Aqueous Chemical Solution Deposition of Novel, Thick and Dense Lattice-Matched Single Buffer Layers Suitable for YBCO Coated Conductors: Preparation and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Method for Cerium Doped Lanthanum Zirconate (LCZO) Solutions
2.2. Preparation Method for Non-Stoichiometric Lanthanum Zirconate Solutions
2.3. Solution Stability, Influence of Complexant and Polymer
2.4. Cleaning of Substrates
2.5. Solution Deposition and Heat-Treatment
2.6. Characterization
3. Results and Discussion
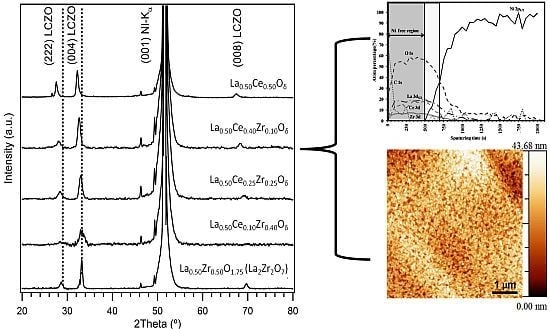
3.1. Crystallinity and Orientation
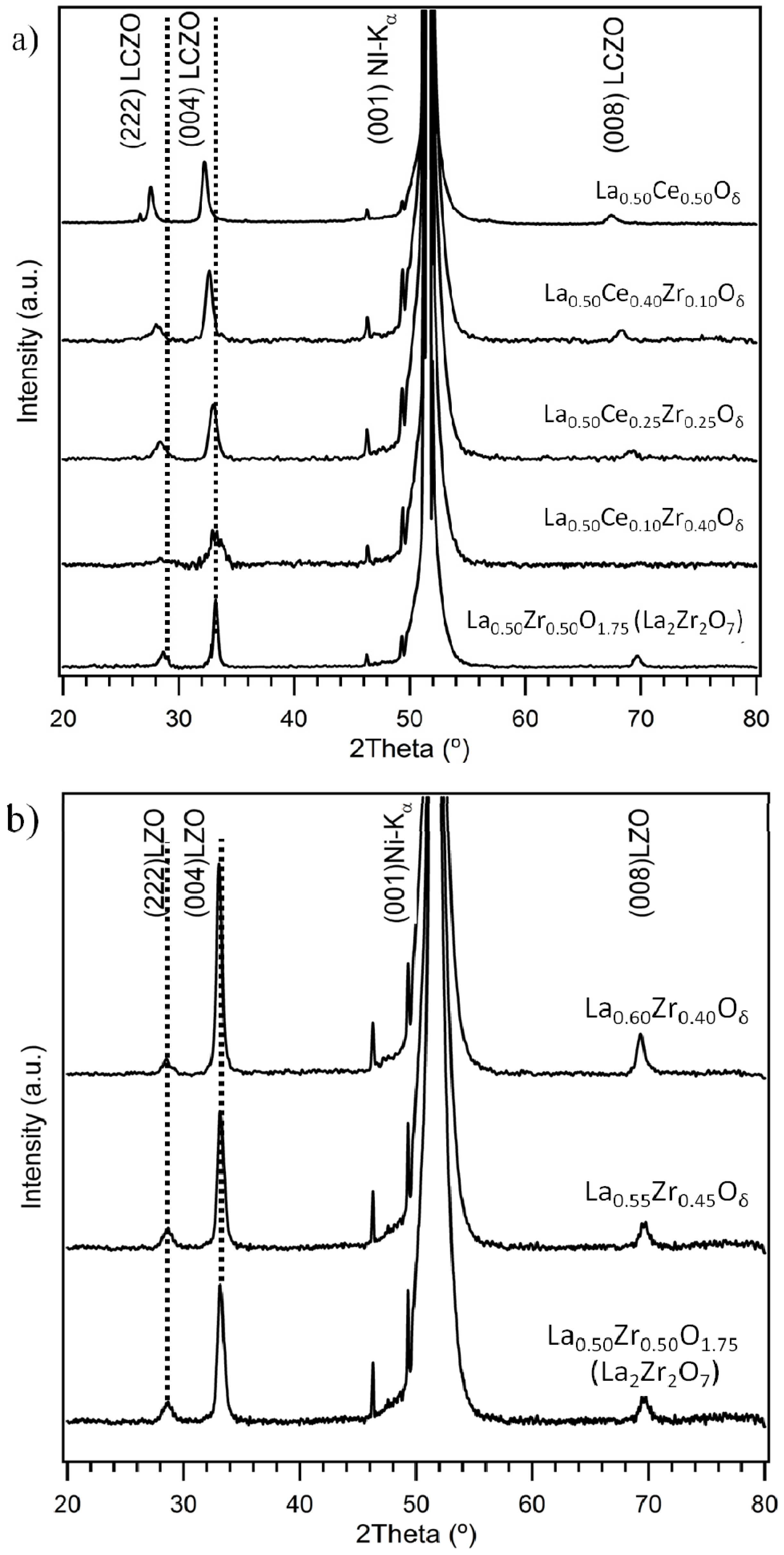

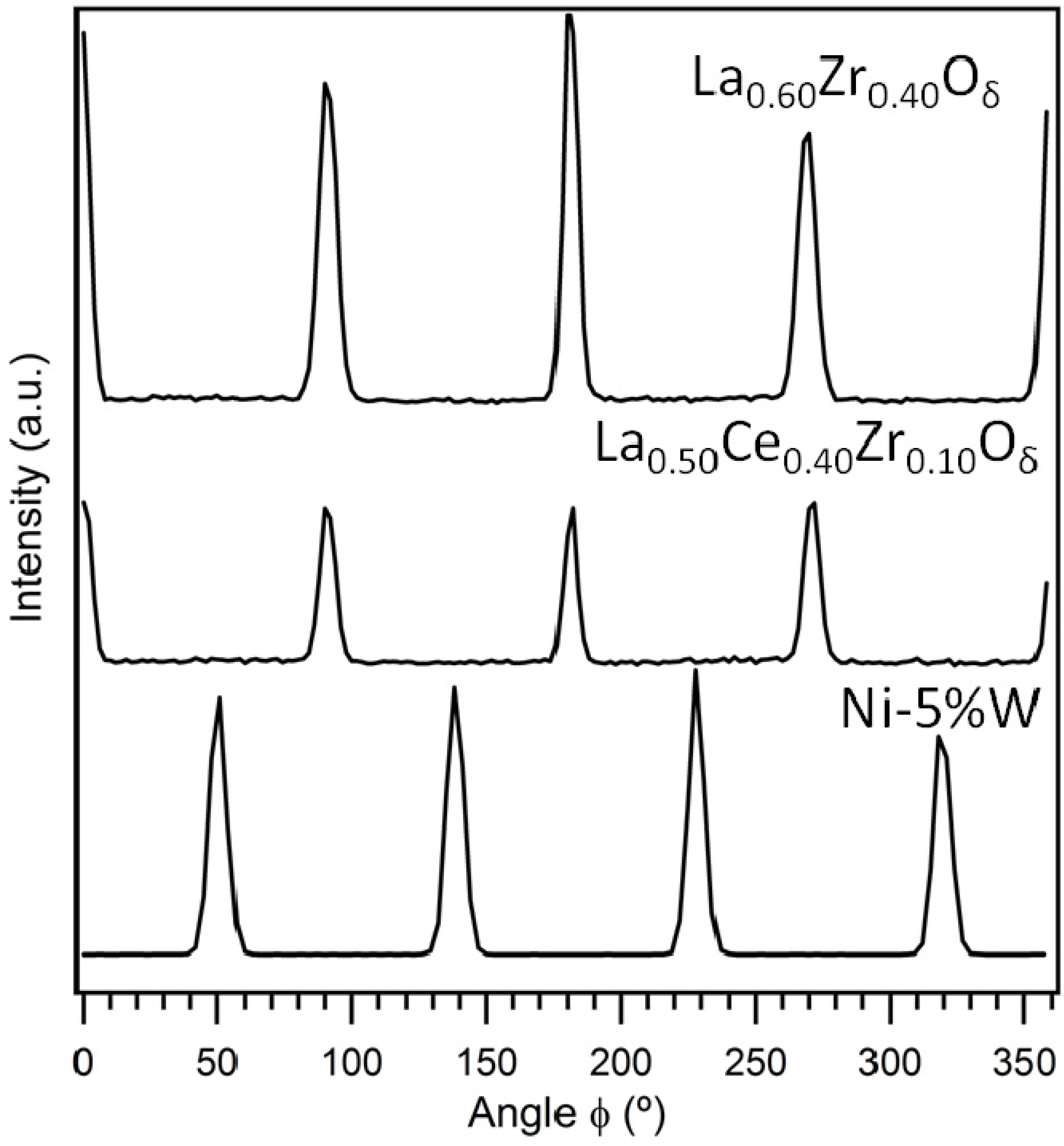
3.1.1. Lattice Mismatch Calculation for LCZO Thin Films on Ni-5% W


| Sample | Lattice parameter a (Å) | Lattice mismatch with Ni-5% W (%) (3.54 Å) | Lattice mismatch with Y1Ba2Cu3O6+x (%) (3.8578 Å) |
|---|---|---|---|
| La0.50Zr0.50O1.75 | 10.79 | 7.76 | −1.11 |
| La0.50Ce0.10Zr0.40Oy | 10.82 | 8.06 | −0.84 |
| La0.50Ce0.25Zr0.25Oy | 10.85 | 8.35 | −0.58 |
| La0.50Ce0.40Zr0.10Oy | 10.99 | 9.76 | 0.72 |
3.1.2. Lattice Mismatch Calculation for Non-Stoichiometric LZO Thin Films on Ni-5% W
| Sample | Lattice parameter a (Å) | Lattice mismatch with Ni-5% W (%) (3.54 Å) | Lattice mismatch with Y1Ba2Cu3O6+x (%) (3.8578 Å) |
|---|---|---|---|
| La0.50Zr0.50O1.75 | 10.79 | 7.76 | −1.11 |
| La0.55Zr0.45Oy | 10.83 | 8.20 | −1.06 |
| La0.60Zr0.40Oy | 10.89 | 8.39 | −0.99 |
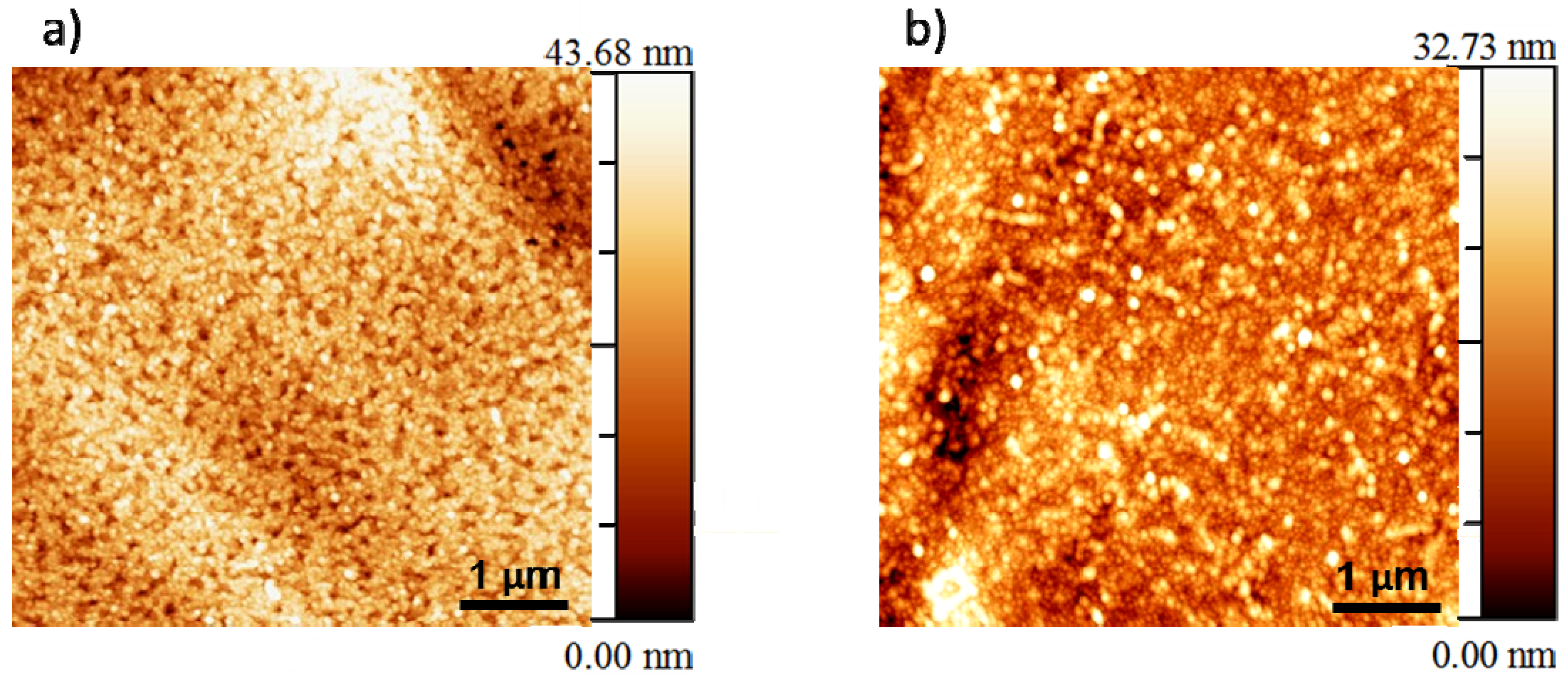
3.2. Microstructural Analysis

3.3. Buffer Layer Action: XPS Study
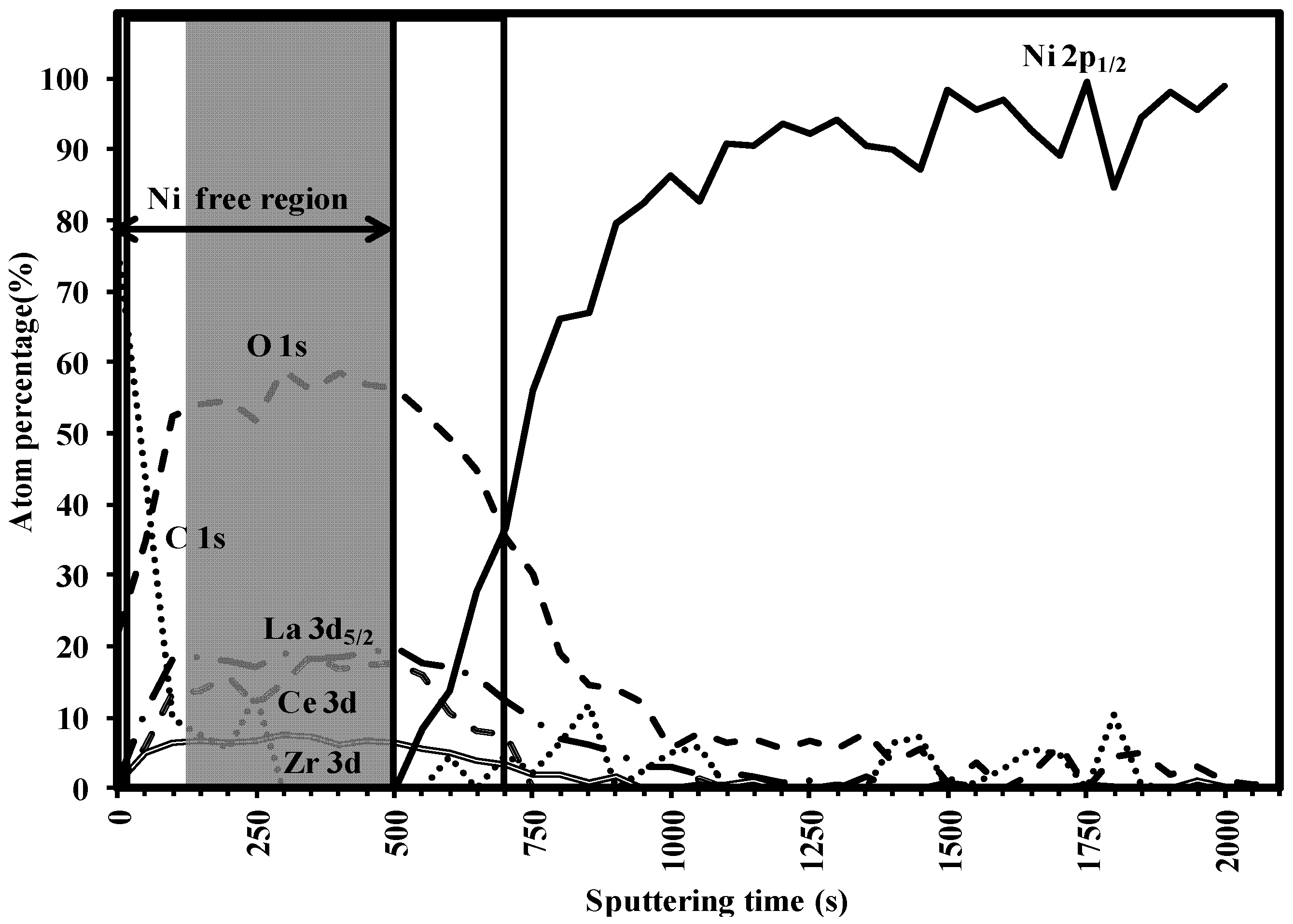
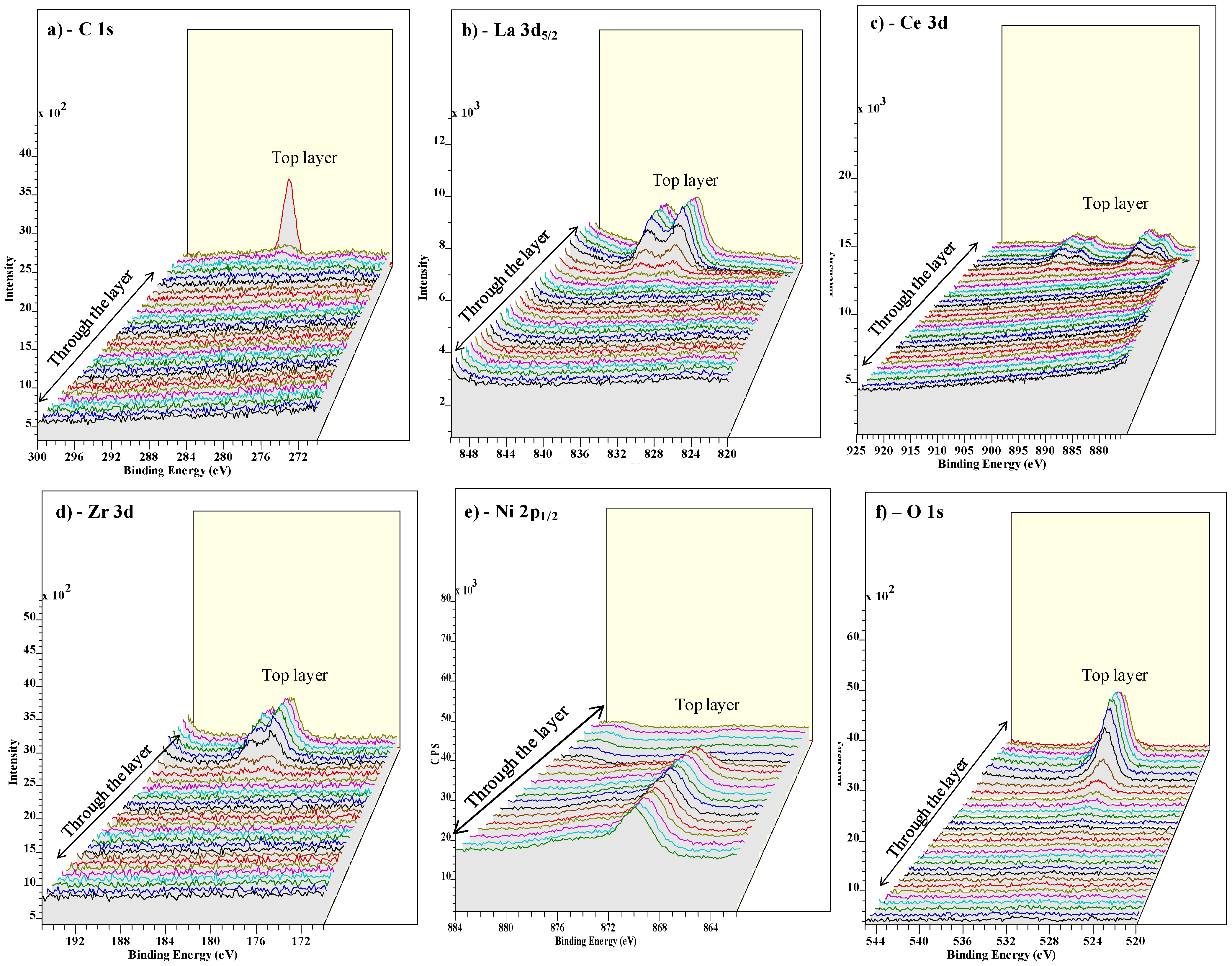
4. Conclusions
Acknowledgements
References
- Goyal, A.; Norton, D.P.; Christen, D.K.; Specht, E.D.; Paranthaman, M.; Kroeger, D.M.; Budai, J.D.; He, Q.; List, F.A.; Feenstra, R.; et al. Epitaxial superconductors on rolling-assisted biaxially-textured substrates (RABiTS): A route towards high critical current density wire. Appl. Supercond. 1996, 61, 403–427. [Google Scholar]
- Cloet, V.; Feys, J.; Hühne, R.; Hoste, S.; van Driessche, I. Thin La2Zr2O7 films made from a water-based solution. J. Solid State Chem. 2009, 182, 37–42. [Google Scholar]
- Knoth, K.; Hühne, R.; Oswald, S.; Schultz, L.; Holzapfel, B. Detailed investigations on La2Zr2O7 buffer layers for YBCO-coated conductors prepared by chemical solution deposition. Acta Mater. 2007, 55, 517–529. [Google Scholar]
- Knoth, K.; Schlobach, B.; Hühne, R.; Schultz, L.; Holzapfel, B. La2Zr2O7 and Ce-Gd-O buffer layers for YBCO coated conductors using chemical solution deposition. Phys. C 2005, 426, 979–984. [Google Scholar] [CrossRef]
- Narayanan, V.; Lommens, P.; de Buysser, K.; Hühne, R.; van Driessche, I. Thick lanthanum zirconate buffer layers from water-based precursor solutions on Ni-5% W substrates. J. Solid State Chem. 2011, 184, 2887–2896. [Google Scholar] [CrossRef]
- Sathyamurthy, S.; Paranthaman, M.; Heatherly, L.; Martin, P.M.; Specht, E.D.; Goyal, A.; Kodenkandath, T.; Li, X.; Rupich, M.W. Solution-processed lanthanum zirconium oxide as a barrier layer for high Ic-coated conductors. J. Mater. Res. 2006, 21, 910–914. [Google Scholar]
- Van de Velde, N.; van de Vyver, D.; Brunkahl, O.; Hoste, S.; Bruneel, E.; van Driessche, I. CeO2 buffer layers for HTSC by an aqueous sol-gel method—Chemistry and microstructure. Eur. J. Inorg. Chem. 2010, 2010, 233–241. [Google Scholar]
- Thuy, T.T.; Lommens, P.; Narayanan, V.; van de Velde, N.; de Buysser, K.; Herman, G.G.; Cloet, V. A nitrilo-tri-acetic-acid/acetic acid route for the deposition of epitaxial cerium oxide films as high temperature superconductor buffer layers. J. Solid State Chem. 2010, 183, 2154–2160. [Google Scholar] [CrossRef]
- Coll, M.; Gazquez, J.; Hühne, R.; Holzapfel, B.; Morilla, Y.; Garcia-Lopez, J.; Pomar, A.; Sandiumenge, F.; Puig, T.; Obradors, X. All chemical YBa2Cu3O7 superconducting multilayers: Critical role of CeO2 cap layer flatness. J. Mater. Res. 2009, 24, 1446–1455. [Google Scholar]
- Engel, S.; Knoth, K.; Hühne, R.; Schultz, L.; Holzapfel, B. An all chemical solution deposition approach for the growth of highly textured CeO2 cap layers on La2Zr2O7-buffered long lengths of biaxially textured Ni-W substrates for YBCO-coated conductors. Supercond. Sci. Technol. 2005, 18, 1385–1390. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, X.; Fang, H.; Putman, P.T.; Salama, K. Development of single solution buffer layers on textured Ni substrate for HTS coated conductors. IEEE Trans. Appl. Supercond. 2005, 15, 2711–2714. [Google Scholar]
- Paranthaman, M.P.; Sathyamurthy, S.; Li, X.; Specht, E.D.; Wee, S.H.; Cantoni, C.; Goyal, A.; Rupich, M.W. Modified lanthanum zirconium oxide buffer layers for low-cost, high performance YBCO coated conductors. Phys. C 2010, 470, 352–356. [Google Scholar]
- Akin, Y.; Heiba, Z.K.; Sigmund, W.; Hascicek, Y.S. Engineered oxide thin films as 100% lattice match buffer layers for YBCO coated conductors. Solid State Electron. 2003, 47, 2171–2175. [Google Scholar] [CrossRef]
- Narayanan, V.; Lommens, P.; de Buysser, K.; Hühne, R.; Vanpoucke, D.; Molina, L.; van Tendeloo, G.; van der Voort, P.; van Driessche, I. Aqueous CSD approach for the growth of novel, lattice-tuned LaxCe1−xOδ epitaxial layers. J. Mater. Chem. 2012, 22, 8476–8483. [Google Scholar]
- Yue, Z.; Li, X.; Khoryushin, A.; He, D.; Andersen, N.H.; Hansen, J.B.; Grivel, J. Development of all chemical solution derived Ce0.9La0.1O2−y/GdcZrcO7 buffer layer stack for coated conductors: Influence of the post-annealing process on surface crystallinity. Supercond. Sci. Technol. 2012, 25, 015008. [Google Scholar] [CrossRef]
- Li, G.; Pu, M.H.; Du, X.H.; Sun, R.P.; Zhang, H.; Zhao, Y. Deposition of potential Y2−xBixO3 buffer layers for YBCO coated conductor. Phys. C 2007, 463, 589–593. [Google Scholar]
- Arda, L.; Heiba, Z.K. Rare-earth mixed oxide thin films as 100% lattice match buffer layers for YBa2Cu3O7−x coated conductors. Thin Solid Films 2010, 518, 3345–3350. [Google Scholar] [CrossRef]
- Paranthaman, M.P.; Qiu, X.; List, F.A.; Kim, K.; Zhang, Y.; Li, X.; Sathyamurthy, S.; Thieme, C.; Rupich, M.W. Development of solution buffer layers for RABiTS based YBCO coated conductors. IEEE Trans. Appl. Supercond. 2011, 21, 3059–3061. [Google Scholar]
- Bhuiyan, M.S.; Paranthaman, M.; Salama, K. Solution-derived textured oxide thin films—A review. Supercond. Sci. Technol. 2006, 19, R1–R21. [Google Scholar] [CrossRef]
- Van Driessche, I.; Penneman, G.; Bruneel, E.; Hoste, S. Non vacuum-based deposition techniques for superconducting ceramic coatings. Pure Appl. Chem. 2002, 74, 2101–2109. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, D. Effect of rare earth doping on thermo-physical properties of lanthanum zirconate ceramic for thermal barrier coatings. J. Rare Earths 2008, 26, 770–774. [Google Scholar] [CrossRef]
- Yi, G.; Sayer, M. An acetic acid/water based sol-gel PZT process I: Modification of Zr and Ti alkoxides with acetic acid. J. Sol-Gel Sci. Technol. 1996, 6, 65–74. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants; Plenum press: New York, NY, USA, 1975. [Google Scholar]
- Kozuka, H.; Higuchi, A. Single-layer submicron-thick BaTiO3 coatings from poly(vinylpyrrolidone)-containing sols: Gel-to-ceramic film conversion, densification, and dielectric properties. J. Mater. Res. 2001, 16, 3116–3123. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705:1–013705:8. [Google Scholar]
- Narayanan, V.; de Buysser, K.; Bruneel, E.; van Driessche, I. X-ray photoelectron spectroscopy (XPS) depth profiling for evaluation of La2Zr2O7 buffer layer capacity. Materials 2012, 5, 364–376. [Google Scholar] [CrossRef] [Green Version]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Narayanan, V.; Van Steenberge, S.; Lommens, P.; Van Driessche, I. Aqueous Chemical Solution Deposition of Novel, Thick and Dense Lattice-Matched Single Buffer Layers Suitable for YBCO Coated Conductors: Preparation and Characterization. Nanomaterials 2012, 2, 298-311. https://doi.org/10.3390/nano2030298
Narayanan V, Van Steenberge S, Lommens P, Van Driessche I. Aqueous Chemical Solution Deposition of Novel, Thick and Dense Lattice-Matched Single Buffer Layers Suitable for YBCO Coated Conductors: Preparation and Characterization. Nanomaterials. 2012; 2(3):298-311. https://doi.org/10.3390/nano2030298
Chicago/Turabian StyleNarayanan, Vyshnavi, Sigelinde Van Steenberge, Petra Lommens, and Isabel Van Driessche. 2012. "Aqueous Chemical Solution Deposition of Novel, Thick and Dense Lattice-Matched Single Buffer Layers Suitable for YBCO Coated Conductors: Preparation and Characterization" Nanomaterials 2, no. 3: 298-311. https://doi.org/10.3390/nano2030298