Al2O3 Nanoparticle Addition to Commercial Magnesium Alloys: Multiple Beneficial Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Monolithic AZ31, ZK60A and Derived Nanocomposites
2.2. Microstructural Characteristics
| Material | Al2O3 (vol.%) | Grain characteristics a | Microhardness (HV) | |
|---|---|---|---|---|
| Size (μm) | Aspect ratio | |||
| AZ31 | – | 4.0 ± 0.9 | 1.4 | 64 ± 4 |
| AZ31/1.5 vol.% Al2O3 | 1.50 | 2.3 ± 0.7 | 1.6 | 83 ± 5 (+30) b |
| ZK60A | – | 11.1 ± 3.0 | 1.5 | 117 ± 6 |
| ZK60A/1.5 vol.% Al2O3 | 1.50 | 10.7 ± 2.5 | 1.3 | 135 ± 8 (+15) |
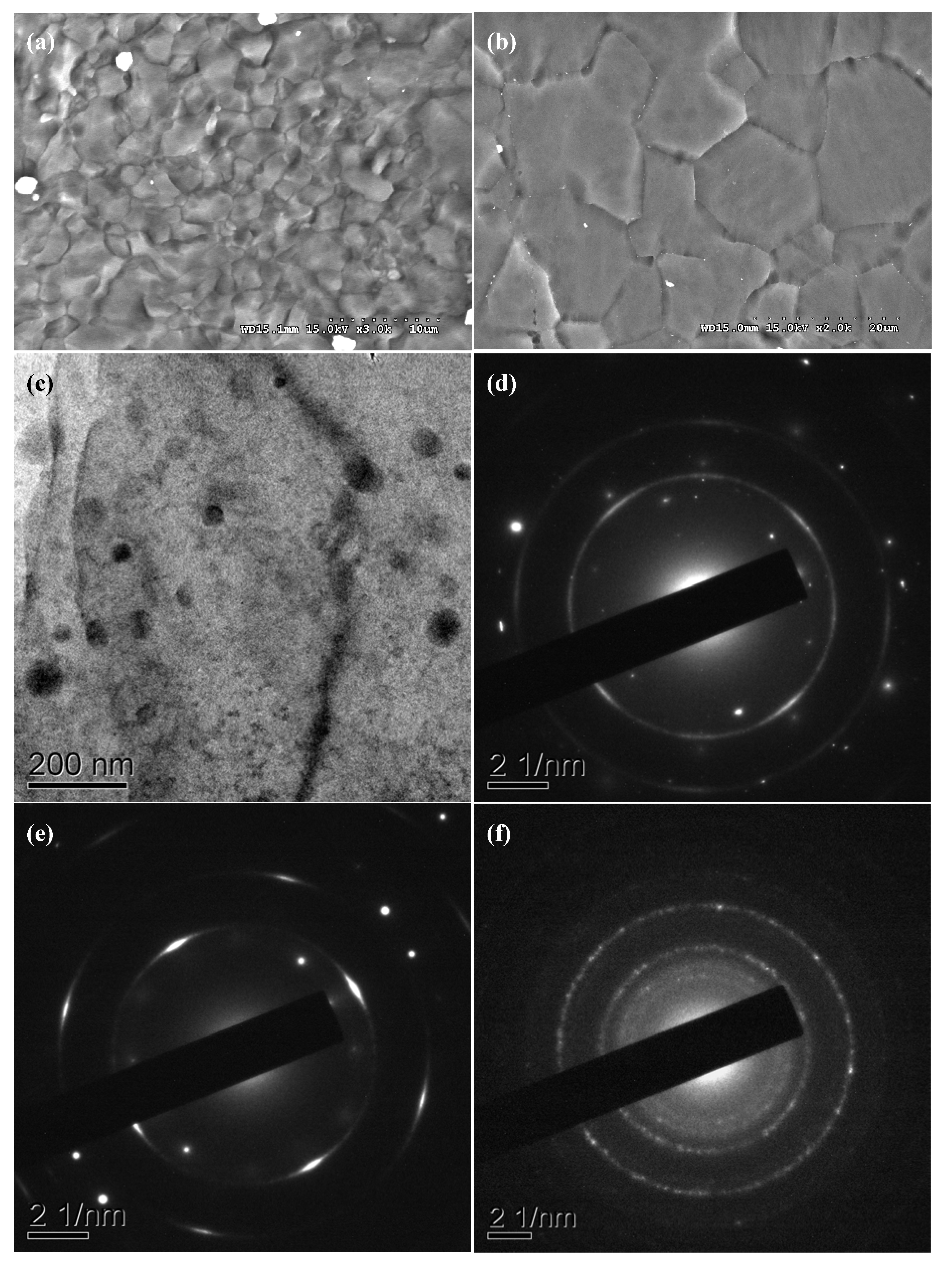
| k space (nm−1) | d (Å) | Phase | Plane | Reference d (Å) | JCPDS card # |
|---|---|---|---|---|---|
| AZ31/1.5 vol.% Al2O3 nanocomposite | |||||
| 34.80 | 1.806 | Al12Mg17 | (5 3 0) | 1.810 | 011128 |
| 39.10 | 1.607 | Al2O3 | (1 1 6) | 1.602 | 431484 |
| 39.39 | 1.595 | Mg | (1 1 0) | 1.605 | 350821 |
| 39.59 | 1.587 | Al12Mg17 | (6 2 2) | 1.600 | 011128 |
| 42.48 | 1.479 | Mg | (1 0 3) | 1.473 | 350821 |
| 49.70 | 1.264 | Al12Mg17 | (6 6 0) | 1.250 | 011128 |
| 64.69 | 0.971 | MgO | (3 3 1) | 0.966 | 450946 |
| 65.66 | 0.957 | Mg | (2 0 4) | 0.951 | 350821 |
| ZK60A/1.5 vol.% Al2O3 nanocomposite | |||||
| 37.37 | 1.682 | MgZn2 | (2 1 1) | 1.677 | 340457 |
| 39.39 | 1.595 | Mg | (1 1 0) | 1.605 | 350821 |
| 40.55 | 1.549 | Al2O3 | (2 1 3) | 1.553 | 260031 |
| 64.69 | 0.971 | MgO | (3 3 1) | 0.966 | 450946 |
| 65.66 | 0.957 | Mg | (2 0 4) | 0.951 | 350821 |
| 67.60 | 0.930 | MgZn2 | (4 1 3) | 0.932 | 340457 |
| 71.16 | 0.883 | Mg | (3 0 2) | 0.873 | 350821 |
| Al2O3 nanoparticle reinforcement | |||||
| 36.00 | 1.745 | Al2O3 | (0 2 4) | 1.740 | 431484 |
| 40.55 | 1.549 | Al2O3 | (2 1 3) | 1.553 | 260031 |
| 49.30 | 1.275 | Al2O3 | (2 0 8) | 1.276 | 431484 |
| 69.30 | 0.907 | Al2O3 | (3 2 4) | 0.908 | 431484 |
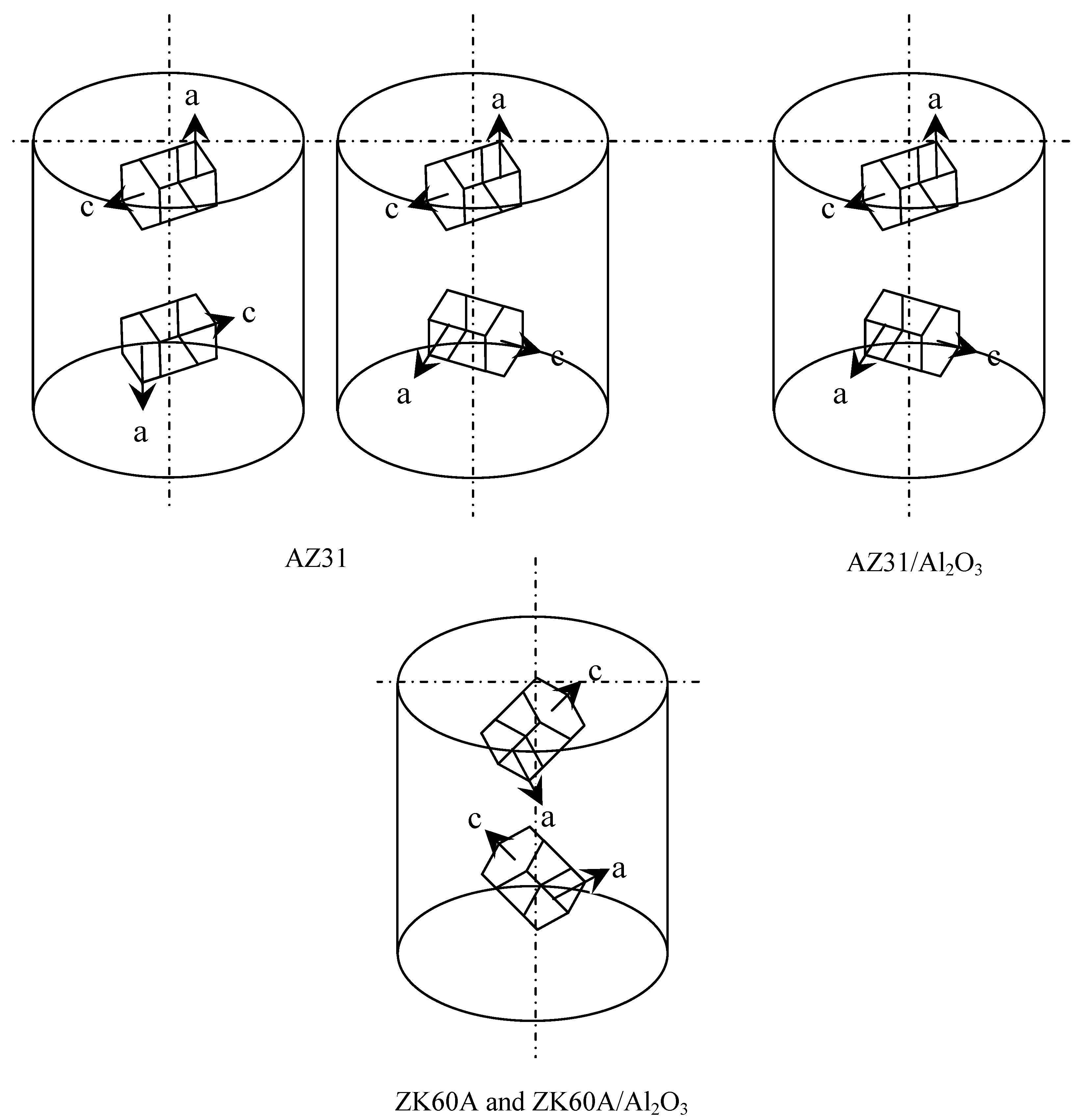
| Material | Section a | Plane | Average I/Imaxb |
|---|---|---|---|
| AZ31 | T | 1 0 −1 0 prism | 1.00 |
| 0 0 0 2 basal | 0.16 | ||
| 1 0 −1 1 pyramidal | 0.81 | ||
| L | 1 0 −1 0 prism | 0.27 | |
| 0 0 0 2 basal | 0.93 | ||
| 1 0 −1 1 pyramidal | 1.00 | ||
| AZ31/1.5 vol.% Al2O3 | T | 1 0 −1 0 prism | 1.00 |
| 0 0 0 2 basal | 0.18 | ||
| 1 0 −1 1 pyramidal | 0.72 | ||
| L | 1 0 −1 0 prism | 0.23 | |
| 0 0 0 2 basal | 0.64 | ||
| 1 0 −1 1 pyramidal | 1.00 | ||
| ZK60A | T | 1 0 −1 0 prism | 0.12 |
| 0 0 0 2 basal | 0.25 | ||
| 1 0 −1 1 pyramidal | 1.00 | ||
| L | 1 0 −1 0 prism | 0.24 | |
| 0 0 0 2 basal | 0.77 | ||
| 1 0 −1 1 pyramidal | 1.00 | ||
| ZK60A/1.5 vol.% Al2O3 | T | 1 0 −1 0 prism | 0.26 |
| 0 0 0 2 basal | 0.12 | ||
| 1 0 −1 1 pyramidal | 1.00 | ||
| L | 1 0 −1 0 prism | 0.30 | |
| 0 0 0 2 basal | 0.48 | ||
| 1 0 −1 1 pyramidal | 1.00 |
2.3. Hardness
2.4. Tensile Behavior
| Material | 0.2% TYS (MPa) | UTS (MPa) | Failure Strain (%) | WOF (MJ/m3) a |
|---|---|---|---|---|
| AZ31 | 172 ± 15 | 263 ± 12 | 10.4 ± 3.9 | 26 ± 9 |
| AZ31/1.5 vol.% Al2O3 | 204 ± 8 (+19) b | 317 ± 5 (+21) | 22.2 ± 2.4 (+113) | 68 ± 7 (+162) |
| ZK60A | 182 ± 4 | 271 ± 1 | 6.7 ± 1.0 | 17 ± 3 |
| ZK60A/1.5 vol.% Al2O3 | 175 ± 2 (−4) | 305 ± 2 (+13) | 18.1 ± 0.9 (+170) | 51 ± 3 (+200) |
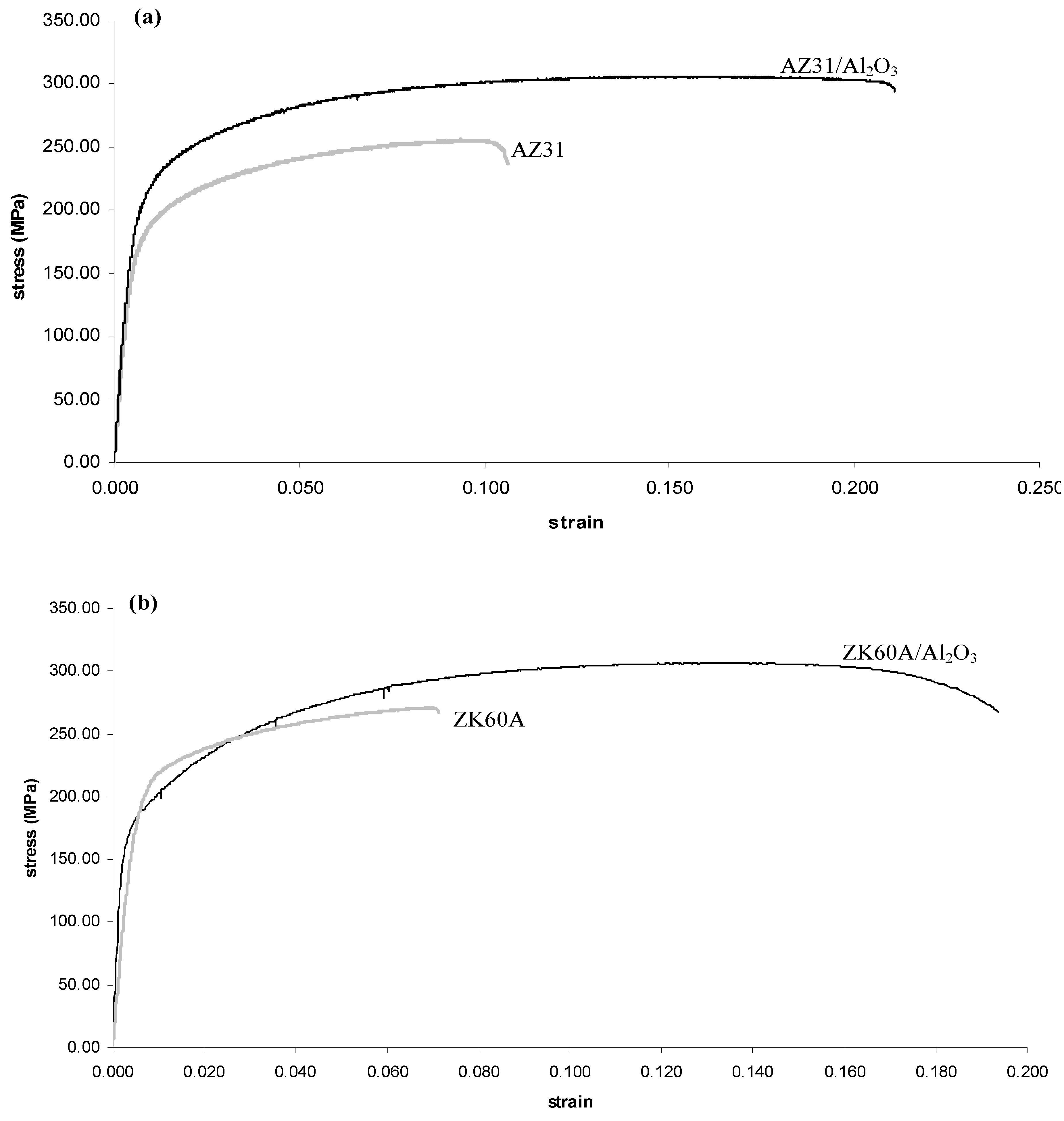
2.5. Compressive Behavior
| Material | 0.2% CYS (MPa) | UCS (MPa) | Failure Strain (%) | Work of Fracture, WOF (MJ/m3) a |
|---|---|---|---|---|
| AZ31 | 93 ± 9 | 486 ± 4 | 19.7 ± 7.2 | 76 ± 14 |
| AZ31/1.5 vol.% Al2O3 | 98 ± 2 (+5)b | 509 ± 12 (+5) | 19.0 ± 2.7 (−4) | 84 ± 15 (+11) |
| ZK60A | 93 ± 8 | 498 ± 16 | 23.2 ± 4.6 | 89 ± 12 |
| ZK60A/1.5 vol.% Al2O3 | 84 ± 7 (−10) | 532 ± 13 (+7) | 26.7 ± 0.3 (+15) | 112 ± 3 (+26) |
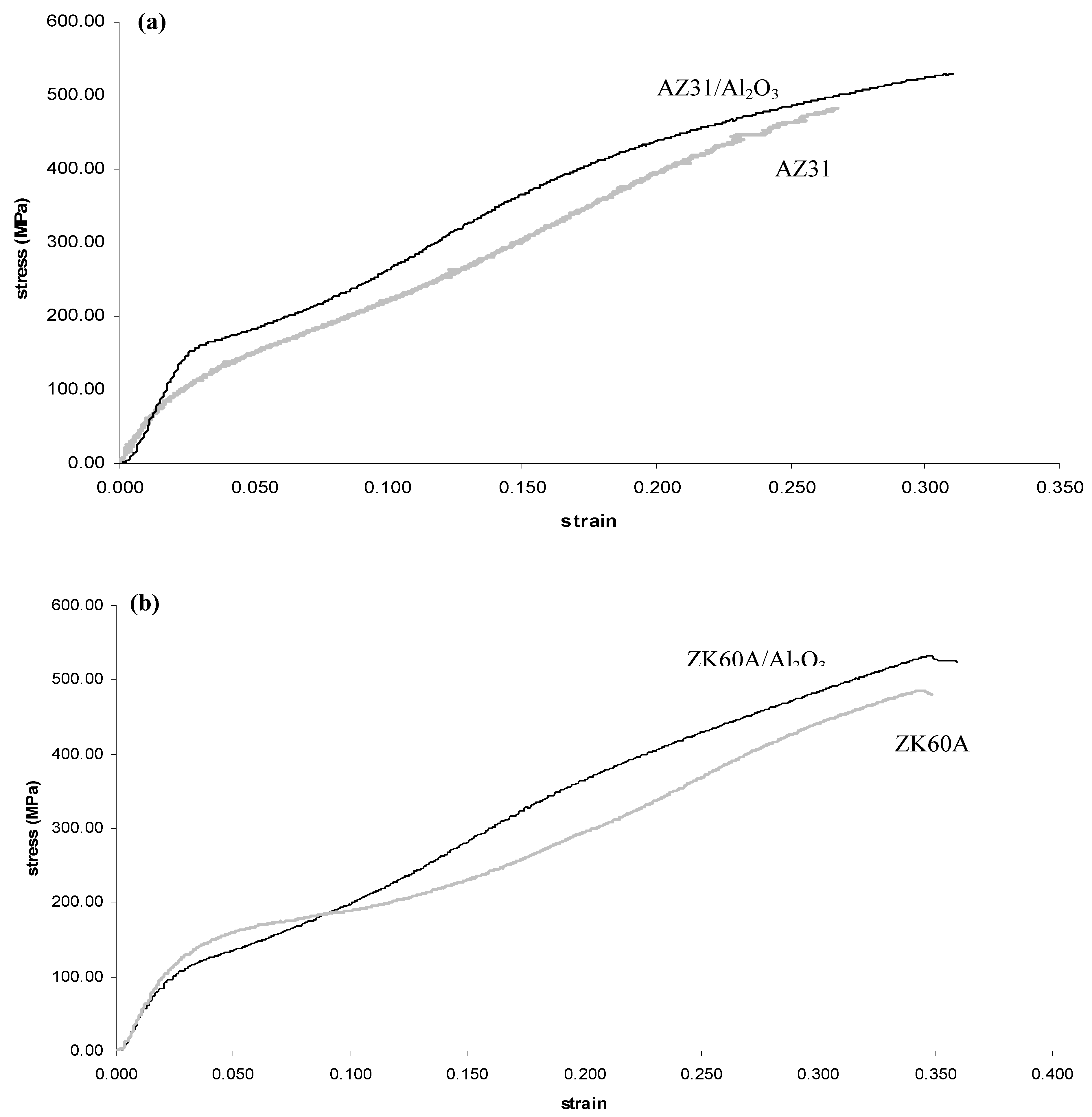
2.6. Tensile/Compressive Yield Strength Anisotropy
3. Experimental Section
3.1. Materials
3.2. Processing
3.3. Microstructural Characterization
3.4. Hardness
3.5. Tensile Testing
3.6. Compressive Testing
4. Conclusions
4.1. AZ31/Al2O3 Nanocomposite
4.2. ZK60A/Al2O3 Nanocomposite
Acknowledgments
References
- Morisada, Y.; Fujii, H.; Nagaoka, T.; Fukusumi, M. Effect of friction stir processing with SiC particles on microstructure and hardness of AZ31. Mater. Sci. Eng. A 2006, 433, 50–54. [Google Scholar] [CrossRef]
- Morisada, Y.; Fujii, H.; Nagaoka, T.; Fukusumi, M. Nanocrystallized magnesium alloy—Uniform dispersion of C60 molecules. Scr. Mater. 2006, 55, 1067–1070. [Google Scholar] [CrossRef]
- Morisada, Y.; Fujii, H.; Nagaoka, T.; Fukusumi, M. MWCNTs/AZ31 surface composites fabricated by friction stir processing. Mater. Sci. Eng. A 2006, 419, 344–348. [Google Scholar] [CrossRef]
- Lapovok, R.; Thomson, P.F.; Cottam, R.; Estrin, Y. Processing routes leading to superplastic behaviour of magnesium alloy ZK60. Mater. Sci. Eng. A 2005, 410–411, 390–393. [Google Scholar]
- Kim, W.J.; Kim, M.J.; Wang, J.Y. Superplastic behavior of a fine-grained ZK60 magnesium alloy processed by high-ratio differential speed rolling. Mater. Sci. Eng. A 2009, 527, 322–327. [Google Scholar] [CrossRef]
- Watanabe, H.; Mukai, T.; Higashi, K. Superplasticity in a ZK60 magnesium alloy at low temperatures. Scr. Mater. 1999, 40, 477–484. [Google Scholar] [CrossRef]
- Nieh, T.G.; Schwartz, A.J.; Wadsworth, J. Superplasticity in a 17 vol.% SiC particulate-reinforced ZK60A magnesium composite (ZK60/SiC/17p). Mater. Sci. Eng. A 1996, 208, 30–36. [Google Scholar] [CrossRef]
- Yan, F.; Wu, K.; Wu, G.L.; Lee, B.L.; Zhao, M. Superplastic deformation behavior of a 19.7 vol.% β-SiCw/ZK60 composite. Mater. Lett. 2003, 57, 1992–1996. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, X.; Min, Z.; Kun, W. Superplasticity and texture of SiC whiskers in a magnesium-based composite. Scr. Mater. 2005, 53, 361–365. [Google Scholar] [CrossRef]
- Sasaki, G.; Wang, W.G.; Hasegawa, Y.; Choi, Y.B.; Fuyama, N.; Matsugi, K.; Yanagisawa, O. Surface treatment of Al18B4O33 whisker and development of Al18B4O33/ZK60 magnesium alloy matrix composite. J. Mater. Proc. Technol. 2007, 187–188, 429–432. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Godbole, C.; Paramsothy, M.; Gupta, M. The role of aluminum oxide particulate reinforcements on cyclic fatigue and final fracture behavior of a novel magnesium alloy. Mater. Sci. Eng. A 2012, 532, 196–211. [Google Scholar] [CrossRef]
- Tjong, S.C. Novel nanoparticle-reinforced metal matrix composites with enhanced mechanical properties. Adv. Eng. Mater. 2007, 9, 639–652. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Effect of type of primary processing on the microstructure, CTE and mechanical properties of magnesium/alumina nanocomposites. Comp. Struct. 2006, 72, 19–26. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Effect of different types of nano-size oxide particulates on microstructural and mechanical properties of elemental Mg. J. Mater. Sci. 2006, 41, 2229–2236. [Google Scholar] [CrossRef]
- Paramsothy, M.; Chan, J.; Kwok, R.; Gupta, M. The synergistic ability of Al2O3 nanoparticles to enhance mechanical response of hybrid alloy AZ31/AZ91. J. Alloys Compd. 2011, 509, 7572–7578. [Google Scholar]
- Hassan, S.F.; Gupta, M. Development of ductile magnesium composite materials using titanium as reinforcement. J. Alloys Compd. 2002, 345, 246–251. [Google Scholar] [CrossRef]
- Perez, P.; Garces, G.; Adeva, P. Mechanical properties of a Mg-10 (vol.%) Ti composite. Compos. Sci. Technol. 2004, 64, 145–151. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Gupta, M. Enhancing thermal stability, modulus and ductility of magnesium using molybdenum as reinforcement. Adv. Eng. Mater. 2005, 7, 250–256. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Development of a novel magnesium/nickel composite with improved mechanical properties. J. Alloys Compd. 2002, 335, L10–L15. [Google Scholar] [CrossRef]
- Huard, G.; Angers, R.; Krishnadev, M.R.; Tremblay, R.; Dube, D. SiCp/Mg composites made by low-energy mechanical processing. Can. Metall. Quart. 1999, 38, 193–200. [Google Scholar] [CrossRef]
- Avedesian, M.M.; Baker, H. ASM Specialty Handbook: Magnesium and Magnesium Alloys; ASM International®: Ohio, OH, USA, 1999; pp. 12–25, 165–172, 226–248, 258–263. [Google Scholar]
- Tham, L.M.; Gupta, M.; Cheng, L. Influence of processing parameters during disintegrated melt deposition processing on near net shape synthesis of aluminium based metal matrix composites. Mater. Sci. Technol. 1999, 15, 1139–1146. [Google Scholar]
- Han, B.Q.; Dunand, D.C. Microstructure and mechanical properties of magnesium containing high volume fractions of yttria dispersoids. Mater. Sci. Eng. A 2000, 277, 297–304. [Google Scholar] [CrossRef]
- Wettability at High Temperatures; Pergamon Materials Series; Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. (Eds.) Pergamon: New York, NY, USA, 1999; Volume 3.
- Gilchrist, J.D. Extraction Metallurgy, 3rd ed; Pergamon: New York, NY, USA, 1989. [Google Scholar]
- Gupta, M.; Lai, M.O.; Soo, C.Y. Effect of type of processing on the microstructural features and mechanical properties of A1-Cu/SiC metal matrix composites. Mater. Sci. Eng. A 1996, 210, 114–122. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Effect of particulate size of Al2O3 reinforcement on microstructure and mechanical behavior of solidification processed elemental Mg. J. Alloys Compd. 2006, 419, 84–90. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Enhancing physical and mechanical properties of Mg using nanosized Al2O3 particulates as reinforcement. Metall. Mater. Trans. A 2005, 36, 2253–2258. [Google Scholar] [CrossRef]
- Hull, D.; Bacon, D.J. Introduction to Dislocations, 4th ed; Butterworth-Heinemann: Oxford, UK, 2002; pp. 43, 231. [Google Scholar]
- Mukai, T.; Yamanoi, M.; Watanabe, H.; Higashi, K. Ductility enhancement in AZ31 magnesium alloy by controlling its grain structure. Scr. Mater. 2001, 45, 89–94. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Properties and deformation behaviour of Mg-Y2O3 nanocomposites. Acta Mater. 2007, 55, 5115–5121. [Google Scholar] [CrossRef]
- Szaraz, Z.; Trojanova, Z.; Cabbibo, M.; Evangelista, E. Strengthening in a WE54 magnesium alloy containing SiC particles. Mater. Sci. Eng. A 2007, 462, 225–229. [Google Scholar] [CrossRef]
- Dai, L.H.; Ling, Z.; Bai, Y.L. Size-dependant inelastic behavior of particle-reinforced metal-matrix composites. Comp. Sci. Technol. 2001, 61, 1057–1063. [Google Scholar] [CrossRef]
- Jayaramanavar, P.; Paramsothy, M.; Balaji, A.; Gupta, M. Tailoring the tensile/compressive response of magnesium alloy ZK60A using Al2O3 nanoparticles. J. Mater. Sci. 2010, 45, 1170–1178. [Google Scholar]
- Hassan, S.F.; Gupta, M. Development of nano-Y2O3 containing magnesium nanocomposites using solidification processing. J. Alloys Compd. 2007, 429, 176–183. [Google Scholar] [CrossRef]
- Reed-Hill, R.E. Physical Metallurgy Principles, 2nd ed; D Van Nostrand Company: New York, NY, USA, 1964; pp. 192, 267, 725. [Google Scholar]
- Laser, T.; Hartig, C.; Nurnberg, M.R.; Letzig, D.; Bormann, R. The influence of calcium and cerium mischmetal on the microstructural evolution of Mg-3Al-1Zn during extrusion and resulting mechanical properties. Acta Mater. 2008, 56, 2791–2798. [Google Scholar] [CrossRef]
- Bohlen, J.; Yi, S.B.; Swiostek, J.; Letzig, D.; Brokmeier, H.G.; Kainer, K.U. Microstructure and texture development during hydrostatic extrusion of magnesium alloy AZ31. Scr. Mater. 2005, 53, 259–264. [Google Scholar] [CrossRef]
- Gupta, M.; Lai, M.O.; Lim, S.C. Regarding the processing associated microstructure and mechanical properties improvement of an Al-4.5 Cu alloy. J. Alloys Compd. 1997, 260, 250–255. [Google Scholar] [CrossRef]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Paramsothy, M.; Chan, J.; Kwok, R.; Gupta, M. Al2O3 Nanoparticle Addition to Commercial Magnesium Alloys: Multiple Beneficial Effects. Nanomaterials 2012, 2, 147-162. https://doi.org/10.3390/nano2020147
Paramsothy M, Chan J, Kwok R, Gupta M. Al2O3 Nanoparticle Addition to Commercial Magnesium Alloys: Multiple Beneficial Effects. Nanomaterials. 2012; 2(2):147-162. https://doi.org/10.3390/nano2020147
Chicago/Turabian StyleParamsothy, Muralidharan, Jimmy Chan, Richard Kwok, and Manoj Gupta. 2012. "Al2O3 Nanoparticle Addition to Commercial Magnesium Alloys: Multiple Beneficial Effects" Nanomaterials 2, no. 2: 147-162. https://doi.org/10.3390/nano2020147





