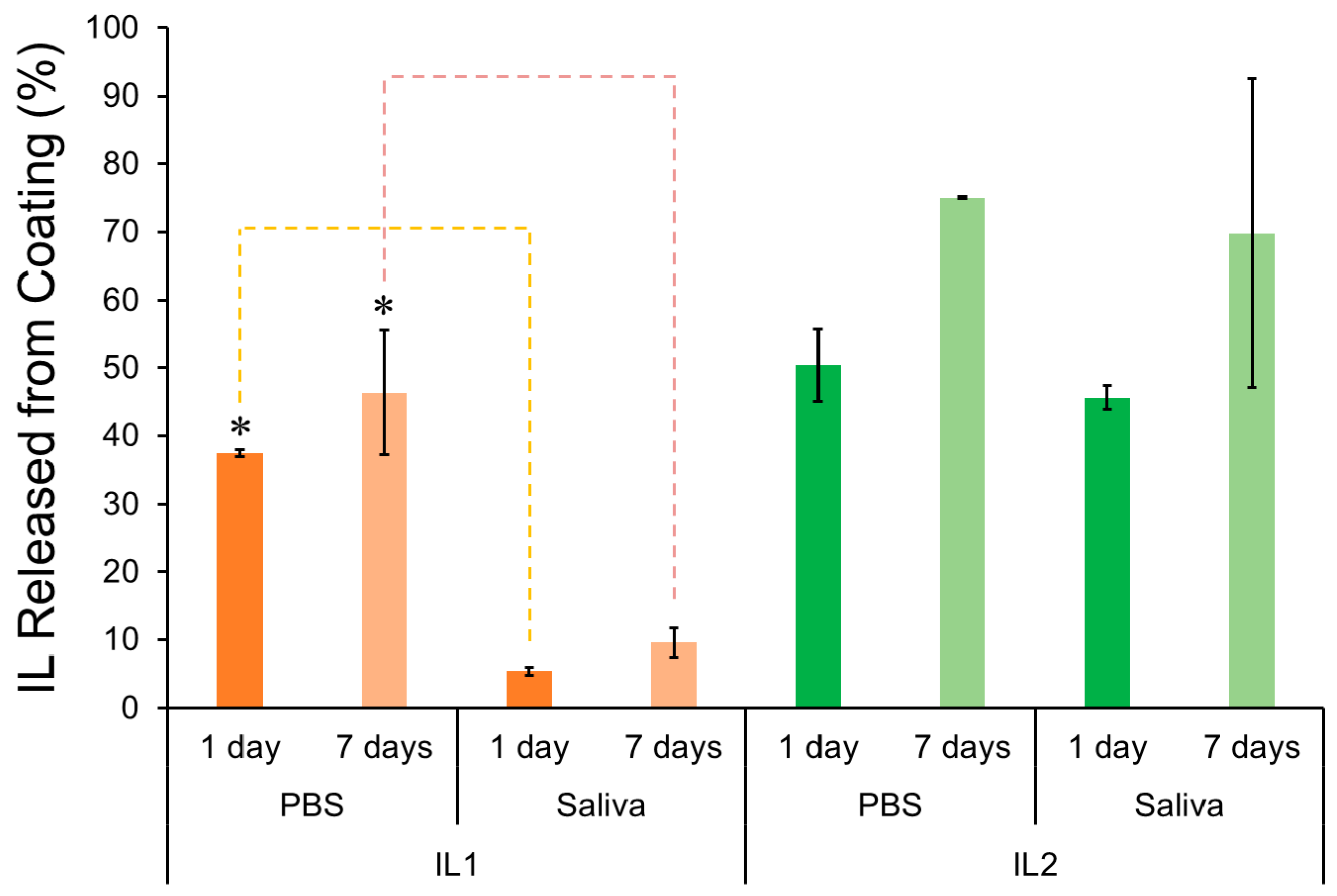
2.1.2. Release Profile
2.2. Mammalian and Bacterial Cell Activity
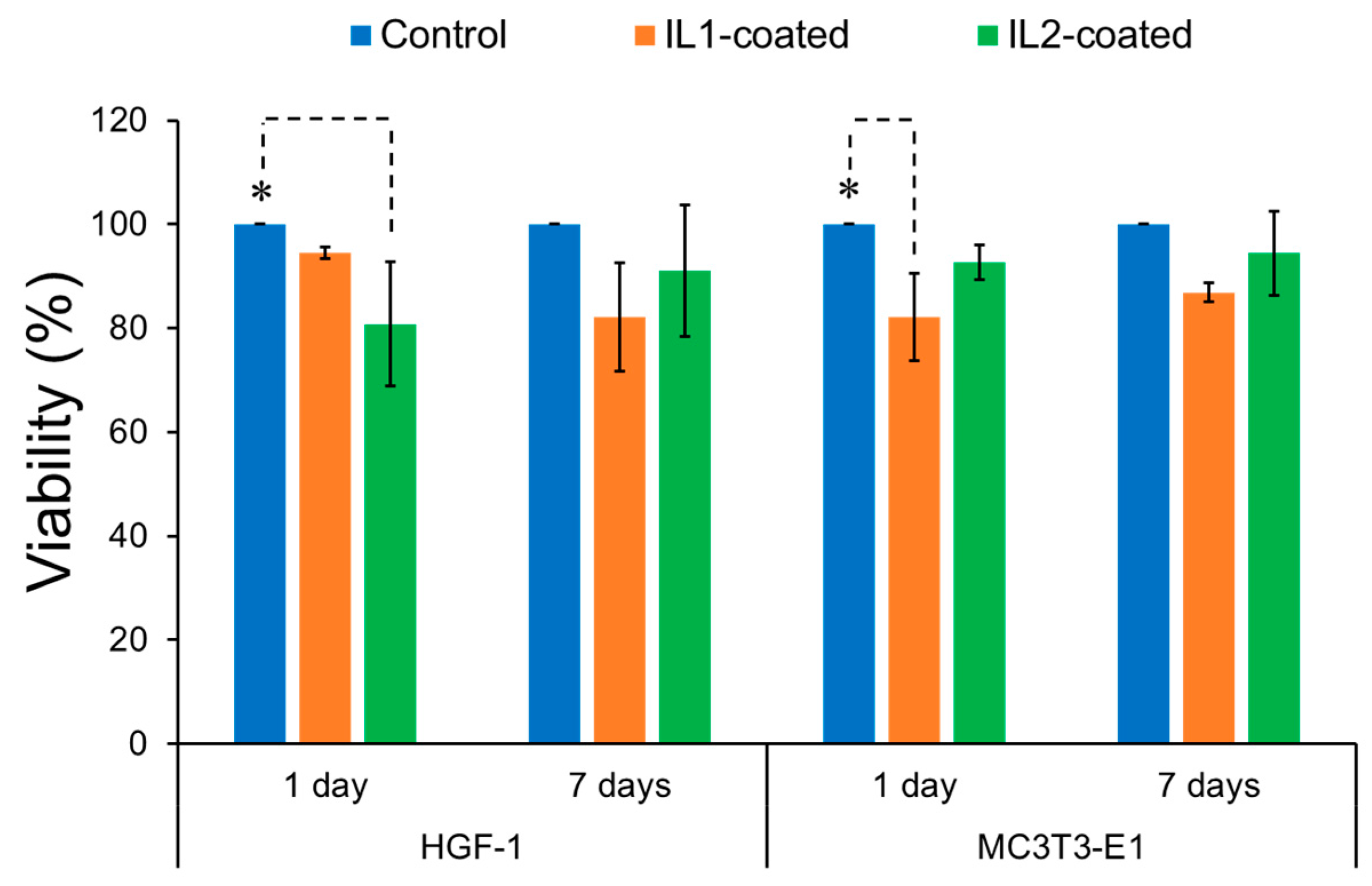
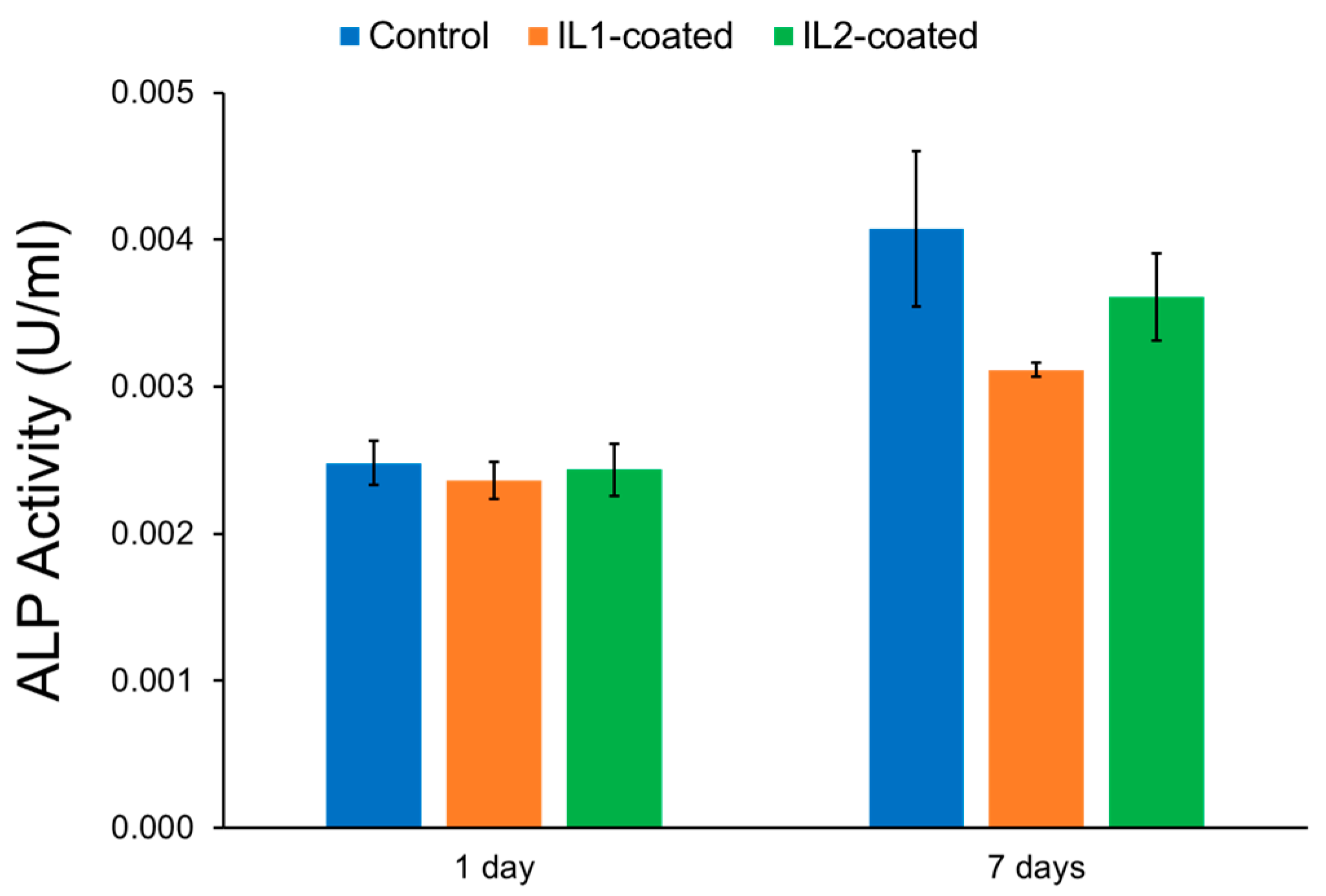
2.2.1. Mammalian Cell Activity
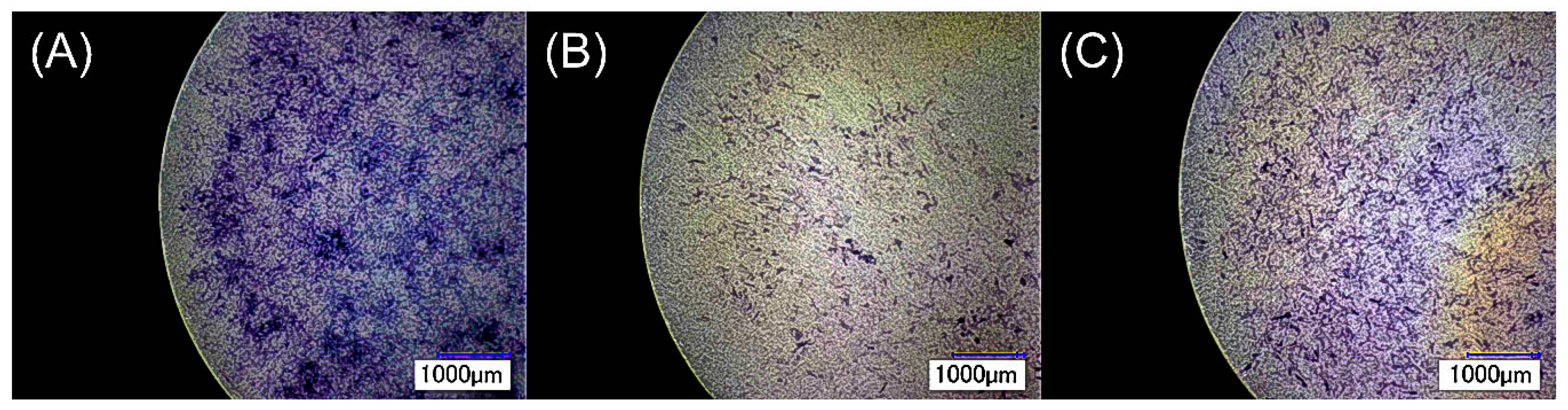
2.2.2. Bacterial Cell Activity
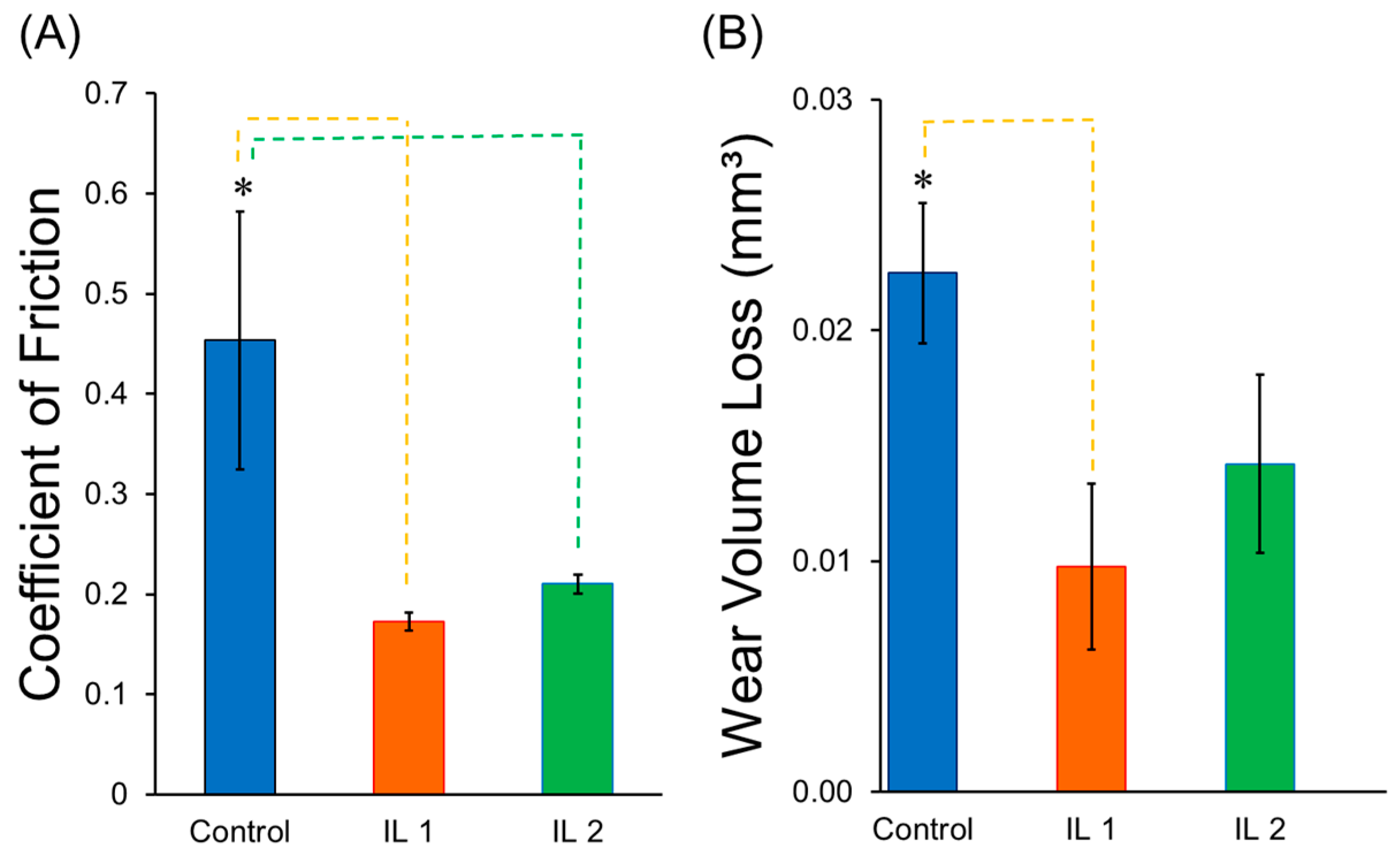
2.3. Tribological Behavior
3. Materials and Methods
3.1. Sample Preparation
3.2. Morphological and Physico-Chemical Characterization of IL-Coated Zirconia
3.2.1. X-ray Photoelectron Spectroscopy
3.2.2. Release Profile
3.3. Anti-Biofilm Activity of IL-Coated Zirconia
3.4. Evaluation of Mammalian Cell Behavior on IL-Coated Zirconia
3.4.1. Cell Culture
3.4.2. Cell Viability
3.4.3. ALP Activity
3.5. Investigation of the Tribological Behavior of IL-Coated Zirconia
- R = Radius of the wear track formed on zirconia specimen.
- r = Diameter of the spherical pin.
- d = Width of the wear track on zirconia specimen or the diameter of the wear scar on the ball.
- h = Wear depth of the flat wear scar on the ball.
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chehroudi, B.; Gould, T.R.L.; Brunette, D.M. The Role of Connective Tissue in Inhibiting Epithelial Downgrowth on Titanium-Coated Percutaneous Implants. J. Biomed. Mater. Res. 1992, 26, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; Poubel, L.A.C.; Ferreira, V.F.; Barboza, E.S.P. Evaluation of Survival and Success Rates of Dental Implants Reported in Longitudinal Studies with a Follow-Up Period of at Least 10 Years: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Heydecke, G.; Kohal, R.; Gläser, R. Optimal Esthetics in Single-Tooth Replacement with the Re-Implant System: A Case Report. Int. J. Prosthodont. 1999, 12, 184–189. [Google Scholar] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium Allergy in Dental Implant Patients: A Clinical Study on 1500 Consecutive Patients. Clin. Oral Implants Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium Allergy: A Literature Review. Indian J. Dermatol. 2014, 59, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Lalor, P.A.; Revell, P.A.; Gray, A.B.; Wright, S.; Railton, G.T.; Freeman, M.A. Sensitivity to Titanium. A Cause of Implant Failure? J. Bone Jt. Surg. Br. 1991, 73, 25–28. [Google Scholar]
- Piconi, C.; Maccauro, G. Zirconia as a Ceramic Biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Andreiotelli, M.; Kohal, R.-J. Fracture Strength of Zirconia Implants after Artificial Aging. Clin. Implant Dent. Relat. Res. 2009, 11, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Wolkewitz, M.; Tsakona, A. The Effects of Cyclic Loading and Preparation on the Fracture Strength of Zirconium-Dioxide Implants: An In Vitro Investigation. Clin. Oral Implants Res. 2011, 22, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Spies, B.C.; Nold, J.; Vach, K.; Kohal, R.-J. Two-Piece Zirconia Oral Implants Withstand Masticatory Loads: An Investigation in the Artificial Mouth. J. Mech. Behav. Biomed. Mater. 2016, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mihatovic, I.; Golubovic, V.; Becker, J.; Schwarz, F. Bone Tissue Response to Experimental Zirconia Implants. Clin. Oral Investig. 2017, 21, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Van Der Mei, H.C.; Subbiahdoss, G.; De Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft Tissue Integration Versus Early Biofilm Formation on Different Dental Implant Materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; van der Mei, H.C.; Rustema-Abbing, M.; Busscher, H.J.; Ren, Y. Osteoblast Integration of Dental Implant Materials after Challenge by Sub-Gingival Pathogens: A Co-Culture Study In Vitro. Int. J. Oral Sci. 2015, 7, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.-P.; Kiattavorncharoen, S.; Lauer, H.-C.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of Zirconia Implants Compared with Titanium: An in Vivo Study. Head Face Med. 2008, 4, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A Systematic Review of the Clinical Survival of Zirconia Implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia Dental Implants: Where Are We Now, and Where Are We Heading? Periodontology 2000 2017, 73, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.L.; Brook, I.M.; Palmquist, A.; van Noort, R.; Moharamzadeh, K. The Biological Seal of the Implant-Soft Tissue Interface Evaluated in a Tissue-Engineered Oral Mucosal Model. J. R. Soc. Interface 2012, 9, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Turkyilmaz, I.; Soganci, G. Rationale for Dental Implants. In Current Concepts in Dental Implantology; Turkyilmaz, I., Ed.; InTech: Rijeka, Croatia, 2015; pp. 1–25. [Google Scholar]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current Trends in Dental Implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Factors Influencing Early Dental Implant Failures. J. Dent. Res. 2016, 95, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors Associated with Early and Late Failure of Dental Implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; De Soete, M.; van Steenberghe, D. Infectious Risks for Oral Implants: A Review of the Literature. Clin. Oral Implants Res. 2002, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pye, A.D.; Lockhart, D.E.A.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A Review of Dental Implants and Infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Mouhyi, J.; Dohan Ehrenfest, D.M.; Albrektsson, T. The Peri-Implantitis: Implant Surfaces, Microstructure, and Physicochemical Aspects. Clin. Implant Dent. Relat. Res. 2012, 14, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial Adhesion on Commercially Pure Titanium and Zirconium Oxide Disks: An In Vivo Human Study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial Colonization of Zirconia Ceramic Surfaces: An In Vitro and In Vivo Study. Int. J. Oral Maxillofac. Implants 2002, 17, 793–798. [Google Scholar] [PubMed]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In Vitro Biofilm Formation On Titanium And Zirconia Implant Surfaces. J. Periodontol. 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Jung, G.-Y.; Kim, D.-J.; Han, J.-S. Initial Bacterial Adhesion on Resin, Titanium and Zirconia in Vitro. J. Adv. Prosthodont. 2011, 3, 81–84. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.R.; Pozzer, L.; Cavalieri-Pereira, L.; de Moraes, P.H.; Olate, S.; de Albergaría Barbosa, J.R. Bacterial Adhesion and Colonization Differences between Zirconia and Titanium implant Abutments: An In Vivo Human Study. J. Periodontal Implant Sci. 2012, 42, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Gindri, I.M.; Siddiqui, D.A.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Ionic Liquid Coatings for Titanium Surfaces: Effect of IL Structure on Coating Profile. ACS Appl. Mater. Interfaces 2015, 7, 27421–27431. [Google Scholar] [CrossRef] [PubMed]
- Gindri, I.M.; Siddiqui, D.A.; Bhardwaj, P.; Rodriguez, L.C.; Palmer, K.L.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Dicationic Imidazolium-Based Ionic Liquids: A New Strategy for Non-Toxic and Antimicrobial Materials. RSC Adv. 2014, 4, 62594–62602. [Google Scholar] [CrossRef]
- Gindri, I.M.; Palmer, K.L.; Siddiqui, D.A.; Aghyarian, S.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Evaluation of Mammalian and Bacterial Cell Activity on Titanium Surface Coated with Dicationic Imidazolium-Based Ionic Liquids. RSC Adv. 2016, 6, 36475–36483. [Google Scholar] [CrossRef]
- Siddiqui, D.A.; Gindri, I.M.; Rodrigues, D.C. Corrosion and Wear Performance of Titanium and Cobalt Chromium Molybdenum Alloys Coated with Dicationic Imidazolium-Based Ionic Liquids. J. Bio-Tribo-Corros. 2016, 2, 1–14. [Google Scholar] [CrossRef]
- Gindri, I.M.; Siddiqui, D.A.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Improvement of Tribological and Anti-Corrosive Performance of Titanium Surfaces Coated with Dicationic Imidazolium-Based Ionic Liquids. RSC Adv. 2016, 6, 78795–78802. [Google Scholar] [CrossRef]
- Cremer, T.; Stark, M.; Deyko, A.; Steinrück, H.-P.; Maier, F. Liquid/Solid Interface of Ultrathin Ionic Liquid Films: [C1C1Im][Tf2N] and [C8C1Im][Tf2N] on Au(111). Langmuir 2011, 27, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Wibmer, L.; Calderón, S.K.; Deyko, A.; Maier, F.; Steinrück, H.-P. Interfaces of Ionic Liquids and Transition Metal Surfaces—Adsorption, Growth, and Thermal Reactions of Ultrathin [C1C1Im][Tf2N] Films on Metallic and Oxidised Ni(111) Surfaces. Phys. Chem. Chem. Phys. 2012, 14, 5153–5163. [Google Scholar] [CrossRef] [PubMed]
- Villar-Garcia, I.J.; Smith, E.F.; Taylor, A.W.; Qiu, F.; Lovelock, K.R.J.; Jones, R.G.; Licence, P. Charging of Ionic Liquid Surfaces under X-Ray Irradiation: The Measurement of Absolute Binding Energies by XPS. Phys. Chem. Chem. Phys. 2011, 13, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Hurisso, B.B.; Lovelock, K.R.J.; Licence, P. Amino Acid-Based Ionic Liquids: Using XPS to Probe the Electronic Environment via Binding Energies. Phys. Chem. Chem. Phys. 2011, 13, 17737–17748. [Google Scholar] [CrossRef] [PubMed]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS Studies of Metal Oxides, Hydroxides and Peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J.; Chian, K.S.; Low, D.K.Y.; Lim, G.C.; Zheng, H.Y. The Formation of a Hydroxyl Bond and the Effects Thereof on Bone-like Apatite Formation on a Magnesia Partially Stabilized Zirconia (MgO-PSZ) Bioceramic Following CO2 Laser Irradiation. J. Mater. Sci. Mater. Med. 2004, 15, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Kerber, S.J.; Bruckner, J.J.; Wozniak, K.; Seal, S.; Hardcastle, S.; Barr, T.L. The Nature of Hydrogen in X-Ray Photoelectron Spectroscopy: General Patterns from Hydroxides to Hydrogen Bonding. J. Vac. Sci. Technol. A 1996, 14, 1314–1320. [Google Scholar] [CrossRef]
- Schmidt, M. X-ray Photoelectron Spectroscopy Studies on Adsorption of Amino Acids from Aqueous Solutions onto Oxidised Titanium Surfaces. Arch. Orthop. Trauma Surg. 2001, 121, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Bolis, V.; Cerrato, G.; Magnacca, G.; Morterra, C. Surface Acidity of Metal Oxides. Combined Microcalorimetric and IR-Spectroscopic Studies of Variously Dehydrated Systems. Thermochim. Acta 1998, 312, 63–77. [Google Scholar] [CrossRef]
- Budyak, I.L.; Zhuravleva, A.; Gierasch, L.M. The Role of Aromatic—Aromatic Interactions in Strand–Strand Stabilization of β-Sheets. J. Mol. Biol. 2013, 425, 3522–3535. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M.; Miura, T.; Kato, T.; Saito, A.; Yoshinari, M. In Vitro Adherence of Periodontopathic Bacteria to Zirconia and Titanium Surfaces. Dent. Mater. J. 2013, 32, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Winter, W.; Klein, D.; Karl, M. Micromotion of Dental Implants: Basic Mechanical Considerations. J. Med. Eng. 2013, 2013, 265412. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.T.; Abbey, S.; Hallab, N.J.; Hall, D.J.; Sukotjo, C.; Wimmer, M.A. Influence of pH on the Tribocorrosion Behavior of CpTi in the Oral Environment: Synergistic Interactions of Wear and Corrosion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liang, Y.; Liu, W. Ionic Liquid Lubricants: Designed Chemistry for Engineering Applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, P.P.K.; Gindri, I.M.; Siddiqui, D.A.; Rodrigues, D.C. Dicationic Imidazolium-Based Ionic Liquid Coatings on Zirconia Surfaces: Physico-Chemical and Biological Characterization. J. Funct. Biomater. 2017, 8, 50. https://doi.org/10.3390/jfb8040050
Sandhu PPK, Gindri IM, Siddiqui DA, Rodrigues DC. Dicationic Imidazolium-Based Ionic Liquid Coatings on Zirconia Surfaces: Physico-Chemical and Biological Characterization. Journal of Functional Biomaterials. 2017; 8(4):50. https://doi.org/10.3390/jfb8040050
Chicago/Turabian StyleSandhu, Pavan P. K., Izabelle M. Gindri, Danyal A. Siddiqui, and Danieli C. Rodrigues. 2017. "Dicationic Imidazolium-Based Ionic Liquid Coatings on Zirconia Surfaces: Physico-Chemical and Biological Characterization" Journal of Functional Biomaterials 8, no. 4: 50. https://doi.org/10.3390/jfb8040050




