Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management
Abstract
:1. Introduction
2. Stages of Wound Healing
Pathologic Wound Healing
3. The Role of Growth Factors in the Wound Healing Process
3.1. Platelet-Derived Growth Factor (PDGF)
3.2. Epidermal Growth Factor (EGF)
3.3. Fibroblast Growth Factor (FGF)
3.4. Insulin Growth Factor (IGF)
3.5. Vascular Endothelial Growth Factor (VEGF)
3.6. Transforming Growth Factor-β
3.7. Hepatocyte Growth Factor (HGF)
3.8. Keratinocyte Growth Factor (KGF)
4. Platelet Rich Plasma: Preparation Methods and Therapeutic Formulations
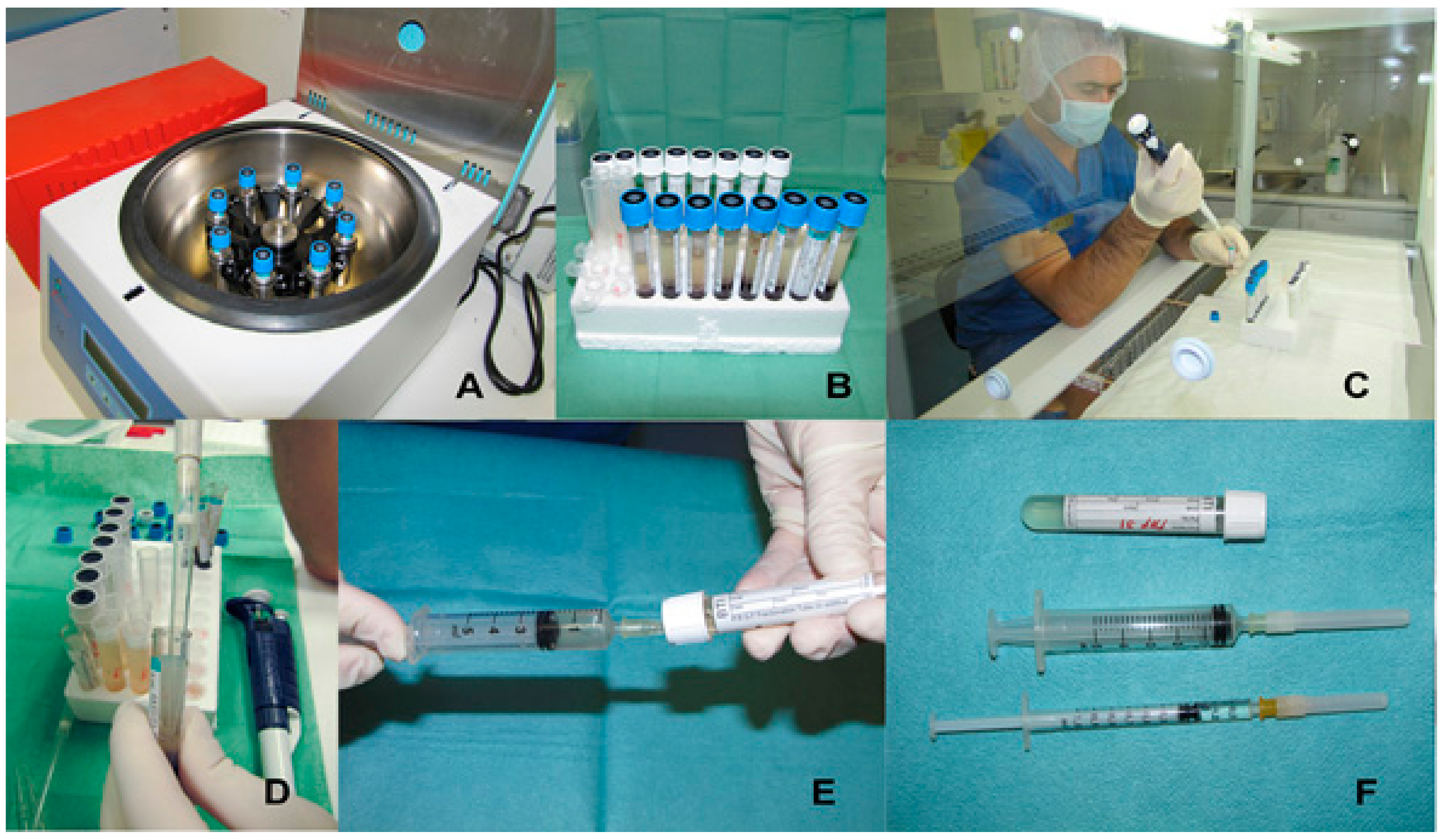
4.1. Preparation Methods
4.1.1. PRGF-System® (BTI Biotechnology Institute, Vitoria, Spain)
4.1.2. Platelet Concentrate Collection System (PCCS®Kit) (3i–Implant Innovations, Palm Beach Gardens, FL, USA)
4.1.3. Gravitational Platelet Separation (GPS® System) (Biomet Merck Biomaterials, Darmstadt, Germany)
4.1.4. Smart PReP® System (Harvest Technologies Corporation, Munich, Germany)
4.1.5. Plateltex® (Plateltex, Bratislava, Slovakia)
4.2. Therapeutic Formulations
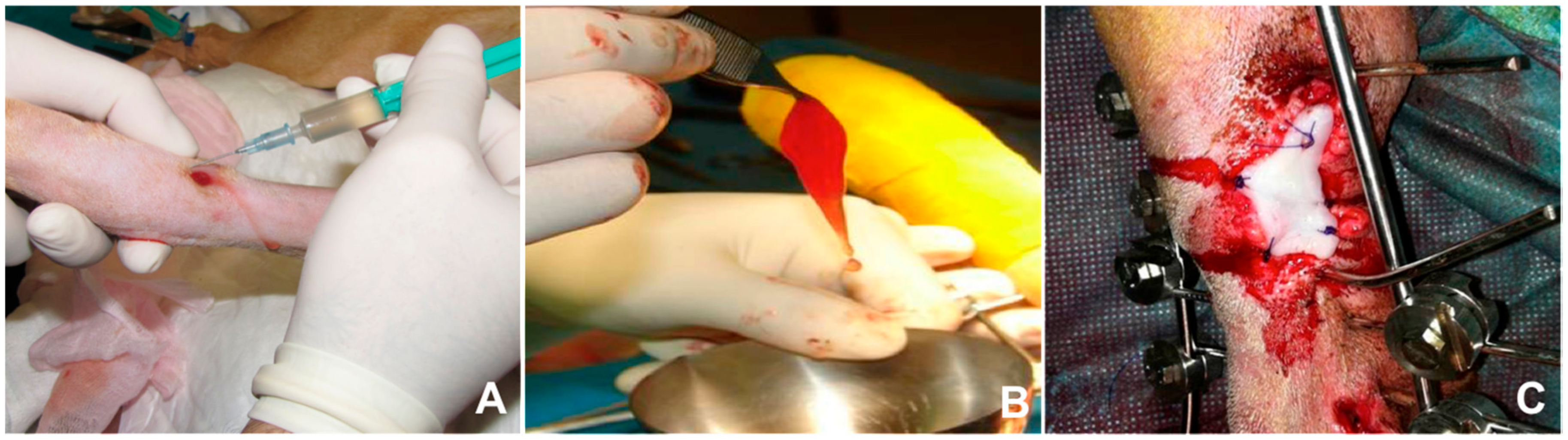
5. The Use of Platelet-Rich Plasma in Cutaneous Wound Healing
6. Future Perspectives in Wound Repair
7. Conclusions
Conflicts of Interest
References
- Crovetti, G.; Martinelli, G.; Issi, M.; Barone, M.; Guizzardi, M.; Campanati, B.; Moroni, M.; Carabelli, A. Platelet gel for healing cutaneous chronic wounds. Transfus. Apher. Sci. 2004, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Theoret, C. Tissue engineering in wound repair: The three “R”s—Repair, replace, regenerate. Vet. Surg. 2009, 38, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.U.; Greiser, U.; Wang, W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014, 22, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Huang, Y.; Zhang, H. Application of stems cells in wound Healing—An update. Wound Repair Regen. 2014, 22, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Trans. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Nauta, A.; Wong, V.; Glotzbach, J.; Gurtner, G.C.; Longaker, M.T. The role of stem cells in cutaneous wound healing: What do we really know? Plast. Reconstr. Surg. 2011, 127 (Suppl. 1), 10S–20S. [Google Scholar] [CrossRef] [PubMed]
- Maan, Z.N.; Januszyk, M.; Rennert, R.C.; Duscher, D.; Rodrigues, M.; Fujiwara, T.; Ho, N.; Whitmore, A.; Hu, M.S.; Longaker, M.T.; et al. Noncontact, low-frequency ultrasound therapy enhances neovascularization and wound healing in diabetic mice. Plast. Reconstr. Surg. 2014, 134, 402e–411e. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.; Vileikyte, L.; Ragnarson-Tennvall, G. The global burden of diabetic foot disease. Lancet 2005, 12, 1719–1724. [Google Scholar] [CrossRef]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The resource impact of wounds on health-care providers in Europe. J. Wound Care 2009, 18, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kozlik, M.; Wojcicki, P. The use of stem cells in plastic and reconstructive surgery. Adv. Clin. Exp. Med. 2014, 23, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sanchez, M.; Nurden, A.T.; Nurden, P.; Orive, G.; Andia, I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006, 24, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, C.; Renner, R.; Milkova, L.; Simon, J.C. Regenerative medicine in dermatology: Biomaterials, tissue engineering, stem cells, gene transfer and beyond. Exp. Dermatol. 2010, 19, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control. Release 2012, 157, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Howard, D.; Brooks, R.A.; Wardale, J.; Henson, F.M.; Getgood, A.; Rushton, N. The role of platelet rich plasma in musculoskeletal science. JRSM Short Rep. 2012, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast. Reconstr. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Johnson, M.L.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, A.; Abdullah, K.M. Wound healing: The role of growth factors. Drugs Today 2003, 39, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Koveker, G.B. Growth factors in clinical practice. Int. J. Clin. Pract. 2000, 54, 590–593. [Google Scholar] [PubMed]
- Arwert, E.N.; Hoste, E.; Watt, F.M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer 2012, 12, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Papanas, N.; Maltezos, E. Growth factors in the treatment of diabetic foot ulcers: New technologies, any promises? Int. J. Low. Extrem. Wounds 2007, 6, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.S.; Evans, G.R. Advances in wound healing: A review of current wound healing products. Plast. Surg. Int. 2012, 2012, 190436. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; McCarthy, T.; Centrella, M. Growth factors and the regulation of bone remodeling. J. Clin. Investig. 1988, 81, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Zielins, E.R.; Atashroo, D.A.; Maan, Z.N.; Duscher, D.; Walmsley, G.G.; Hu, M.; Senarath-Yapa, K.; McArdle, A.; Tevlin, R.; Wearda, T.; et al. Wound healing: An update. Regen. Med. 2014, 9, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Villela, D.L.; Santos, V.L. Evidence on the use of platelet-rich plasma for diabetic ulcer: A systematic review. Growth Factors 2010, 28, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Medici, D.; Serra, M.; Panizza, R.; Rivara, G.; Orecchia, S.; Libener, R.; Cattana, E.; Levis, A.; Betta, P.G.; et al. The use of autologous platelet gel to treat difficult-to-heal wounds: A pilot study. Transfusion 2004, 44, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Ostvar, O.; Shadvar, S.; Yahaghi, E.; Azma, K.; Fayyaz, A.F.; Ahmadi, K.; Nowrouzian, I. Effect of platelet-rich plasma on the healing of cutaneous defects exposed to acute to chronic wounds: A clinico-histopathologic study in rabbits. Diagn. Pathol. 2015, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Reddy, M.S.; Geurs, N.; Palcanis, K.G.; Lemons, J.E.; Rahemtulla, F.G.; Ho, K.J.; Chen, D.T.; Davis, C.R. Feldman, D.S. Efficacy of platelet-rich plasma on wound healing in rabbits. J. Periodontol. 2008, 79, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Pallua, N.; Wolter, T.; Markowicz, M. Platelet-rich plasma in burns. Burns 2010, 36, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Sommeling, C.E.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Stillaert, F.B.; Monstrey, S. The use of platelet-rich plasma in plastic surgery: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Gentile, P.; Scioli, M.G.; Grimaldi, M.; Casciani, C.U.; Spagnoli, L.G.; Orlandi, A. Application of platelet-rich plasma in plastic surgery: Clinical and in vitro evaluation. Tissue Eng. Part C Methods 2009, 15, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Kiritsi, D.; Nystrom, A. The role of TGFbeta in wound healing pathologies. Mech. Ageing Dev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative Skin Wound Healing in Mammals: State-of-the-Art on Growth Factor and Stem Cell Based Treatments. Cell. Physiol. Biochem. 2015, 36, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Jee, C.H.; Eom, N.Y.; Jang, H.M.; Jung, H.W.; Choi, E.S.; Won, J.H.; Hong, I.H.; Kang, B.T.; Jeong, D.W.; Jung, D.I. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J. Vet. Sci. 2016, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.R. Inflammation and healing. In Pathologic Basis of Veterinary Disease, 5th ed.; Zachary, F.J., McGavin, D.M., Eds.; Elsevier: St. Louis, MO, USA, 2011; pp. 60–88. [Google Scholar]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686, discussion 86. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.T.; Schultz, G.S. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am. J. Surg. 1993, 165, 728–737. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S.; Lupher, M.; Thannickal, V.J.; Wynn, T.A. Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 2013, 8, 241–276. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Farghali, H.A.; AbdElKader, N.A.; Khattab, M.S.; AbuBakr, H.O. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Grey, J.E.; Harding, K.G. Recent advances and emerging treatments. Br. Med. J. 2006, 332, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Marston, W.; Armstrong, D.G. Wound care: The role of advanced wound-healing technologies. J. Am. Podiatr. Med. Assoc. 2010, 100, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Markova, A.; Mostow, E.N. US skin disease assessment: Ulcer and wound care. Dermatol. Clin. 2012, 30, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Wolf, L.; Deckenback, J.; Hamblin, M.R.; Herman, I.M. Human platelet-rich plasma-and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PLoS ONE 2012, 7, e32146. [Google Scholar]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- De La Mata, J. Platelet rich plasma. A new treatment tool for the rheumatologist? Reumatol. Clin. 2013, 9, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Smith, R.K. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet. Clin. N. Am. Equine Pract. 2008, 24, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Roubelakis, M.G.; Trohatou, O.; Roubelakis, A.; Mili, E.; Kalaitzopoulos, I.; Papazoglou, G.; Pappa, K.I.; Anagnou, N.P. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev. 2014, 10, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Cross, K.J.; Mustoe, T.A. Growth factors in wound healing. Surg. Clin. N. Am. 2003, 83, 531–545. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 2: Role of growth factors in normal and pathological wound healing: Therapeutic potential and methods of delivery. Adv. Skin Wound Care 2012, 25, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Moulin, V. Growth factors in skin wound healing. Eur. J. Cell. Biol. 1995, 68, 1–7. [Google Scholar] [PubMed]
- Dituri, F.; Mazzocca, A.; Lupo, L.; Edling, C.E.; Azzariti, A.; Antonaci, S.; Falasca, M.; Giannelli, G. PI3K class IB controls the cell cycle checkpoint promoting cell proliferation in hepatocellular carcinoma. Int. J. Cancer 2012, 130, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Kiritsy, C.P.; Lynch, A.B.; Lynch, S.E. Role of growth factors in cutaneous wound healing: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 729–760. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.E.; Nixon, J.C.; Colvin, R.B.; Antoniades, H.N. Role of platelet-derived growth factor in wound healing: Synergistic effects with other growth factors. Proc. Natl. Acad. Sci. USA 1987, 84, 7696–7700. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef] [PubMed]
- Hoosgood, G. Wound repair and specific tissue response to injury. In Textbook of Small Animal Surgery, 3rd ed.; Slater, D., Ed.; Saunders: Philadelphia, PA, USA, 2003; pp. 66–86. [Google Scholar]
- Steed, D.L. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast. Reconstr. Surg. 2006, 117, 143S–149S, discussion 150S–151S. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.F.; Tarpley, J.E.; Tseng, J.; Bready, J.; Chang, D.; Kenney, W.C.; Rudolph, R.; Robson, M.C.; Vande Berg, J.; Reid, P.; et al. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J. Clin. Investig. 1995, 96, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Girdler, N.M.; McGurk, M.; Aqual, S.; Prince, M. The effect of epidermal growth factor mouthwash on cytotoxic-induced oral ulceration. A phase I clinical trial. Am. J. Clin. Oncol. 1995, 18, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.L.; Nanney, L.B.; Griffen, J.; Cramer, A.B.; Yancey, J.M.; Curtsinger, L.J., 3rd.; Holtzin, L.; Schultz, G.S.; Jurkiewicz, M.J.; Lynch, J.B. Enhancement of wound healing by topical treatment with epidermal growth factor. N. Engl. J. Med. 1989, 321, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lew, D.H.; Tark, K.C.; Rah, D.K.; Hong, J.P. Effect of recombinant human epidermal growth factor against cutaneous scar formation in murine full-thickness wound healing. J. Korean Med. Sci. 2010, 25, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bian, H.; Qi, S.; Xu, Y.; Tang, J.; Li, T.; Liu, X. Effects of basic fibroblast growth factor on the expression of extracellular matrix and matrix metalloproteinase-1 in wound healing. Clin. Exp. Dermatol. 2008, 33, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Chang, Z.; Peng, B.; Xia, Q.; Lu, W.; Huang, P.; Tsao, M.S.; Chiao, P.J. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-kappaB transcription factors. J. Biol. Chem. 2007, 282, 6001–6011. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, R.; Rifkin, D.B. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J. Exp. Med. 1990, 172, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.X.; Lin, C.; Lin, B.B.; Wang, Z.G.; Zhang, H.Y.; Wu, F.Z.; Cheng, Y.; Xiang, L.J.; Guo, D.J.; Luo, X.; et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS ONE 2013, 8, e59966. [Google Scholar] [CrossRef] [PubMed]
- Gartner, M.H.; Benson, J.D.; Caldwell, M.D. Insulin-like growth factors I and II expression in the healing wound. J. Surg. Res. 1992, 52, 389–394. [Google Scholar] [CrossRef]
- Tsuboi, R.; Shi, C.M.; Sato, C.; Cox, G.N.; Ogawa, H. Co-administration of insulin-like growth factor (IGF)-I and IGF-binding protein-1 stimulates wound healing in animal models. J. Investig. Dermatol. 1995, 104, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.L.; Kane, C.D.; Chernausek, S.D.; Greenhalgh, D.G. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice. Am. J. Pathol. 1997, 151, 715–724. [Google Scholar] [PubMed]
- Yu, D.H.; Mace, K.A.; Hansen, S.L.; Boudreau, N.; Young, D.M. Effects of decreased insulin-like growth factor-1 stimulation on hypoxia inducible factor 1-alpha protein synthesis and function during cutaneous repair in diabetic mice. Wound Repair Regen. 2007, 15, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Haase, I.; Evans, R.; Pofahl, R.; Watt, F.M. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J. Cell Sci. 2003, 116, 3227–3238. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G.; Hummel, R.P.; Albertson, S.; Breeden, M.P. Synergistic actions of platelet-derived growth factor and the insulin-like growth factors in vivo. Wound Repair Regen. 1993, 1, 69–81. [Google Scholar] [PubMed]
- Hsu, C.; Chang, J. Clinical implications of growth factors in flexor tendon wound healing. J. Hand Surg. 2004, 29, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Enholm, B.; Alitalo, K.; Paavonen, K. The biology of vascular endothelial growth factors. Cardiovasc. Res. 2005, 65, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Papazoglou, L.G.; Loukopoulos, P.; Kazakos, G.; Chantes, A.; Giannakas, N.; Savvas, I.; Psalla, D.; Kritsepi-Konstantinou, M.; Dionyssiou, D. Locally injected autologous platelet-rich plasma enhanced tissue perfusion and improved survival of long subdermal plexus skin flaps in dogs. Vet. Comp. Orthop. Traumatol. 2014, 27, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Hanft, J.R.; Pollak, R.A.; Barbul, A.; van Gils, C.; Kwon, P.S.; Gray, S.M.; Lynch, C.J.; Semba, C.P.; Breen, T.J. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J. Wound Care 2008, 17, 30–32, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-beta family in wound healing, burns and scarring: A review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar] [PubMed]
- Shah, M.; Foreman, D.M.; Ferguson, M.W. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J. Cell Sci. 1994, 107 Pt 5, 1137–1157. [Google Scholar] [PubMed]
- Le, M.; Naridze, R.; Morrison, J.; Biggs, L.C.; Rhea, L.; Schutte, B.C.; Kaartinen, V.; Dunnwald, M. Transforming growth factor Beta 3 is required for excisional wound repair in vivo. PLoS ONE 2012, 7, e48040. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.; Price, P.; Harding, K.G.; Jiang, W.G. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006, 14, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Andia, I.; Sanchez, M.; Azofra, J.; del Mar Zalduendo, M.; de la Fuente, M.; Nurden, P.; Nurden, A.T. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J. Orthop. Res. 2005, 23, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Borrione, P.; Gianfrancesco, A.D.; Pereira, M.T.; Pigozzi, F. Platelet-rich plasma in muscle healing. Am. J. Phys. Med. Rehabil. 2010, 89, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Johal, H.; Bhandari, M. An evidence-based evaluation on the use of platelet rich plasma in orthopedics—A review of the literature. SICOT J. 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.J.; van Veldhoven, P.L.; Moen, M.H.; Weir, A.; Tol, J.L.; Maffulli, N. Autologous growth factor injections in chronic tendinopathy: A systematic review. Br. Med. Bull. 2010, 95, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Salarinia, R.; Sadeghnia, H.R.; Alamdari, D.H.; Hoseini, S.J.; Mafinezhad, A.; Hosseini, M. Platelet rich plasma: Effective treatment for repairing of spinal cord injury in rat. Acta Orthop. Traumatol. Turc. 2017, 51, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Reffat, S.A.; Hassan, A.; Eskander, F. Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Ann. Vasc. Surg. 2017, 38, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ronci, C.; Ferraro, A.S.; Lanti, A.; Missiroli, F.; Sinopoli, S.; Del Proposto, G.; Cipriani, C.; De Felici, C.; Ricci, F.; Ciotti, M.; et al. Platelet-rich plasma as treatment for persistent ocular epithelial defects. Trans. Apher. Sci. 2015, 52, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Ghoddusi, J.; Maghsudlu, A.; Jafarzadeh, H.; Jafarian, A.; Forghani, M. Histological Evaluation of the Effect of Platelet-rich Plasma on Pulp Regeneration in Nonvital Open Apex Teeth: An Animal Study. J. Contemp. Dent. Pract. 2017, 18, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Jt. Surg. Am. 2017, 99, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Griffeth, R.J.; Garcia-Parraga, D.; Mellado-Lopez, M.; Crespo-Picazo, J.L.; Soriano-Navarro, M.; Martinez-Romero, A.; Moreno-Manzano, V. Platelet-rich plasma and adipose-derived mesenchymal stem cells for regenerative medicine-associated treatments in bottlenose dolphins (Tursiops truncatus). PLoS ONE 2014, 9, e108439. [Google Scholar] [CrossRef] [PubMed]
- Bernuzzi, G.; Tardito, S.; Bussolati, O.; Adorni, D.; Cantarelli, S.; Fagnoni, F.; Rossetti, A.; Azzarone, M.; Ficarelli, E.; Caleffi, E.; et al. Platelet gel in the treatment of cutaneous ulcers: The experience of the Immunohaematology and Transfusion Centre of Parma. Blood Trans. 2010, 8, 237–247. [Google Scholar]
- Leitner, G.C.; Gruber, R.; Neumuller, J.; Wagner, A.; Kloimstein, P.; Hocker, P.; Kormoczi, G.F.; Buchta, C. Platelet content and growth factor release in platelet-rich plasma: A comparison of four different systems. Vox Sang. 2006, 91, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sanchez, M.; Orive, G.; Andia, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007, 28, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sanchez, M.; Orive, G.; Andia, I. Delivering growth factors for therapeutics. Trends Pharmacol. Sci. 2008, 29, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Kleis, W.K.; Hitzler, W.E.; Hafner, G. Comparison of the platelet concentrate collection system with the plasma-rich-in-growth-factors kit to produce platelet-rich plasma: A technical report. Int. J. Oral Maxillofac. Implants 2005, 20, 118–123. [Google Scholar] [PubMed]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, C.C.; Stammers, A.H.; Woods, E.; Yen, B.R.; Klayman, M.; Gilbert, C. Use of platelet gel and its effects on infection in cardiac surgery. J. Extra-Corpor. Technol. 2005, 37, 381–386. [Google Scholar] [PubMed]
- Bielecki, M.; Lebowska, D. New methods of hand mobilization after operative treatment of flexor tendon injuries. Wiad. Lek. 2007, 60, 346–351. [Google Scholar] [PubMed]
- Appel, T.R.; Potzsch, B.; Muller, J.; von Lindern, J.J.; Berge, S.J.; Reich, R.H. Comparison of three different preparations of platelet concentrates for growth factor enrichment. Clin. Oral Implants Res. 2002, 13, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Kleis, W.K. Curasan PRP kit vs. PCCS PRP system. Collection efficiency and platelet counts of two different methods for the preparation of platelet-rich plasma. Clin. Oral Implants Res. 2002, 13, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, S.; Mousavi, M.; Gabler, C.; Erdos, J.; Vecsei, V. A new simplified technique for producing platelet-rich plasma: A short technical note. Eur. Spine J. 2004, 13 (Suppl. 1), S102–S106. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Kleis, W.K.; Buch, R.; Hitzler, W.E.; Hafner, G. The Harvest Smart PRePTM system versus the Friadent-Schutze platelet-rich plasma kit. Clin. Oral Implants Res. 2003, 14, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Balbo, V.; Cattana, E.; Borzini, P. Platelet-rich plasma and platelet gel preparation using Plateltex. Vox Sang. 2008, 94, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.A. Enhancement of osseointegration by generating a dynamic implant surface. J. Oral Implantol. 2006, 32, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Barbero, J.E.; Galindo-Moreno, P.; Avila-Ortiz, G.; Caba, O.; Sanchez-Fernandez, E.; Wang, H.L. Flow cytometric and morphological characterization of platelet-rich plasma gel. Clin. Oral Implants Res. 2006, 17, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Platelet-rich plasma: Underlying biology and clinical correlates. Regen. Med. 2013, 8, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.; Gupta, S.; Bukhari, S.; Ponemone, V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: A case series. J. Biomed. Sci. 2017, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1–9. [Google Scholar] [PubMed]
- Yung, Y.L.; Fu, S.C.; Cheuk, Y.C.; Qin, L.; Ong, M.T.; Chan, K.M.; Yung, P.S. Optimisation of platelet concentrates therapy: Composition, localisation, and duration of action. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2017, 7, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sardari, K.; Reza, M.; Kazemi, H. Effects of platelet rich plasma (PRP) on cutaneous regeneration and wound healing in dogs treated with dexamethasone. Comp. Clin. Pathol. 2011, 20, 155–162. [Google Scholar] [CrossRef]
- Carter, C.A.; Jolly, D.G.; Worden, C.E., Sr.; Hendren, D.G.; Kane, C.J. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp. Mol. Pathol. 2003, 74, 244–255. [Google Scholar] [CrossRef]
- Dionyssiou, D.; Demiri, E.; Foroglou, P.; Cheva, A.; Saratzis, N.; Aivazidis, C.; Karkavelas, G. The effectiveness of intralesional injection of platelet-rich plasma in accelerating the healing of chronic ulcers: An experimental and clinical study. Int. Wound J. 2013, 10, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Ogata, H.; Yazawa, M.; Watanabe, N.; Mori, T.; Nakajima, T. The effects of platelet-rich plasma on cutaneous incisional wound healing in rats. J. Dermatol. Sci. 2005, 40, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Brissett, A.E.; Hom, D.B. The effects of tissue sealants, platelet gels, and growth factors on wound healing. Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Krupski, W.C.; Reilly, L.M.; Perez, S.; Moss, K.M.; Crombleholme, P.A.; Rapp, J.H. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: A preliminary report. J. Vasc. Surg. 1991, 14, 526–532, discussion 532–536. [Google Scholar] [CrossRef]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Babaei, V.; Afradi, H.; Gohardani, H.Z.; Nasseri, F.; Azarafza, M.; Teimourian, S. Management of chronic diabetic foot ulcers using platelet-rich plasma. J. Wound Care 2017, 26, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Hersant, B.; SidAhmed-Mezi, M.; Bosc, R.; Meningaud, J.P. Autologous Platelet-Rich Plasma/Thrombin Gel Combined with Split-Thickness Skin Graft to Manage Postinfectious Skin Defects: A Randomized Controlled Study. Adv. Skin Wound Care 2017, 30, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Cieslik-Bielecka, A.; Skowronski, R.; Jedrusik-Pawlowska, M.; Pierchala, M. The application of L-PRP in AIDS patients with crural chronic ulcers: A pilot study. Adv. Med. Sci. 2017, 63, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Man, D.; Plosker, H.; Winland-Brown, J.E. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast. Reconstr. Surg. 2001, 107, 229–237, discussion 238–239. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Driver, V.R.; Carman, D.; Lucero, B.; Borris-Hale, C.; Fylling, C.P.; Rappl, L.M.; Clausen, P.A. Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: A prospective case series. Ostomy Wound Manag. 2010, 56, 36–44. [Google Scholar]
- Driver, V.R.; Hanft, J.; Fylling, C.P.; Beriou, J.M.; Autologel Diabetic Foot Ulcer Study G. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manag. 2006, 52, 68–70, 72, 74. [Google Scholar]
- Steenvoorde, P.; van Doorn, L.P.; Naves, C.; Oskam, J. Use of autologous platelet-rich fibrin on hard-to-heal wounds. J. Wound Care 2008, 17, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Carias, R.B.; Teixeira, M.V.; da Cruz Pacheco, I.; Correa do Amaral, R.J.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- McAleer, J.P.; Sharma, S.; Kaplan, E.M.; Persich, G. Use of autologous platelet concentrate in a nonhealing lower extremity wound. Adv. Skin Wound Care 2006, 19, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fylling, C.P.; Parnell, L.K. Use of platelet rich plasma gel on wound healing: A systematic review and meta-analysis. Eplasty 2011, 11, e38. [Google Scholar] [PubMed]
- Kim, D.H.; Je, Y.J.; Kim, C.D.; Lee, Y.H.; Seo, Y.J.; Lee, J.H.; Lee, Y. Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Ann. Dermatol. 2011, 23, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Na, J.I.; Choi, J.W.; Choi, H.R.; Jeong, J.B.; Park, K.C.; Youn, S.W.; Huh, C.H. Rapid healing and reduced erythema after ablative fractional carbon dioxide laser resurfacing combined with the application of autologous platelet-rich plasma. Dermatol. Surg. 2011, 37, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, E.P.; Sahin, G.; Aydin, F.; Senturk, N.; Turanli, A.Y. Evaluation of effects of platelet-rich plasma on human facial skin. J. Cosmet. Laser Ther. 2014, 16, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Choi, H.I.; Choi, D.K.; Sohn, K.C.; Im, M.; Seo, Y.J.; Lee, Y.H.; Lee, J.H.; Lee, Y. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol. Surg. 2012, 38, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.W.; Kim, S.A.; Lee, K.S. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int. J. Mol. Med. 2012, 29, 32–36. [Google Scholar] [PubMed]
- DeRossi, R.; Coelho, A.C.; Mello, G.S.; Frazilio, F.O.; Leal, C.R.; Facco, G.G.; Brum, K.B. Effects of platelet-rich plasma gel on skin healing in surgical wound in horses. Acta Cir. Bras. 2009, 24, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, C.; Park, H.M. Curative effect of autologous platelet-rich plasma on a large cutaneous lesion in a dog. Vet. Dermatol. 2009, 20, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound healing in the 21st century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Karayannopoulou, M.; Psalla, D.; Kazakos, G.; Loukopoulos, P.; Giannakas, N.; Savvas, I.; Kritsepi-Konstantinou, M.; Chantes, A.; Papazoglou, L.G. Effect of locally injected autologous platelet-rich plasma on second intention wound healing of acute full-thickness skin defects in dogs. Vet. Comp. Orthop. Traumatol. 2015, 28, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Waller, W.; Lineaweaver, W.C. Growth factors and flap survival. Microsurgery 2004, 24, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Sumi, Y.; Ishihara, M.; Kishimoto, S.; Nakamura, S.; Yanagibayashi, S.; Hattori, H.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. PRP&F/P MPs improved survival of dorsal paired pedicle skin flaps in rats. J. Surg. Res. 2011, 170, e189–e196. [Google Scholar] [PubMed]
- Li, W.; Enomoto, M.; Ukegawa, M.; Hirai, T.; Sotome, S.; Wakabayashi, Y.; Shinomiya, K.; Okawa, A. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve survival rates. Plast. Reconstr. Surg. 2012, 129, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Molina-Minano, F.; Lopez-Jornet, P.; Camacho-Alonso, F.; Vicente-Ortega, V. The use of plasma rich in growth factors on wound healing in the skin: Experimental study in rabbits. Int. Wound J. 2009, 6, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Molina-Minano, F.; Vicente-Ortega, V. Effects of plasma rich in growth factors on wound healing of the tongue. Experimental study on rabbits. Med. Oral Patol. Oral Cir. Bucal 2009, 14, e425–e428. [Google Scholar] [PubMed]
- Abegao, K.G.; Bracale, B.N.; Delfim, I.G.; Santos, E.S.; Laposy, C.B.; Nai, G.A.; Giuffrida, R.; Nogueira, R.M. Effects of heterologous platelet-rich plasma gel on standardized dermal wound healing in rabbits. Acta Cir. Bras. 2015, 30, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Jinming, W.; Caiyue, L.; Baojin, W.; Antang, L.; Yingfan, Z.; Hui, W.; Lie, Z.; Hua, J. Effects of Platelet-Rich Plasma on Tissue Expansion in Rabbits. Aesthet. Plast. Surg. 2017, 41, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Yin, X.; Li, H.; Jia, L.; He, X.; Yan, Y.; Liu, N.; Wan, K.; Li, X.; Lin, S. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann. Dermatol. 2014, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Lee, I.H.; Park, E.S.; Kim, J.Y. Hydrogel and Platelet-Rich Plasma Combined Treatment to Accelerate Wound Healing in a Nude Mouse Model. Arch. Plast. Surg. 2017, 44, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Spence, A.J.; Rodriguez, I.A.; McCool, J.M.; Petrella, R.L.; Garg, K.; Ericksen, J.J.; Bowlin, G.L. A preliminary study on the potential of manuka honey and platelet-rich plasma in wound healing. Int. J. Biomater. 2012, 2012, 313781. [Google Scholar] [CrossRef] [PubMed]
- Isakson, M.; de Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J. Mesenchymal Stem Cells and Cutaneous Wound Healing: Current Evidence and Future Potential. Stem Cells Int. 2015, 2015, 831095. [Google Scholar] [CrossRef] [PubMed]
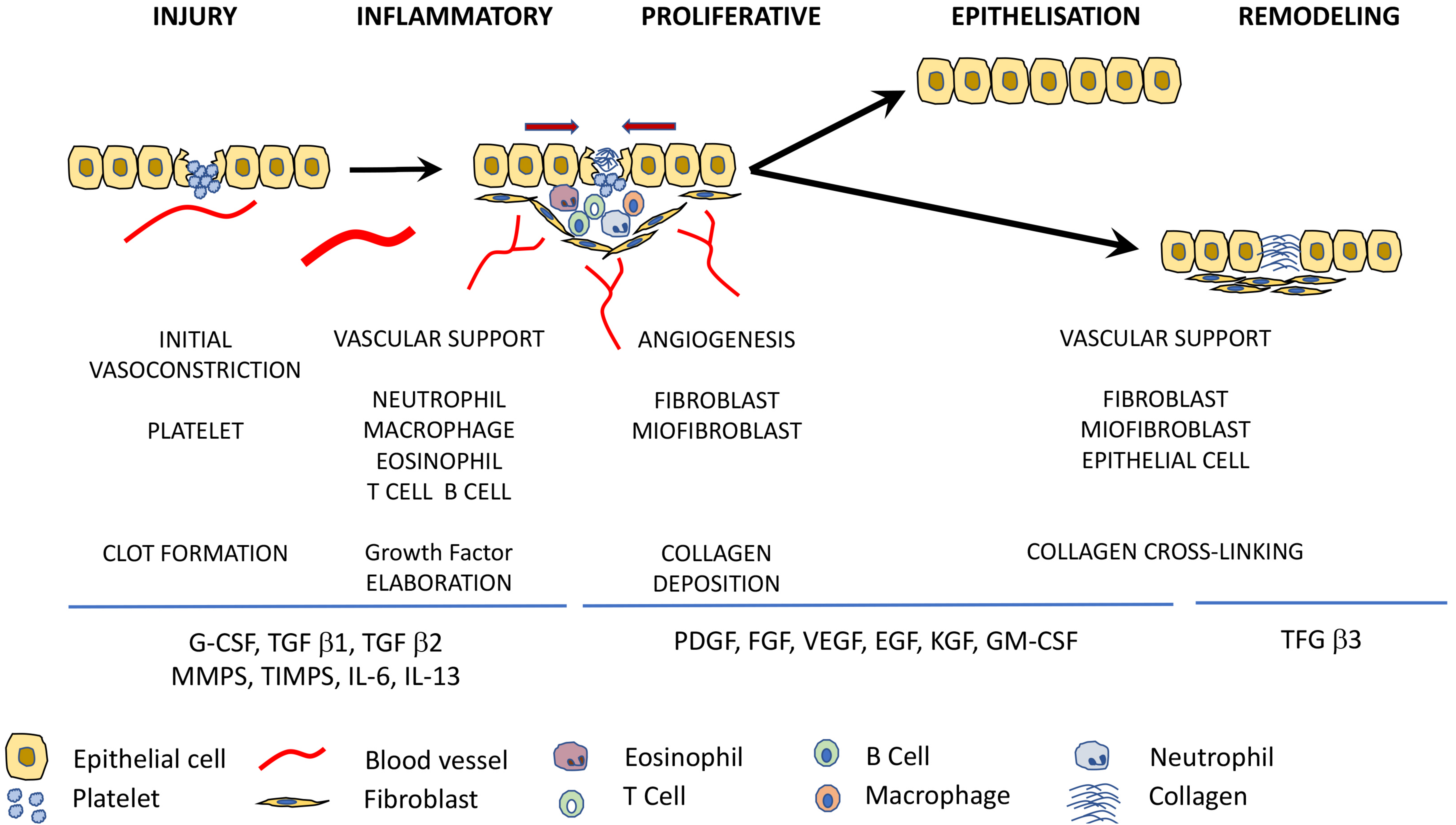
| Wound Healing Stages | Growth Factors |
|---|---|
| Inflammatory phase | G-CSF, TGF-β1, TGF-β2 |
| Proliferative phase | PDGF, FGF, VEGF |
| Epithelialisation | EGF, KGF, GM-CSF |
| Remodeling phase | TGF-β3 |
| GFs | Origin |
|---|---|
| PDGF | Platelets, macrophages, endothelial cells, keratinocytes, muscle cells |
| VEGF | Platelets, keratinocytes, macrophages, fibroblasts |
| FGF | Macrophages, T lymphocytes, mast cells, endothelial cells, fibroblasts, different tissues |
| HGF | Mesenchymal cells |
| TGFβ | Platelets, T lymphocytes, macrophages, endothelial cells, keratinocytes, fibroblasts, muscle cells |
| Method | Platelets (×103/mL) | Leukocytes (×103/mL) | Blood Volume (mL) |
|---|---|---|---|
| PRGF-System® GF-EN | 500 | ≈0 | 10–20 |
| LANDESBERG | 550–900 | N/D | 5 |
| PCSS® | 1100–2200 | 5.5–14.8 | 54 |
| CURASAN PRP® | 1000–2500 | 14.8–33.1 | 8.5 |
| GPS® SYSTEM | 1600 | 31.1 | 54 |
| SMART® PREP SYSTEM | 1250 | 19.2 | 52 |
| FRIADENT-SCHÜTZE PRP® | 1440 | 21.7 | 8.5 |
| PLATELTEX® | 1600 | N/D | N/D |
| SECQUIRE PRP® SYSTEM | N/D | N/D | N/D |
| ARTHREX ACP® | 550 | ≈0 | 9 |
| VIVOSTAT® | N/D | N/D | 120 |
| FIBRINET® | 346 | N/D | 8 |
| REGEN PRP® | 430 | N/D | 10 |
| References | Model | Type of Wound | Functional Effects |
|---|---|---|---|
| Babaei V. et al. 2017 | Human | Diabetic foot ulcers | Improved healing with short recovery time |
| Suthar M. et al. 2017 | Human | Chronic ulcers | Reduction in wound size, pain and inflammation |
| Cieslik-B. et al. 2017 | Human | Chronic ulcers-AIDS | Enhanced neovascularization + reepithelialization |
| Farghali et al. 2017 | Dogs | Full-thickness | Increased wound contraction, reepithelialization, collagen deposition and reduced scar formation |
| Jee CH. et al. 2016 | Dogs | Full-thickness | Increased angiogenesis, granulation tissue, collagen deposition and re-epithelialization |
| Karayannopoulou et al. 2015 | Dogs | Full-thickness-2nd intention | Increased tissue perfusion and better collagen architecture |
| Karayannopoulou et al. 2014 | Dogs | Skin flaps | Increased skin flap survival and reduced oedema |
| Ostvar O. et al. 2015 | Rabbits | Full-thickness | Enhanced angiogenesis and collagen deposition |
| Molina-Miñano et al. 2009 | Rabbits | Full-thickness | Reduced inflammation, increased granulation tissue formation and re-epithelialization |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10. https://doi.org/10.3390/jfb9010010
Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, Carrillo-Poveda JM, Cuervo-Serrato B, Peláez-Gorrea P, Sopena-Juncosa JJ. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. Journal of Functional Biomaterials. 2018; 9(1):10. https://doi.org/10.3390/jfb9010010
Chicago/Turabian StyleChicharro-Alcántara, Deborah, Mónica Rubio-Zaragoza, Elena Damiá-Giménez, José M. Carrillo-Poveda, Belén Cuervo-Serrato, Pau Peláez-Gorrea, and Joaquín J. Sopena-Juncosa. 2018. "Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management" Journal of Functional Biomaterials 9, no. 1: 10. https://doi.org/10.3390/jfb9010010





