1. Introduction
To counterbalance the shrinking market of consumer paper products, the pulp and paper industries are intensifying their research efforts to diversify the use of wood raw material. Strategies to produce value-added products from lignocellulosic biomass have emerged [
1], particularly in the field of development, production and application of nanocellulose [
2,
3]. Literature has shown that cellulose nanofibers can be produced by an oxidation system involving the use of a catalyst of TEMPO, sodium bromide (NaBr) and sodium hypochlorite (NaOCl), followed by mechanical dispersion [
4,
5,
6,
7,
8]. Our recent studies indicate that the oxidation efficiency can be significantly improved with 15% to 30% increase in carboxylate content [
9,
10,
11,
12] when the oxidation was conducted in the presence of ultrasonic cavitation.
Despite numerous studies [
4,
5,
6,
7,
8,
9,
10,
11,
12] on the preparation of cellulose nanofibers, the mechanism of mechanical dispersion has rarely been investigated. The use of a blender does generate great shear rate, but higher shear rate mixers might be more efficient in nanofiber dispersion and are available on the market [
13]. High shear rate equipment such as those provided by IKA Works Inc. (USA) are used in the food [
14] and pharmaceutical fields [
15]. Here are a few examples of applications: homogenization, dispersion, emulsification, grinding, dissolving, chemical reaction and cell disruption [
13].
Some researchers [
16,
17] examined the correlation between the quality of nanocellulose dispersion and rheological measurements. The following exponential relationship between the shear rate (
γ) and shear stress (
σ) has been proposed [
16] and expressed in Equation (1): where
K is the viscosity coefficient and
n, the flow behavior index. By definition, shear thinning is when the viscosity of a fluid decreases with increasing shear rate while shear thickening is the opposite. When using Equation (1), if
n is equal to 1, then the fluid is Newtonian. When it is less than 1, it indicates a shear thinning fluid. In contrast, when
n is greater than 1, it indicates a shear thickening fluid. The reader should note that Equation (1) is not universal but can be successfully applied to a wide range of fluids, which is the case in our study. Rheological measurements can be associated to particle morphology and aggregation potential.
The main objective of this study is to examine the influence of high shear dispersion parameters on the characteristics of nanocellulose produced by TEMPO-mediated oxidation of Kraft pulp in the presence of ultrasound waves.
2. Results and Discussion
Previously, we have shown that it is possible to scale up the production of oxidized cellulose fibers by performing the TEMPO-mediated oxidation in a flow-through sonoreactor system [
18]. The optimal ultrasonic conditions determined in our previous research [
18] on production of oxidized cellulose with carboxylate content of 935 mmol/kg, were applied. The carboxylate content of cellulose produced in our experiments, which is superior to that indicated in the literature, indicates the efficiency of ultrasound conditions of our experiments. As a reference, the carboxyl density of unoxidized bleached Kraft pulps is known to be less than 100 mmol/kg [
19]. According to Saito
et al. [
5], the oxidized fibers with carboxylate content less than 600 mmol/kg were mostly unfibrillated by the mechanical treatment and maintained their original fibrous morphologies. Since our oxidized pulp carried a carboxylate content which is much higher than that reported by Saito
et al. [
5], these oxidized fibers should be readily dispersed.
2.1. Experimental Error
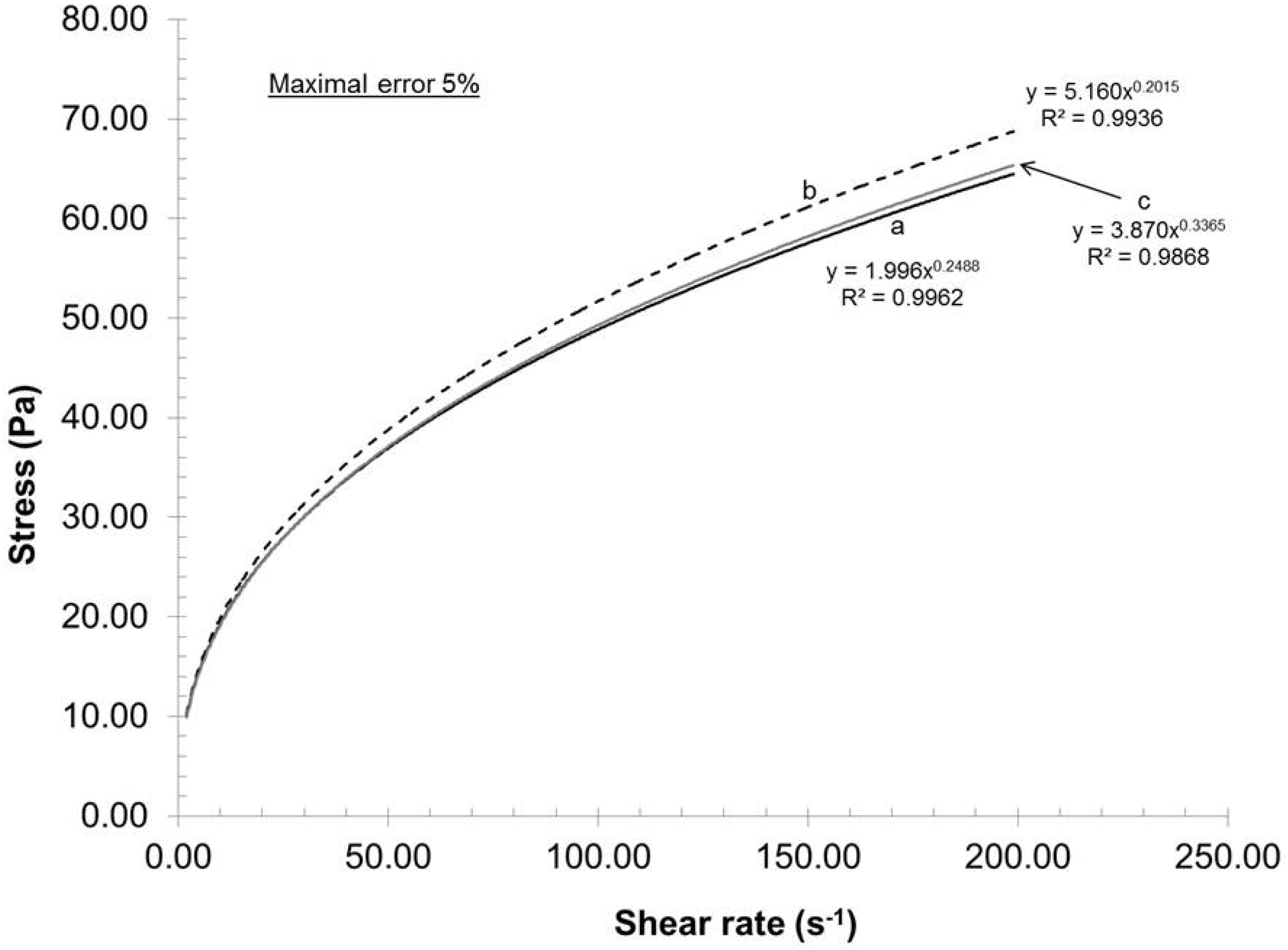
To evaluate the experimental error of the mechanical dispersion and rheological measurements, three trials (a, b, c) were executed using pulps having the same carboxylate content and under identical dispersion conditions (recycle rate, gap, pH and consistency). The experimental error of each trial was then evaluated using a rheometer (ATS RheoSystems) and the curve fitted results are illustrated in
Figure 1. As the correlation coefficient is close to 1, the fitted data trends are identical to experimental data trend; all experimental data points are omitted for the purpose of clarity.
Figure 1.
Experimental error assessment.
Figure 1.
Experimental error assessment.
Figure 1 shows that curves a and c were practically identical, whereas the stress of curve b was in general larger than those of curves a and c. As the gap between the curves increased with increasing shear rate, the maximal experimental error will be reported in this study. From these three experiments, the maximal error achieved was 5% in stress. As a result, based on this value, the entire process can be considered as highly reproducible and the curves reported in this study as representative.
2.2. Optimization of Mechanical Dispersion of Oxidized Fibers Using Rheological Curves
2.2.1. Influence of Consistency
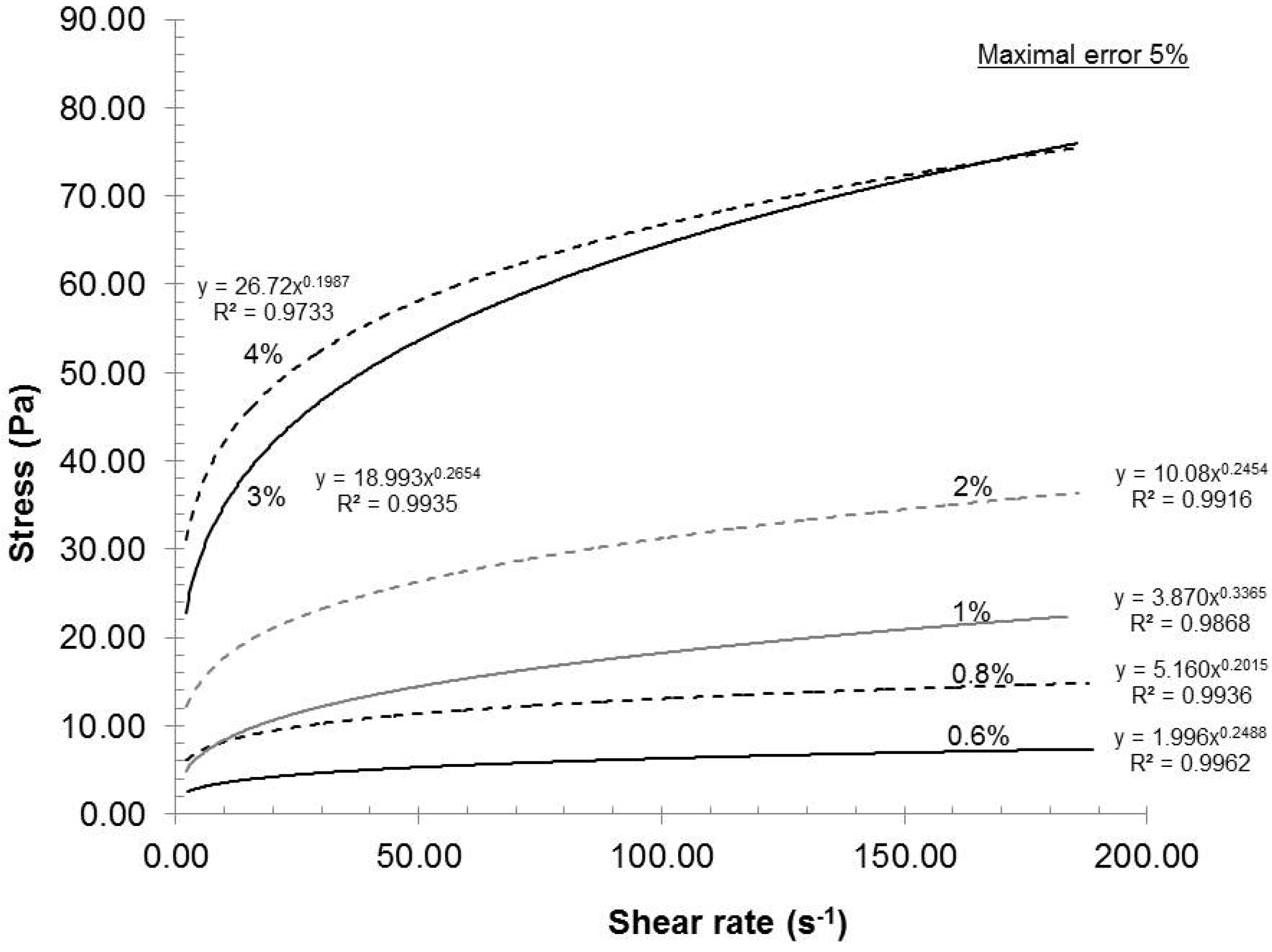
The feed consistency was varied to access its influence on mechanical dispersion of the oxidized pulp, which has a carboxylate content of 935 mmol/kg.
Figure 2 presents the relationship between the consistency and the rheological behavior of the gels under these conditions: Rotor-stator gap 0.042 mm, recycle rate 200 mL/min and pH 7. As seen in
Figure 2, the gels exhibited shear thinning rheological behaviors at all consistencies. Such a result is not uncommon, because the gels are composed of nanofibers and fibers (microfibers) of cylindrical shape with network capabilities (hydrogen bond). When the shear rate increased, the fiber and nanofiber network slowly broke up into individual entities and they oriented themselves in a parallel formation, thus reducing the final viscosity of the gels (
Figure 3). Such behavior is frequently observed in the dispersion of polymer. The polymer elements are similar to the oxidized fibers in shape [
20]. These results indicated that when the shear rate increased, the resulting viscosity decreased while the applied stress increased.
Figure 2.
Effect of consistency on the rheological evolution of the gels.
Figure 2.
Effect of consistency on the rheological evolution of the gels.
Figure 3.
Effect of consistency on the viscosity of the gels.
Figure 3.
Effect of consistency on the viscosity of the gels.
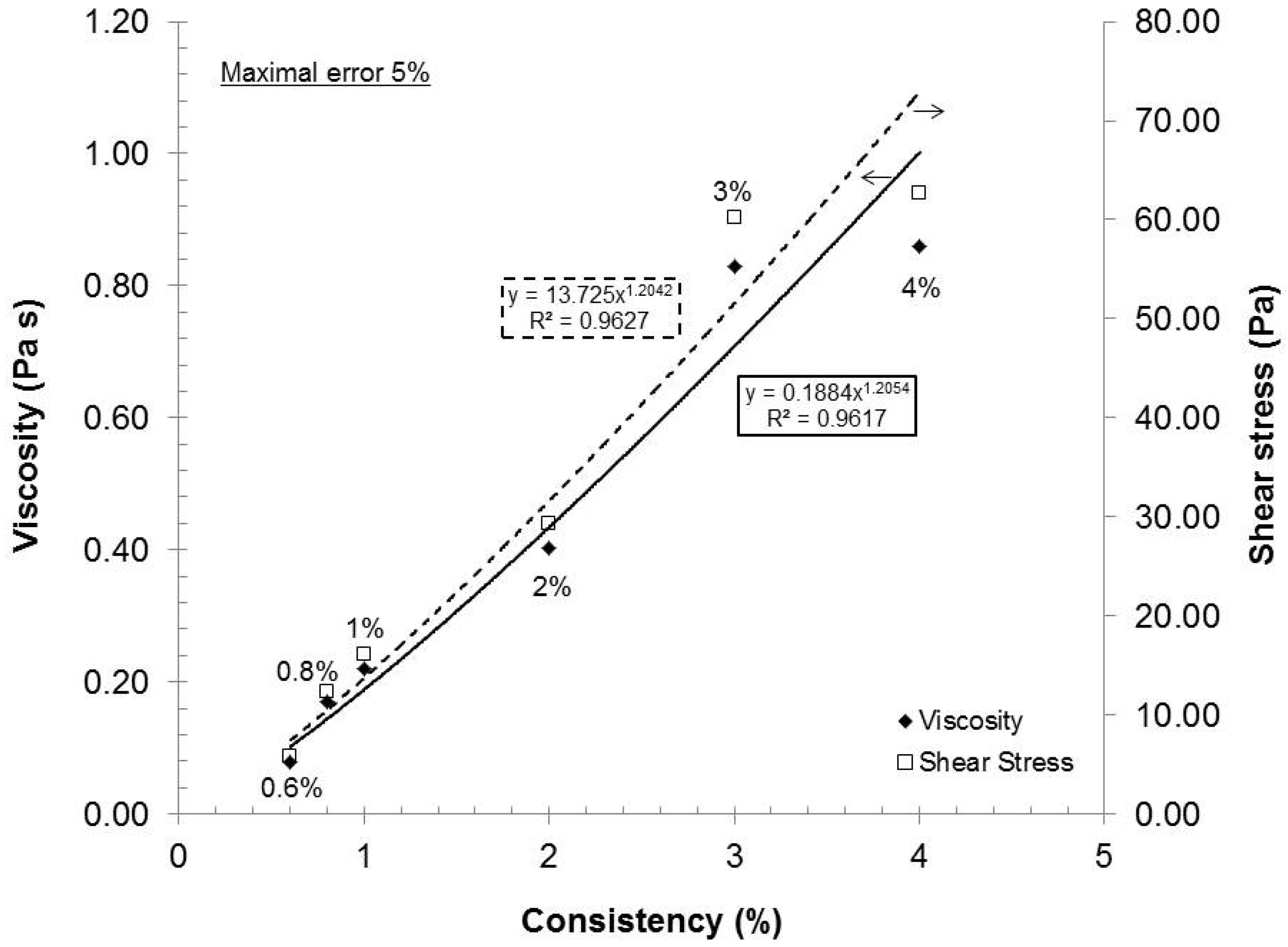
As the consistency increased (increased fiber concentration), the statistical probability of contact, initial viscosity or stress also augmented. The final stress can then be correlated to the network and gel strength or dispersion quality. If we chose an arbitrary shear stress (e.g., 73.42 s
−1) in
Figure 2 and
Figure 3, and compare the resulting viscosities in
Figure 4, we can conclude that the rheological response of stress or viscosity to the shear rate does not follow a true linear trend but rather a subtle power function. Such behavior is expected as the statistical probability of collision between fibers increases exponentially to their number. This characteristic has been observed for TEMPO-oxidized fibers [
16]. From a gel quality standpoint, 4% consistency was the best option. But, at this consistency, the pumping needs a lot of power and, therefore, great energy loss is transferred as heat in the gel. The resulting heating then requires more cooling to maintain the experimental temperature. In this aspect, further laboratory experiments were made at 2% consistency.
Figure 4.
Gel viscosity evolution trend for the various consistencies at shear rate of 73.42 s−1.
Figure 4.
Gel viscosity evolution trend for the various consistencies at shear rate of 73.42 s−1.
2.2.2. Influence of Stator-Rotor Gap
In a rotor and stator configuration, a reduced gap will develop higher shear force that should result in an increase in stress on individual fibers.
Figure 5 presents the rheological curves as a function of gap at 2% consistency, 200 mL/min recycle rate and pH 7. In this figure, we can clearly identify two groups. The biggest gap had resulted in a network with lower stress capacity as compared to the other two gap settings. However, within the maximal error of 5%, the curves for gaps of 0.042 and 0.1 mm were similar. At maximal motor speed, the maximal admissible gap, the gap before losing dispersion similarity to 0.042 mm, will be found between 0.1 and 0.281 mm. Additional reduction in gap will not increase the dispersion quality and, in fact, would increase the heat generated by friction, causing useless energy loss. From a laboratory stand point, the 0.042 mm gap is identified as optimal but further investigation will be required if thermal energy is to be taken into account.
Figure 5.
Effect of stator-rotor gap on the rheological evolution of the gels.
Figure 5.
Effect of stator-rotor gap on the rheological evolution of the gels.
2.2.3. Influence of Recirculation Rate
In the dispersion unit, a volume of 2 L of oxidized pulp suspension circulated in a closed loop for a total time of 1 h. The recirculation rate is, therefore, directly related to the amount of passes or cycles that the pulp will undergo. A low recycle rate will increase the retention time in the dispersion unit, resulting in a lower number of passes. In contrast, a high recycle rate will reduce the retention time and increase the number of passes.
Figure 6 presents the effect of recycle rate on the dispersion quality at 2% consistency, a rotor-stator gap of 0.042 mm and pH 7.
Figure 6.
Effect of recirculation rate on the rheological evolution of the gels.
Figure 6.
Effect of recirculation rate on the rheological evolution of the gels.
From the results shown in
Figure 6 and considering the statistical error, the effect of the retention time was not evident for flow rates between 200 and 800 mL/min. However, as the flow rate increased, the dispersion quality was drastically reduced. Consequently, the condition of fewer passes at high retention time gave better gel quality than those with a greater number of passes at lower retention time. This effect is similar to that of a thermomechanical pulp refiner where the fiber quality is greatly influenced by the feed rate [
21]. A recycle rate of 200 mL/min is then preferable for laboratory experiment as well as for the eventual industrial production because the dispersion stage would require smaller equipment, thus smaller investment.
2.2.4. Influence of Dispersion pH
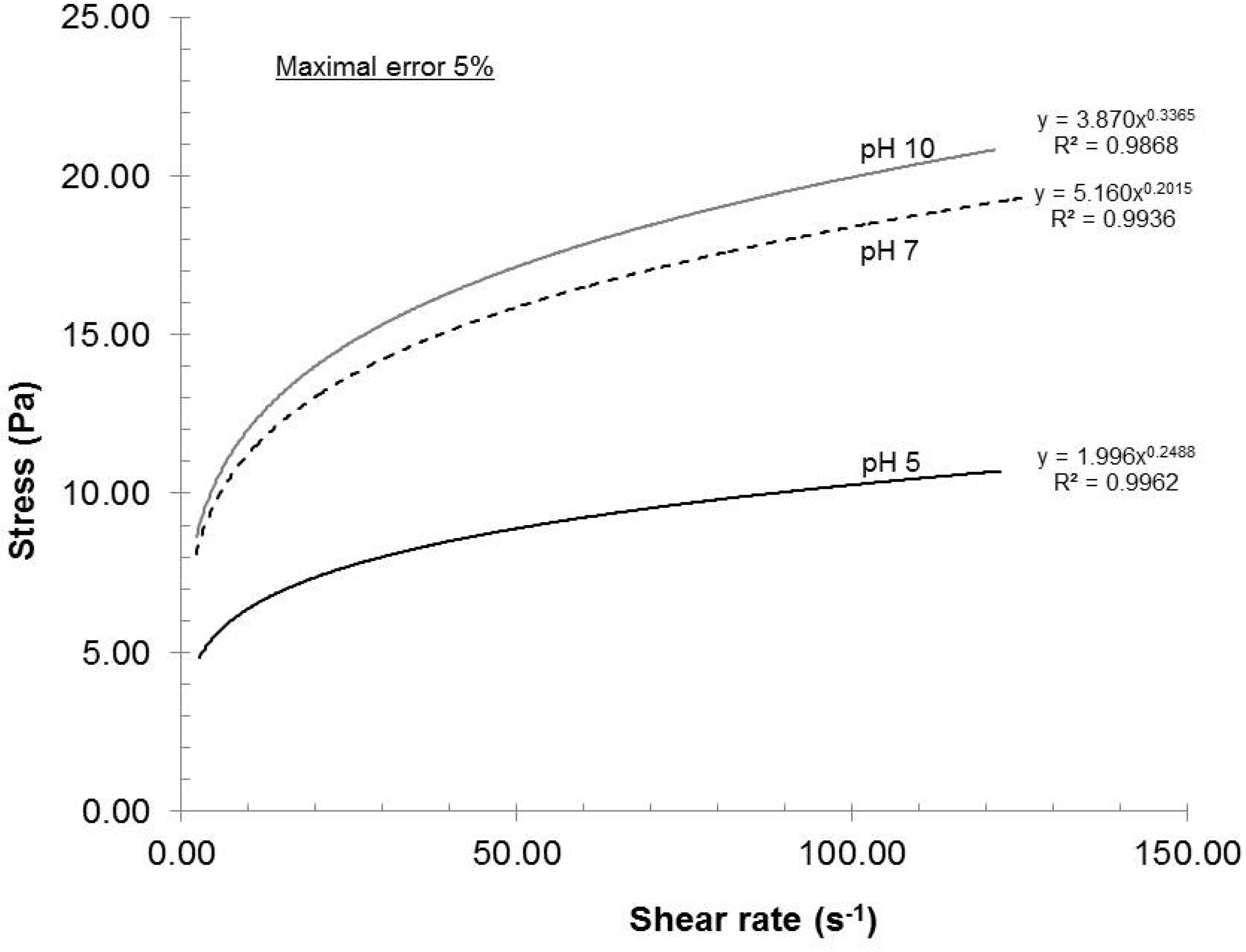
As reported in References [
4] and [
16], the dispersion pH greatly influences the surface charge of the nanofibers. With low pH, the carboxyl groups become protonated and lose their charge. Consequently, the beneficial electrostatic repulsion is greatly diminished and will show up in the rheological profile (
Figure 7).
Figure 7.
Effect of dispersion pH on the rheological evolution of the gels.
Figure 7.
Effect of dispersion pH on the rheological evolution of the gels.
The rheological profiles shown in
Figure 7 are clear examples of this situation. The dispersion quality was the lowest at pH 5 and the highest at pH 10, meaning that a minimum surface charge was observed at pH 5 and a maximum value was noted at pH 10. However, the difference in surface charge between pH 7 and pH 10 was almost within the experimental error. The pH 5 clearly gave the worst quality measured. Although pH 10 yielded the best results, a dispersion pH of 7 should be chosen in order to prevent hydrolysis of pulp when the sample is stored at pH 10.
3. Experimental Section
3.1. Materials
A commercial never-dried bleached Kraft wood pulp (carboxyl content: 53 mmol/kg) was provided by Fraser Paper (Thurso, Canada) and used in this study as native cellulose fibers. Free radical 4-acetamido-TEMPO (2,2,6,6-tetramethylpiperidin-1-oxyl) was purchased from Sigma-Aldrich (Canada) and sodium bromide from Fisher Scientifics (Canada). All chemicals were ACS reagent grade. Sodium hypochlorite at 6.0% was purchased at the supermarket.
3.3. Measurement of Carboxylate Group Content
Carboxylic acid groups generated during oxidation were determined with a Dosimat 765 titrator from Metrohm (USA) and a conductivity meter (Orion 3Star) from Thermo (USA). The carboxylate content of oxidized pulp was measured using conductimetric titration [
22]. Briefly, a sample (2 g, o.d. basis) of oxidized fibers was first washed twice—using 250 mL 0.1 N HCl—to convert the carboxylate groups into free carboxyl. The treated pulp was thoroughly washed twice with distilled water (2 × 500 mL) to remove any excess acid. The washed sample (acidified pulp) was dispersed in 450 mL of 0.001 N NaCl with an addition of 5.0 mL of 0.1 N HCl. The suspension was then titrated with 0.1 N NaOH. After the titration, the sample was thickened by filtration and dried at 105 °C to obtain its o.d. weight for the calculation of carboxylate content.
3.4. High Shear Dispersion
High shear dispersion was performed in a wet colloid milling apparatus (MK 2000/4) from IKA Works, Inc. (USA). The oxidized pulp suspension (2 L) was pumped from an agitated tank between a conical rotor and a stator in the MK mill as shown in
Figure 9a. The gap between the rotor and the stator is adjustable to achieve various shear rates.
Figure 9b shows a schematic of the entire process. The residence time in the MK mill is controlled manually by the recirculation valve and the feed pump speed. The pulp or product then passes through a heat exchanger, cooled with tap water to maintain the temperature at 25 °C. The same cooling water was used to remove heat from the bearing seal loop to ensure proper operation of the mill. In our work, the mill was operated in closed loop for a given amount of time (1 h); in a typical industrial application, the process would be continuous. The experimental conditions explored were: 0.6% to 4% consistency of the feed pulp, 0.042 to 0.281 mm for the stator-rotor gap, 200 to 2400 mL/min for the recirculation rate and at pH 5, 7 and 10. The normal operating conditions of this laboratory setup were: 2% consistency, 0.073 mm gap, 200 mL/min recirculation rate, 25 °C, pH 7 and 1 h.
Figure 9.
(a) Schematic representation of the operation principle; (b) Schematic diagram of the colloid milling apparatus (MK 2000/4) from Process IKA Works, Inc.
Figure 9.
(a) Schematic representation of the operation principle; (b) Schematic diagram of the colloid milling apparatus (MK 2000/4) from Process IKA Works, Inc.
3.5. Rheological Measurements
The quality of the dispersion was measured by rheological measurement by means of a STRESSTECH rheometer (ATS RheoSystems), equipped with a cone-plate measurement head (40 mm diameter, 4° angle). With this measurement head, shear rate from 2 to 200 s−1 were explored upstream and downstream. Sample stress and viscosity were then related to the shear rate to study the rheological behavior such as shear thinning, shear thickening or even Newtonian fluid. The instrument used an automatic real time inertia compensation to make high precision measurements and it was also equipped with a patented quantitative normal force capability. All experiments were carried out at room temperature (23 °C).
4. Conclusions
Since we have determined that the exponent n of the exponential relation (1) is always below 1, all nanofibers gels produced in this study exhibit rheological behavior known as shear thinning. The final stress can then be correlated to the network and gel strength or dispersion quality.
Consistency optimization reveals that the rheological response of stress or viscosity to the shear rate does not follow a linear trend but rather a power function. From a gel quality standpoint, 4% consistency is the best condition; but 2% was chosen for laboratory considerations. The maximal admissible gap is between 0.1 and 0.281 mm; additional reduction does not increase the dispersion quality. From a laboratory standpoint, the 0.042 mm gap is considered optimal; further investigation will be required if energy is to be taken into consideration. A low recycle flow rate (fewer passes at high retention time) has resulted in higher gel quality as compared to a high recycle flow rate (more passes at lower retention time). A recycle rate of 200 mL/min is preferable for laboratory experiments and for eventual industrial production as the dispersion step would require smaller investment. Since the variation of dispersion pH between pH 7 and 10 was determined to have no influence on the dispersion quality and lower pH of 5 is detrimental to the dispersion quality, the dispersion pH of 7 was chosen as optimal.
Hence, based on the rheological measurements and dispersion conditions, the followings operating conditions were identified as optimal in our laboratory apparatus: 0.042 mm gap, 200 mL/min recycle rate, dispersion pH of 7 and a feed consistency of 2%. These experimental values can serve as the basis for scaling up the production of nanocellulose fibers at an industrial level.
Acknowledgments
The authors gratefully acknowledge the financial supports from the Canada Research Chair in Value-added Paper and the Natural Sciences and Engineering Research Council of Canada (NSERC). Grateful thanks go to Michel Paquin, a former colleague, who participated in the early stage of this research project. Many thanks also go to Khalil Jradi for reviewing the content of the manuscript.
References
- Brown, R. Section 2: The Forest Products Industry. In Forest Products Industry Technology Roadmap; The Agenda 2020 Technology Alliance: Washington, DC, USA, 2010; pp. 9–14. [Google Scholar]
- Hamad, W. On the development and applications of cellulosic nanofibrillar and nanocrystalline materials. Can. J. Chem. Eng. 2006, 84, 513–519. [Google Scholar] [CrossRef]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Reduction of water wettability of nanofibrillated cellulose by adsorption of cationic surfactants. Cellulose 2010, 18, 257–270. [Google Scholar]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated system. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Mishra, S.; Manent, A.-S.; Chabot, B.; Daneault, C. The use of sodium chlorite in post-oxidation of TEMPO-oxidized pulp: Effect on pulp characteristics and nanocellulose yield. J. Wood Chem. Technol. 2012, 32, 137–148. [Google Scholar] [CrossRef]
- Mishra, S.; Manent, A.-S.; Chabot, B.; Daneault, C. Production of nanocellulose from native cellulose—various options utilizing ultrasound. BioResources 2012, 7, 422–436. [Google Scholar]
- Mishra, S.; Thirree, J.; Manent, A.-S.; Chabot, B.; Daneault, C. Ultrasound–catalyzed TEMPO-mediated oxidation of native cellulose for production of nanocellulose: Effect of process variables. BioResources 2011, 6, 121–143. [Google Scholar]
- Rattaz, A.; Mishra, S.; Chabot, B.; Daneault, C. Cellulose nanofibers by sonocatalysed-TEMPO-oxidation. Cellulose 2011, 18, 585–593. [Google Scholar] [CrossRef]
- Atiemo-Obeng, V.A.; Calabrese, R.V. Rotor–Stator Mixing Devices. In Handbook of Industrial Mixing: Science and Practice; Paul, E.L., Atiemo-Obeng, V.A., Kresta, S.M., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004; pp. 479–505. [Google Scholar]
- Roy, M.K.; Juneja, L.R.; Isobe, S.; Tsushida, T. Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chem. 2009, 114, 263–269. [Google Scholar] [CrossRef]
- Tashtoush, B.; Jacobson, E.L.; Jacobson, M.K. A rapid HPLC method for simultaneous determination of tretinoin and isotretinoin in dermatological formulations. J. Pharm. Biomed. Anal. 2007, 43, 859–864. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Roux, D.; Nishiyama, Y. Rheological properties of microfibrillar suspension of TEMPO-oxidized pulp. Cellulose 2008, 15, 425–433. [Google Scholar] [CrossRef]
- Ishii, D.; Saito, T.; Isogai, A. Viscoelastic evaluation of average length of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2011, 12, 548–550. [Google Scholar] [CrossRef]
- Loranger, E.; Paquin, M.; Daneault, C.; Chabot, B. Comparative study of sonochemical effects in an ultrasonic bath and in a large-scale flow-through sonoreactor. Chem. Eng. J. 2011, 178, 359–365. [Google Scholar] [CrossRef]
- Parks, E.J.; Hebert, R.L. Thermal analysis of ion exchange reaction products of wood pulps with calcium and aluminium cations. Tappi J. 1972, 55, 1510–1514. [Google Scholar]
- Fatkullin, N.; Mattea, C.; Stapf, S. A simple scaling derivation of the shear thinning power-law exponent in entangled polymer melts. Polymer 2011, 52, 3522–3525. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Zha, Q.; Bandekar, R.; Alsaggaf, A.; Nia, Y. Review: Effects of wood quality and refining process on TMP pulp and paper quality. BioResources 2011, 6, 3569–3584. [Google Scholar]
- Katz, S.; Beatson, R.P.; Scallan, A.M. The determination of strong and weak acidic groups in sulfite pulps. Svensk Papperstindning 1984, 6, R48–R53. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).