Preparation and Characteristics of SiOx Coated Carbon Nanotubes with High Surface Area
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of SiOx-CNT
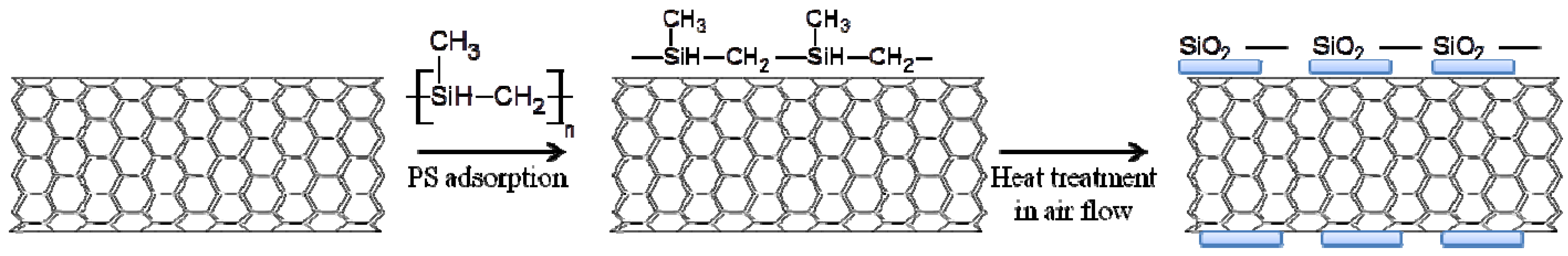
2.3. Characterization
3. Results and Discussion
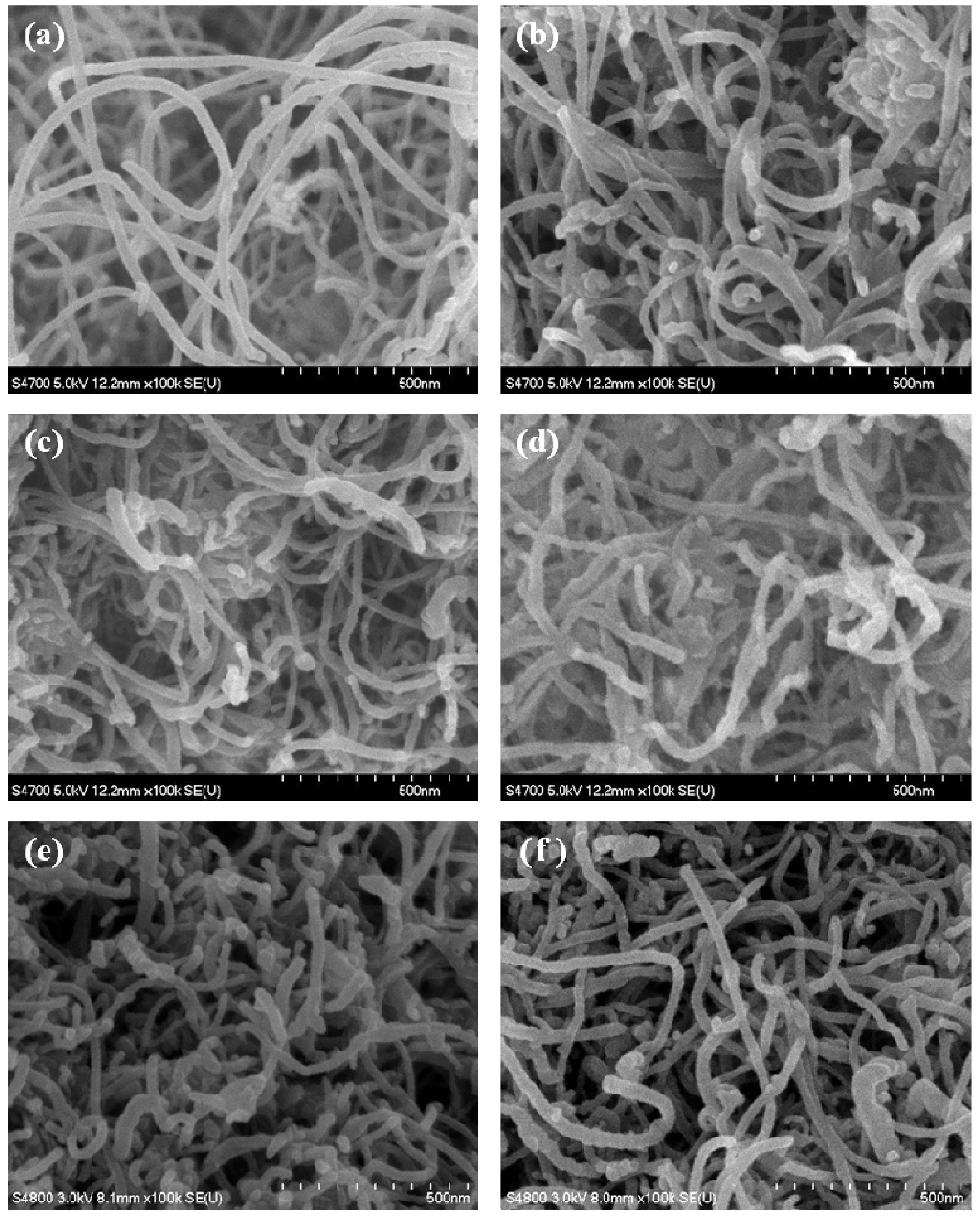
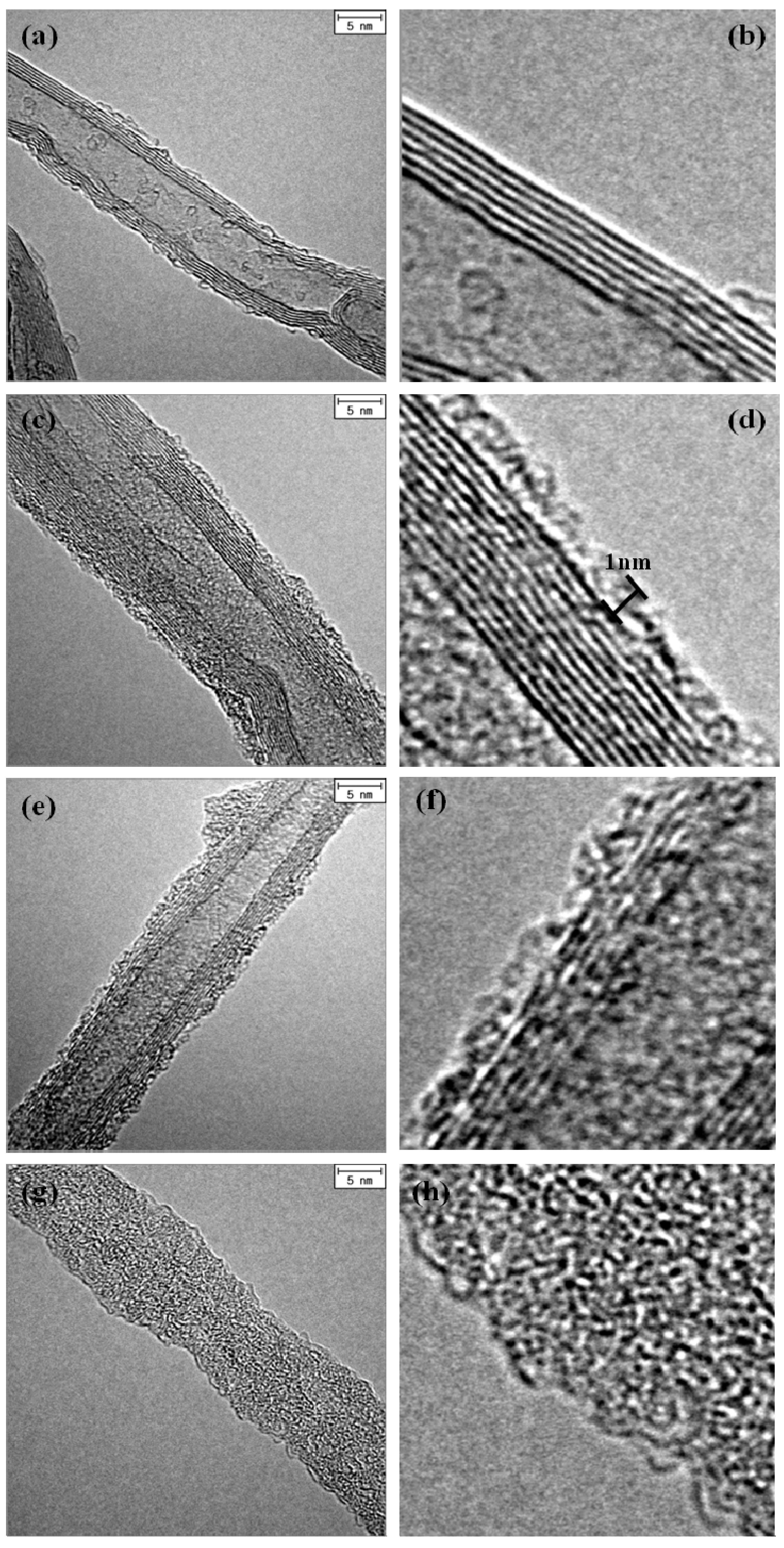
3.1. Morphology of SiOx-CNT Composites
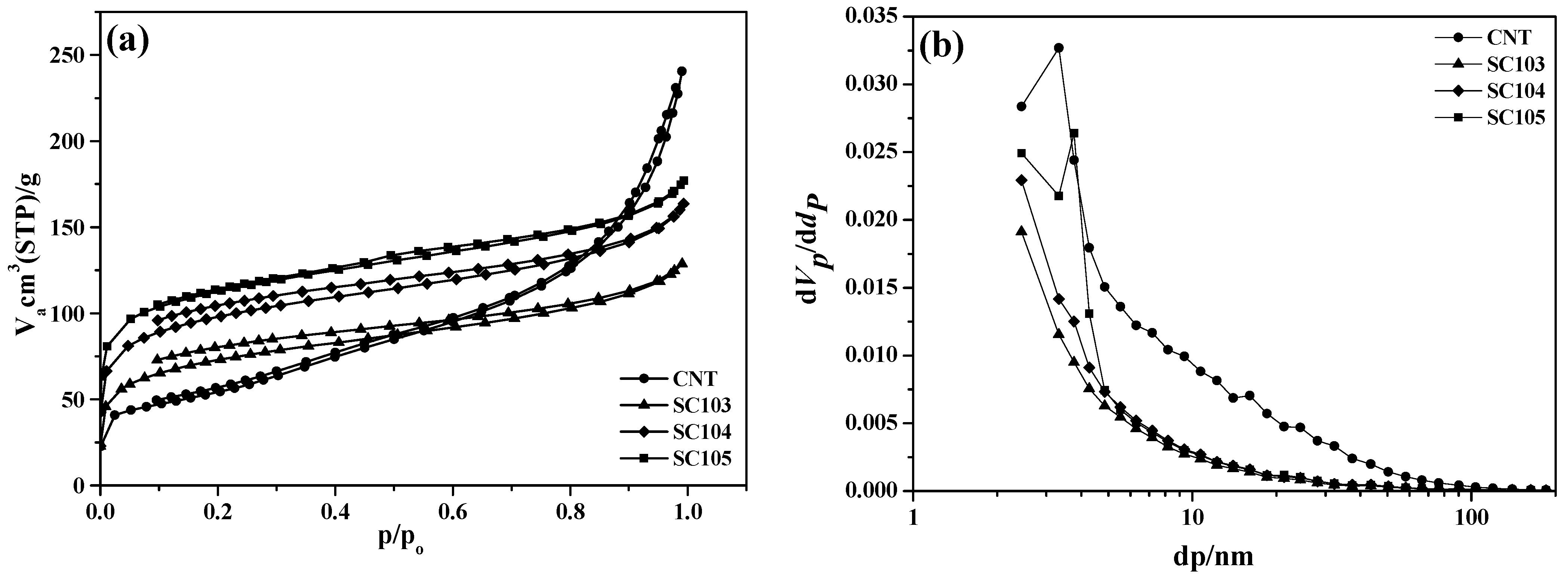
3.2. Surface Properties of SiOx-CNT
| Sample | Content (wt %) | Heat treatment conditions | Specific surface area (m2/g) | Total pore volume (cm3/g) | |
|---|---|---|---|---|---|
| SiOx | CNT | ||||
| CNT | 0 | 100 | – | 198 | 0.372 |
| SC103 | 48.8 | 51.2 | 300 °C, air, 2h | 261 | 0.199 |
| SC104 | 48.8 | 51.2 | 400 °C, air, 2h | 352 | 0.253 |
| SC105 | 48.8 | 51.2 | 500 °C, air, 2h | 405 | 0.273 |
| SC107 | 100 | 0 | 700 °C, air, 2h | 671 | 0.768 |
| SC203 | 32.1 | 67.9 | 300 °C, air, 2h | 214 | 0.317 |
| SC204 | 32.1 | 67.9 | 400 °C, air, 2h | 315 | 0.388 |
| SC205 | 32.1 | 67.9 | 500 °C, air, 2h | 335 | 0.357 |
| SC303 | 26.9 | 23.1 | 300 °C, air, 2h | 220 | 0.344 |
| SC304 | 26.9 | 23.1 | 400 °C, air, 2h | 250 | 0.384 |
| SC305 | 26.9 | 23.1 | 500 °C, air, 2h | 328 | 0.434 |
3.3. XPS Analysis of the Chemical Status of the SiOx-CNT Composites
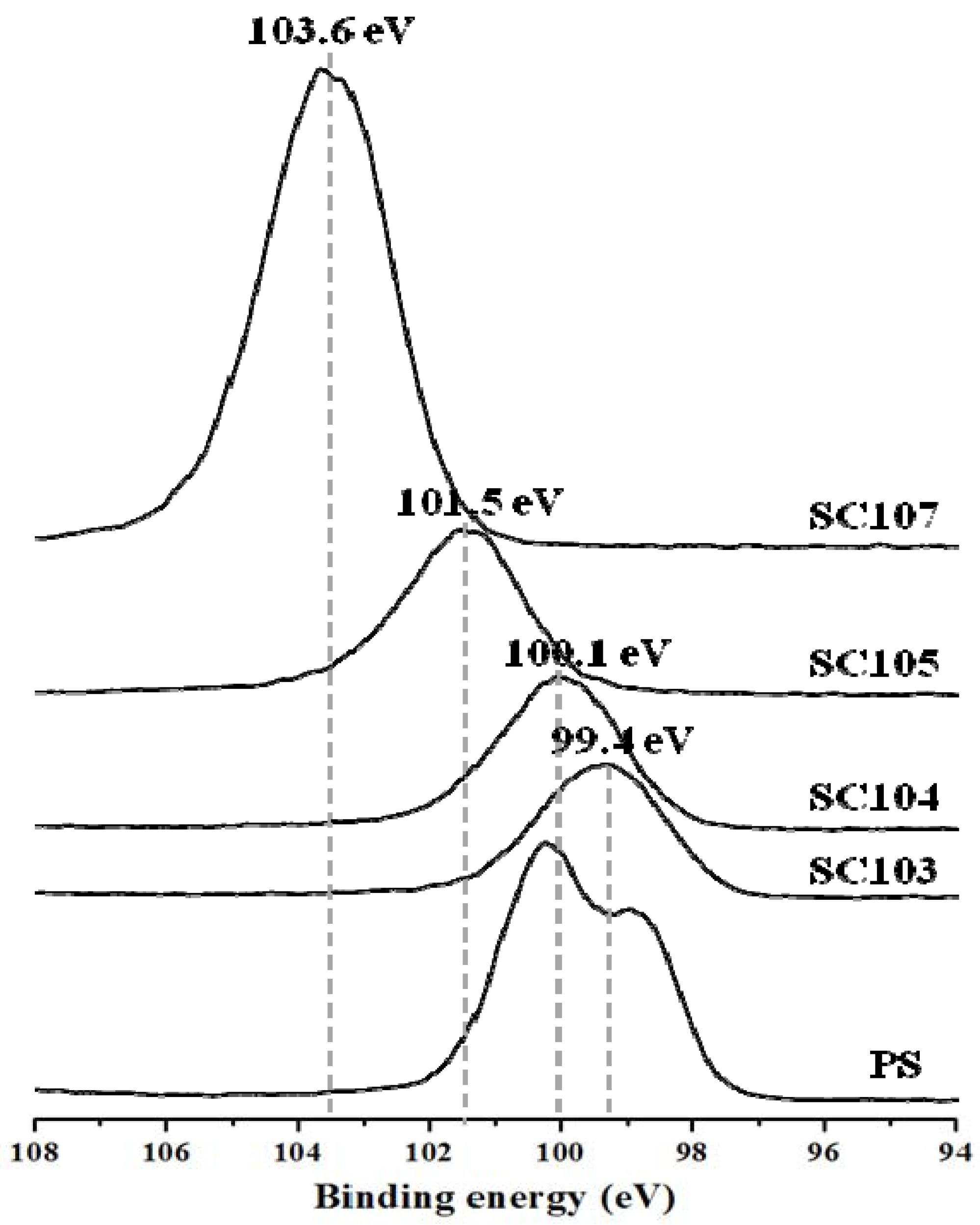
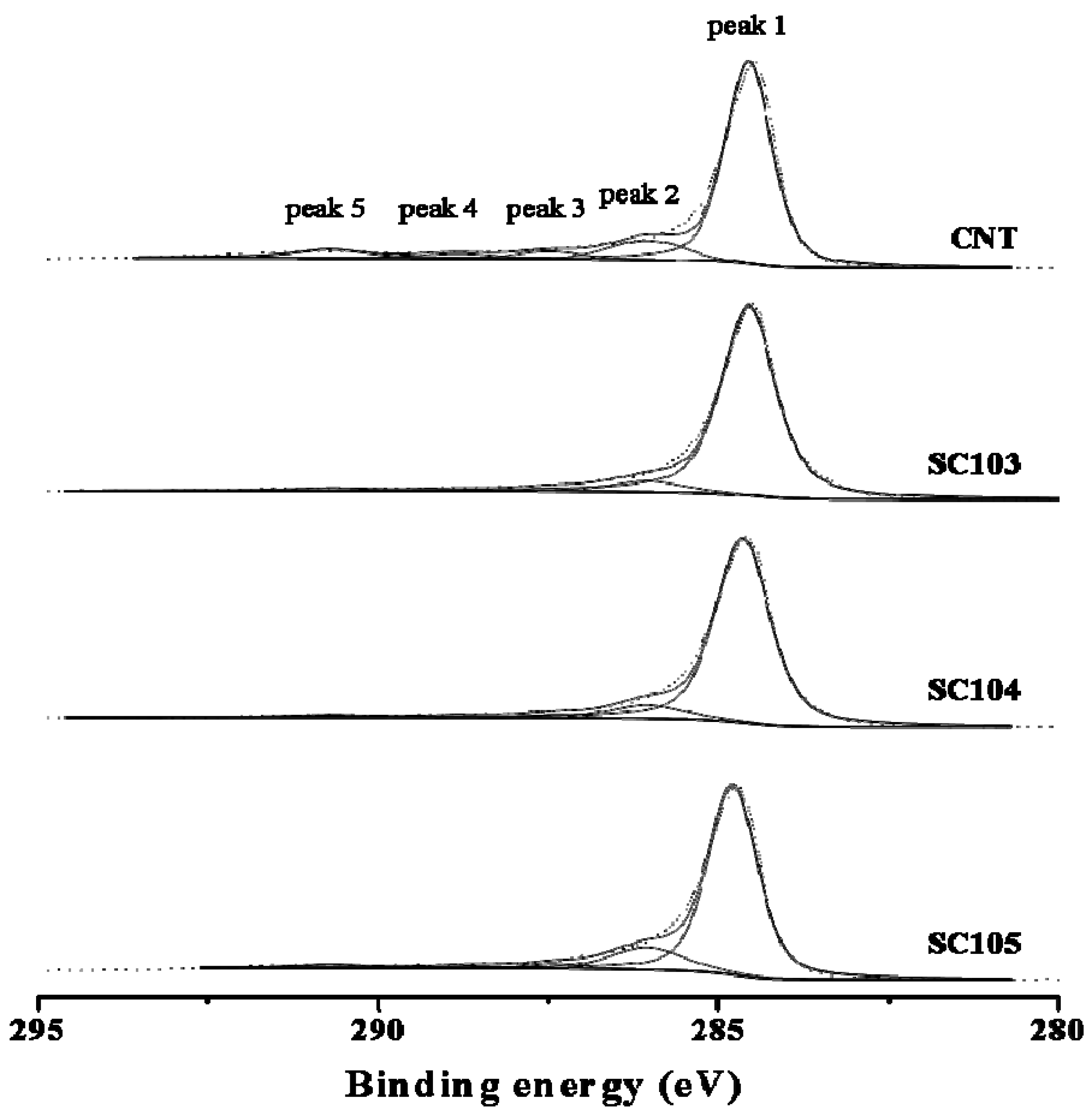
3.4. XRD Analysis of the SiOx-CNT Composites
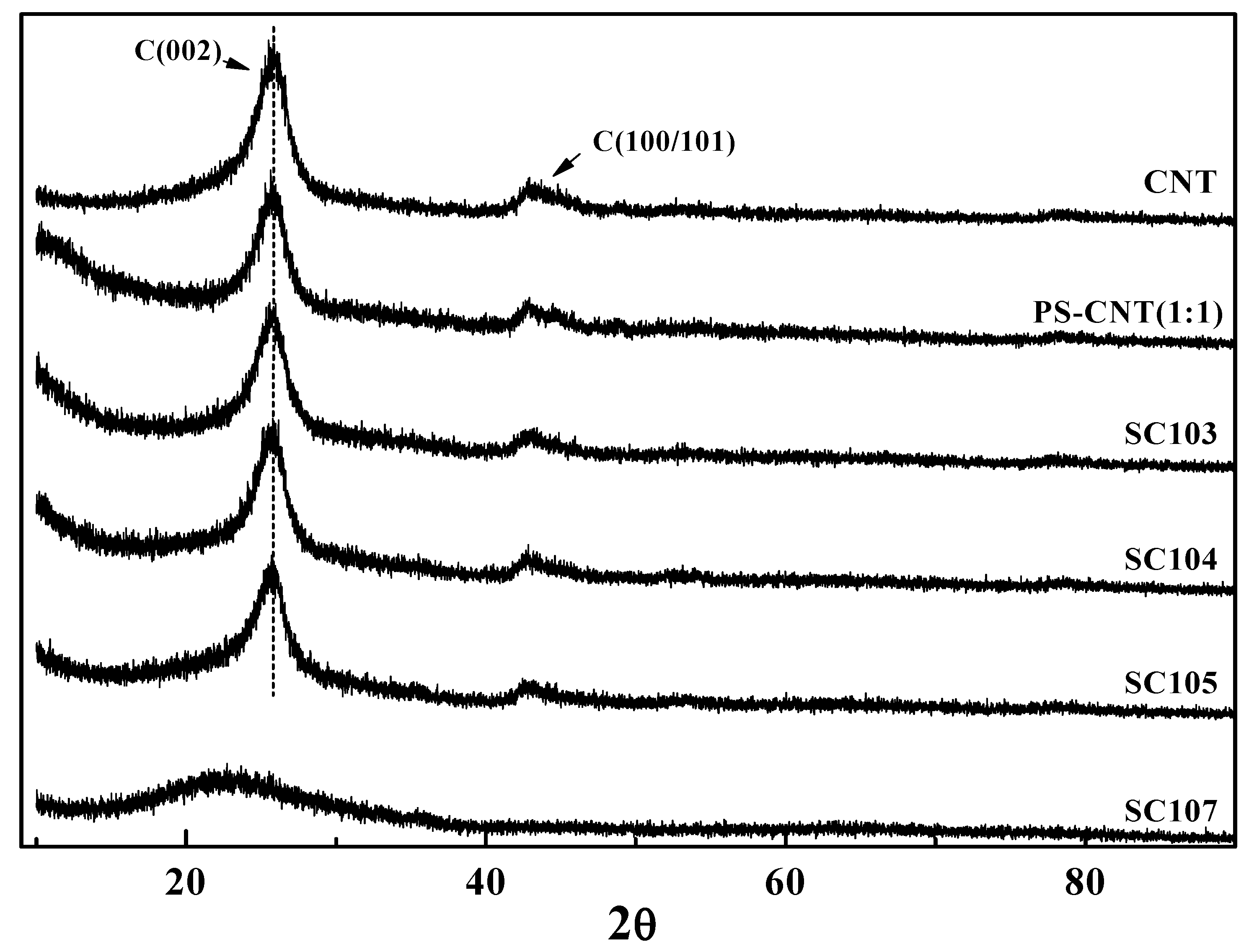
4. Conclusions
Acknowledgments
References
- Song, H.; Qiu, X.; Li, F.; Zhu, W.; Chen, L. Ethanol electro-oxidation on catalysts with TiO2 coated carbon nanotubes as support. Electrochem. Commun. 2007, 9, 1416–1421. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, C.; Wang, T. The enhanced ethanol sensing properties of multi-walled carbon nanotubes/SnO2 core/shell nanostructures. Nanotechnol. 2006, 17, 3012–3017. [Google Scholar] [CrossRef]
- Varghese, O.K.; Kichambre, P.D.; Gong, D.; Ong, K.G.; Dickey, E.C.; Grimes, C.A. Gas sensing characteristics of multi-wall carbon nanotubes. Sens. Actuators B Chem. 2001, 81, 32–41. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1804. [Google Scholar]
- Sinha, N.; Ma, J.; Yeow, J.T.W. Carbon nanotube-based sensors. J. Nanosci. Nanotechnol. 2006, 6, 573–590. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar]
- Jiang, L.; Gao, L. Fabrication and characterization of ZnO-coated multi-walled carbon nanotubes with enhanced photocatalytic activity. Mater. Chem. Phys. 2005, 91, 313–316. [Google Scholar] [CrossRef]
- Gong, K.P.; Yu, P.; Su, L.; Xiong, S.X.; Mao, L.Q. Polymer-assisted synthesis of manganese dioxide/carbon nanotube nanocomposite with excellent electrocatalytic activity toward reduction of oxygen. J. Phys. Chem. C 2007, 111, 1882–1887. [Google Scholar]
- Pan, L.; Konishi, Y.; Tanaka, H. Effect of MgO coating on field emission of a stand-alone carbon nanotube. J. Vac. Sci. Technol. B 2007, 25, 1581–1583. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Dallas, P. Preparation of a water-dispersible carbon nanotube-silica hybrid. Carbon 2007, 45, 2136–2139. [Google Scholar] [CrossRef]
- Bottini, M.; Tautz, L.; Huynh, H.; Monosov, E.; Bottini, N.; Dawson, M.I.; Bellucci, S.; Mustelin, T. Covalent decoration of multi-walled carbon nanotubes with silica nanoparticles. Chem. Commun. 2005, 6, 758–760. [Google Scholar]
- Riggs, J.E.; Walker, D.B.; Carroll, D.L.; Sun, Y.P. Optical limiting properties of suspended and solubilized carbon nanotubes. J. Phys. Chem. B 2000, 104, 7071–7076. [Google Scholar]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of nanotube. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar]
- Hou, P.X.; Liu, C.; Cheng, H.M. Purification of carbon nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 2, 180–192. [Google Scholar] [CrossRef]
- Gojny, F.H.; Nastalczyk, J.; Roslaniec, Z.; Schulte, K. Surface modified multi-walled carbon nanotubes in CNT/epoxy composites. Chem. Phys. Lett. 2003, 370, 820–824. [Google Scholar] [CrossRef]
- Liu, L.; Qin, Y.; Guo, Z.X.; Zhu, D. Reduction of solubilized multi-walled carbon nanotubes. Carbon 2003, 41, 331–335. [Google Scholar] [CrossRef]
- Fu, K.F.; Sun, Y.P. Dispersion and solubilization of carbon nanotubes. J. Nanosci. Nanotechnol. 2003, 3, 351–364. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Georgakilas, V.; Prato, M. Soluble carbon nanotubes. Chem. Eur. J. 2003, 9, 4000–4008. [Google Scholar] [CrossRef]
- Shallenberger, J.R. Determination of chemistry and microstructure in SiOx (0.1 < x < 0.8) films by X-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 1996, 14, 693–698. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Llewellyn, S.A.; Tobias, G.; Ward, M.A.H.; Huh, Y.; Green, M.L.H. The role of carboxylated carbonaceous fragments in the functionalization and spectroscopy of a single-walled carbon-nanotube material. Adv. Mater. 2007, 19, 883–887. [Google Scholar]
- Gardner, S.D.; Singamsetty, C.S.K.; Booth, G.L.; He, G.; Pittman, C.U. Surface characterization of carbon fibers using angle-resolved XPS and ISS. Carbon 1995, 33, 587–595. [Google Scholar] [CrossRef]
- Wang, X.; Miao, X.; Li, Z.; Deng, W. Fabrication of mesoporous silica hollow spheres using triblock copolymer PEG–PPG–PEG as template. J. Non-Cryst. Solids 2010, 356, 898–905. [Google Scholar]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, A.; Lim, S.; Peck, D.-H.; Kim, S.-K.; Lee, B.; Jung, D. Preparation and Characteristics of SiOx Coated Carbon Nanotubes with High Surface Area. Nanomaterials 2012, 2, 206-216. https://doi.org/10.3390/nano2020206
Kim A, Lim S, Peck D-H, Kim S-K, Lee B, Jung D. Preparation and Characteristics of SiOx Coated Carbon Nanotubes with High Surface Area. Nanomaterials. 2012; 2(2):206-216. https://doi.org/10.3390/nano2020206
Chicago/Turabian StyleKim, Aeran, Seongyop Lim, Dong-Hyun Peck, Sang-Kyung Kim, Byungrok Lee, and Doohwan Jung. 2012. "Preparation and Characteristics of SiOx Coated Carbon Nanotubes with High Surface Area" Nanomaterials 2, no. 2: 206-216. https://doi.org/10.3390/nano2020206




