Spontaneous Deposition of Prussian Blue on Multi-Walled Carbon Nanotubes and the Application in an Amperometric Biosensor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Deposition of Prussian Blue on Different Electrode Substrates
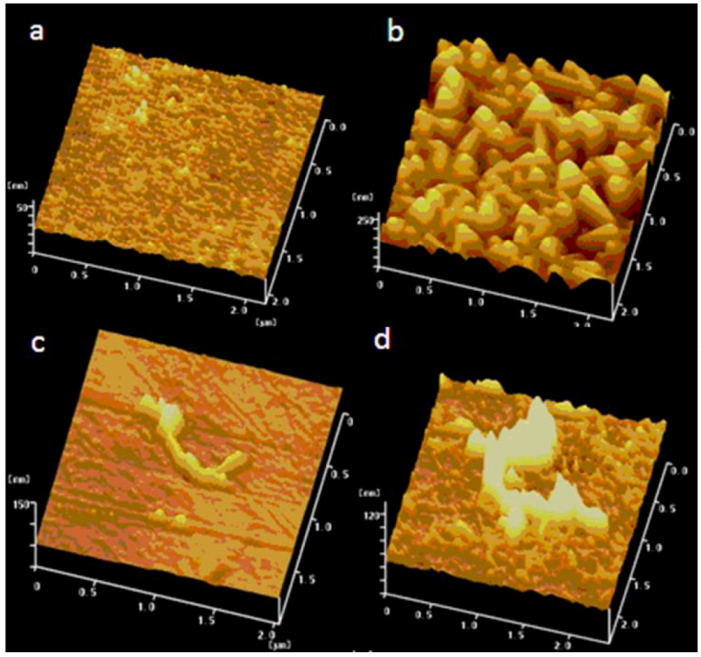
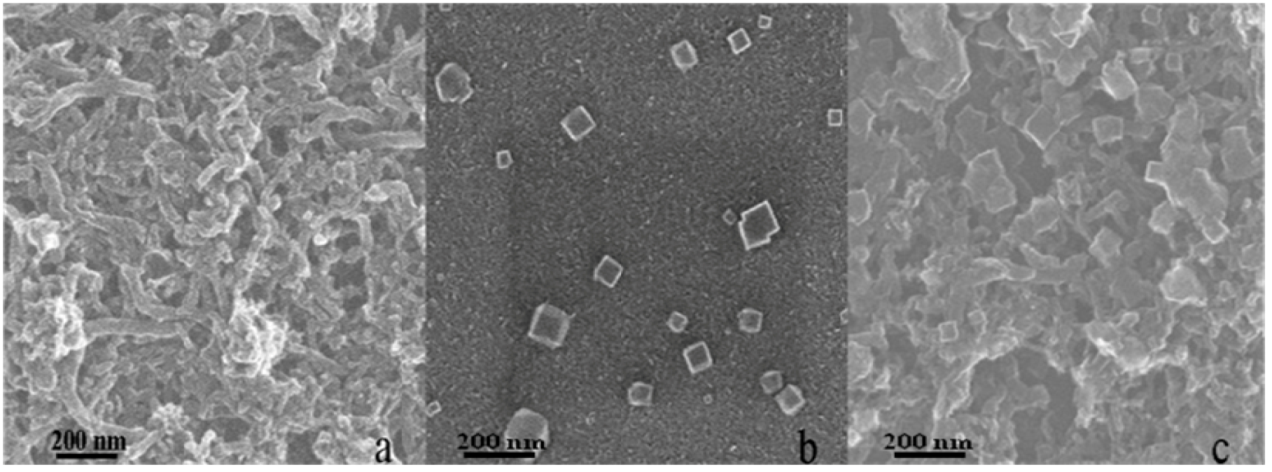
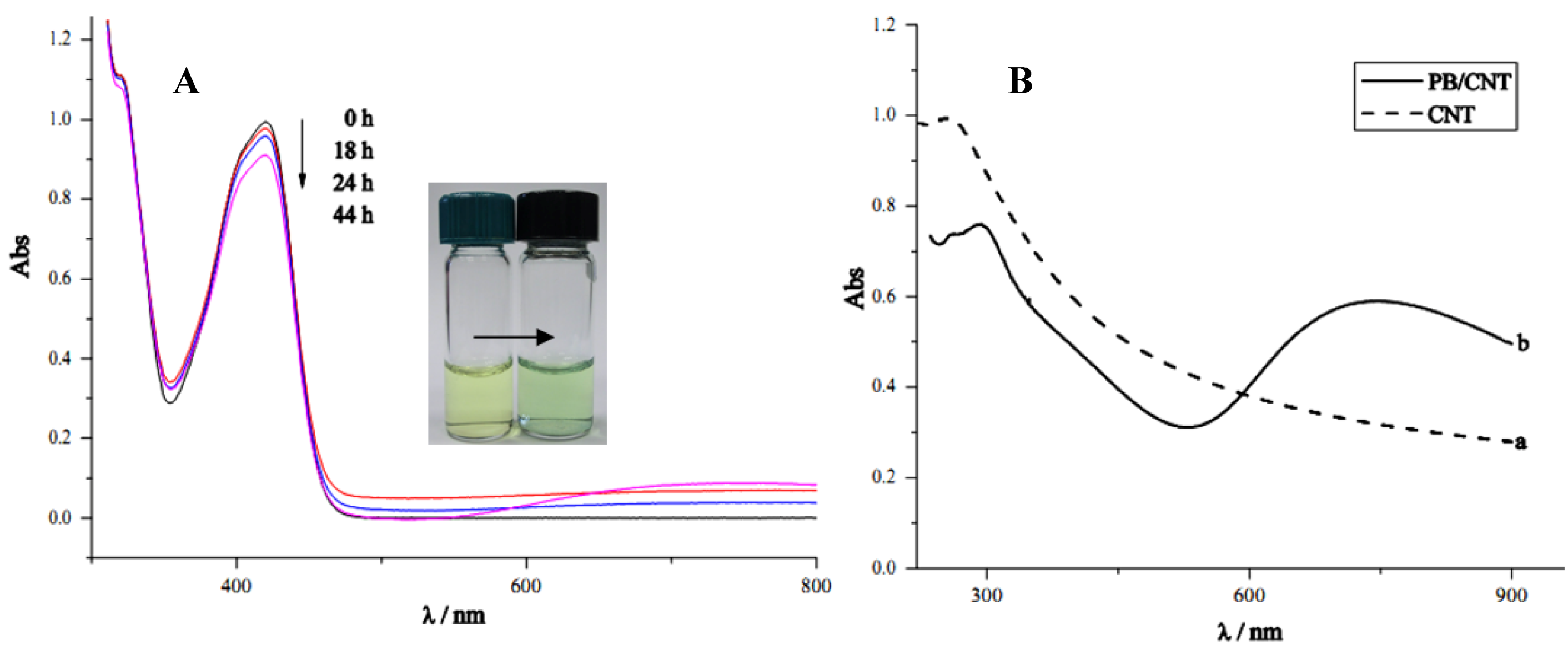
2.2. Mechanism for the Spontaneous Deposition of Prussian Blue
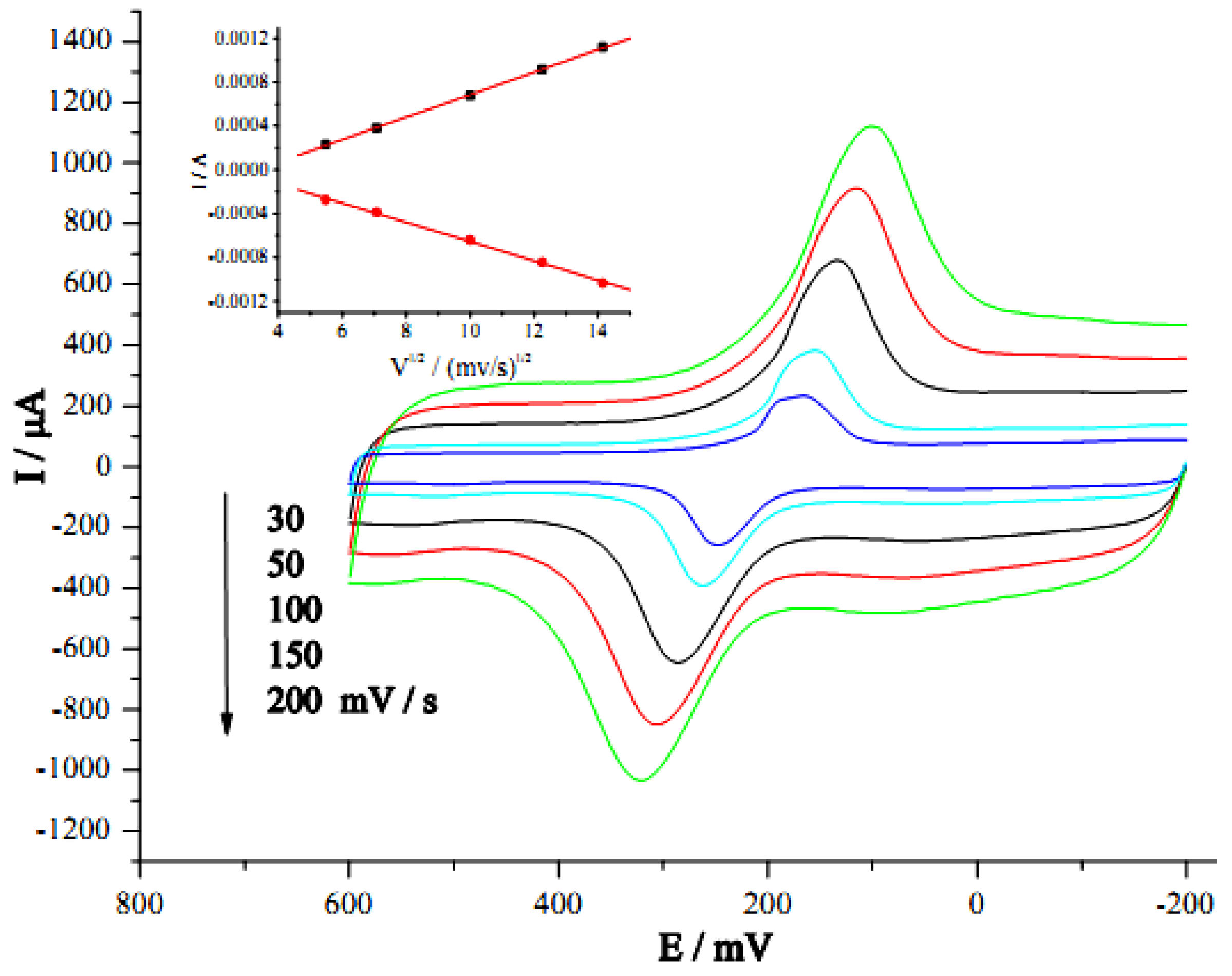
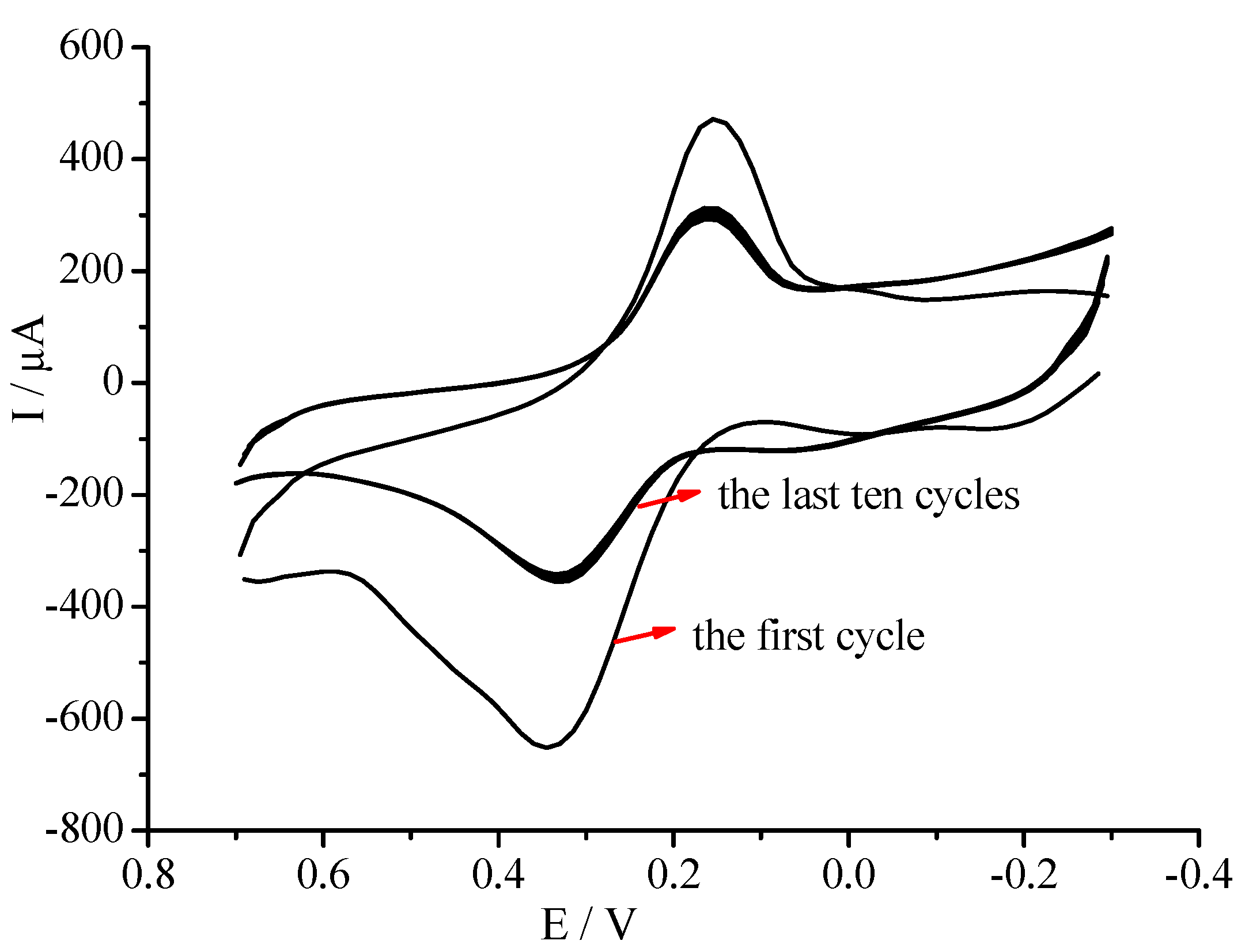
2.3. Electrochemical and Electrocatalytic Properties of the GC/CNT/PB Modified Electrode
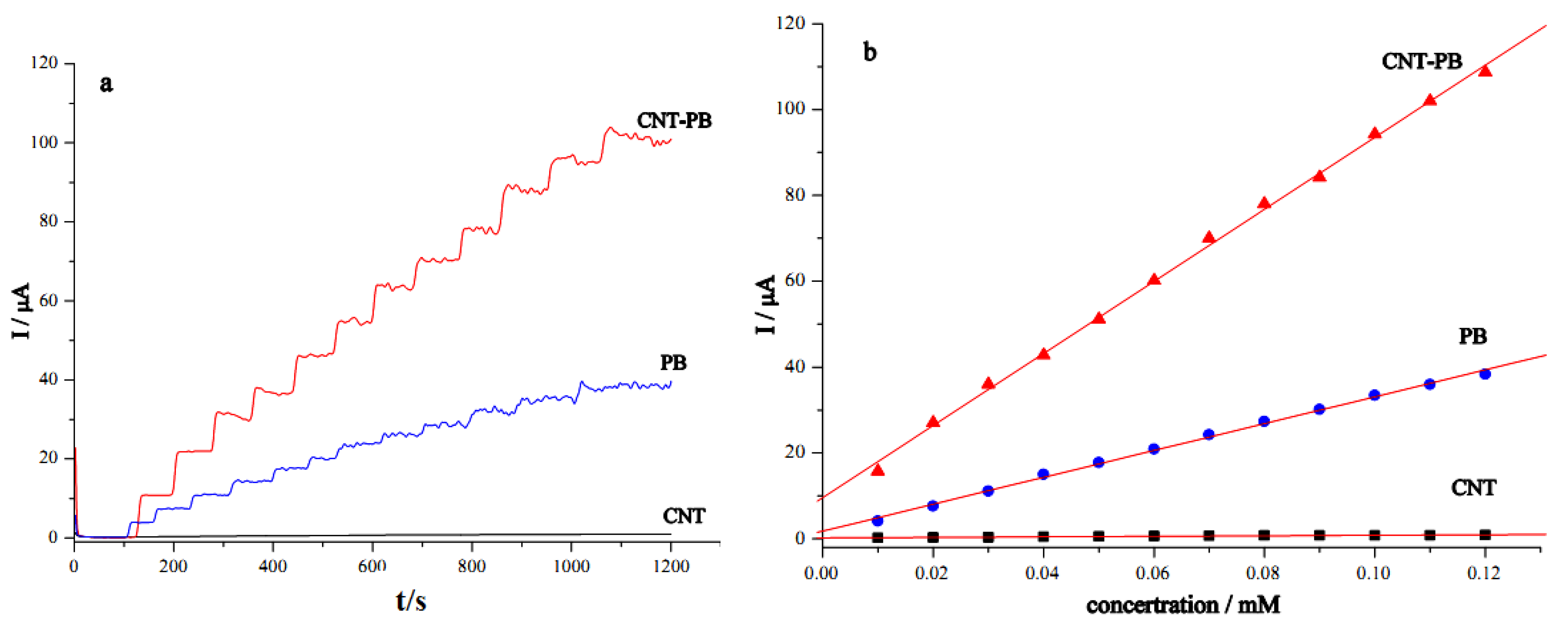
| Electrode | GC/CNT | GC/PB | GC/CNT/PB |
|---|---|---|---|
| Sensitivity (AM−1cm−2) | 0.82 | 4.7 | 13.0 |
| Linear range (μM) | 10–60 | 10–120 | 10–120 |
| Detection limit (μM) | 2 | 2 | 1 |
| Response time (s) | <0.2 | <0.2 | <0.2 |
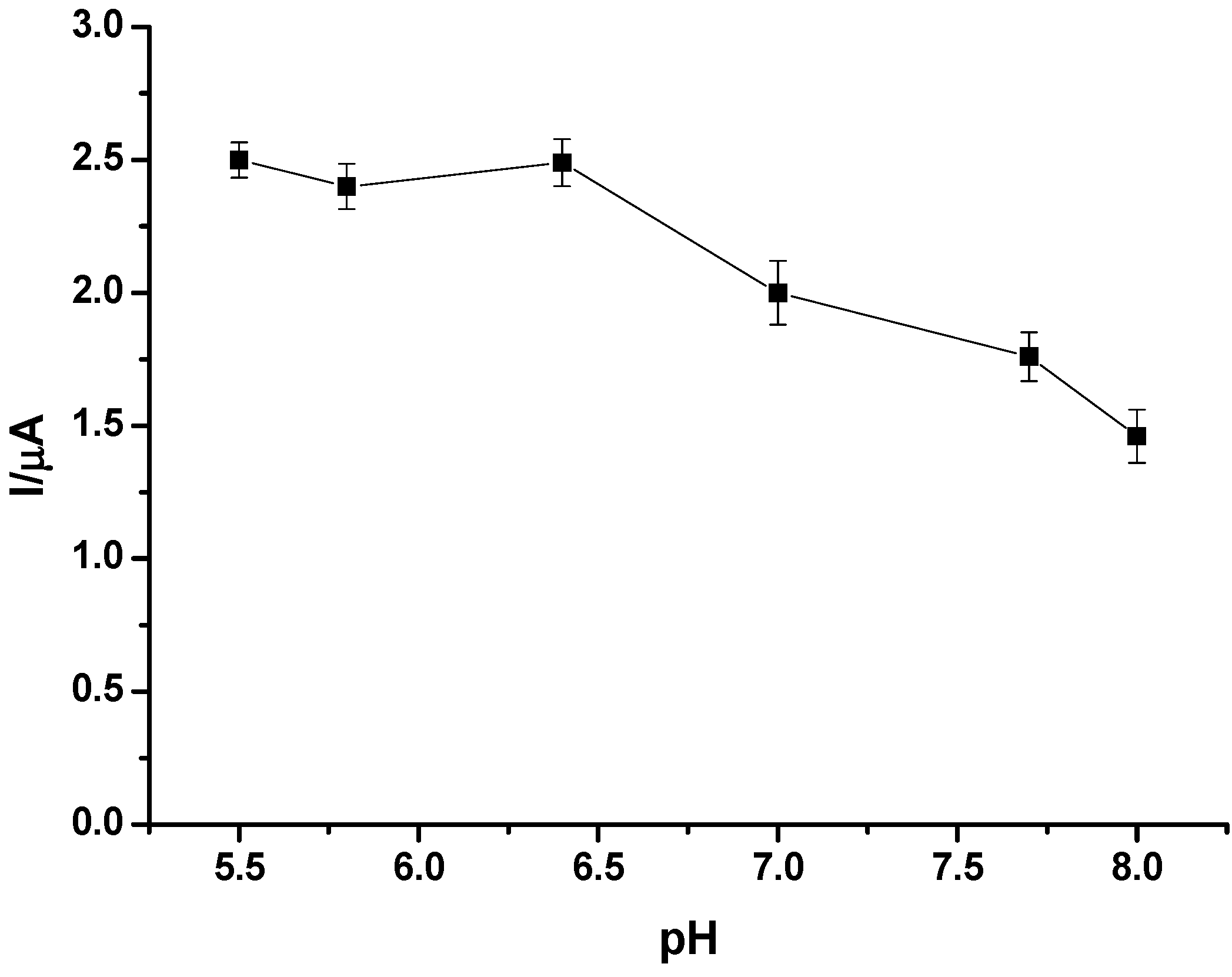
2.4. Preparation and Performance Characteristics of the GC/CNT/PB/PPD-GOD Glucose Biosensor
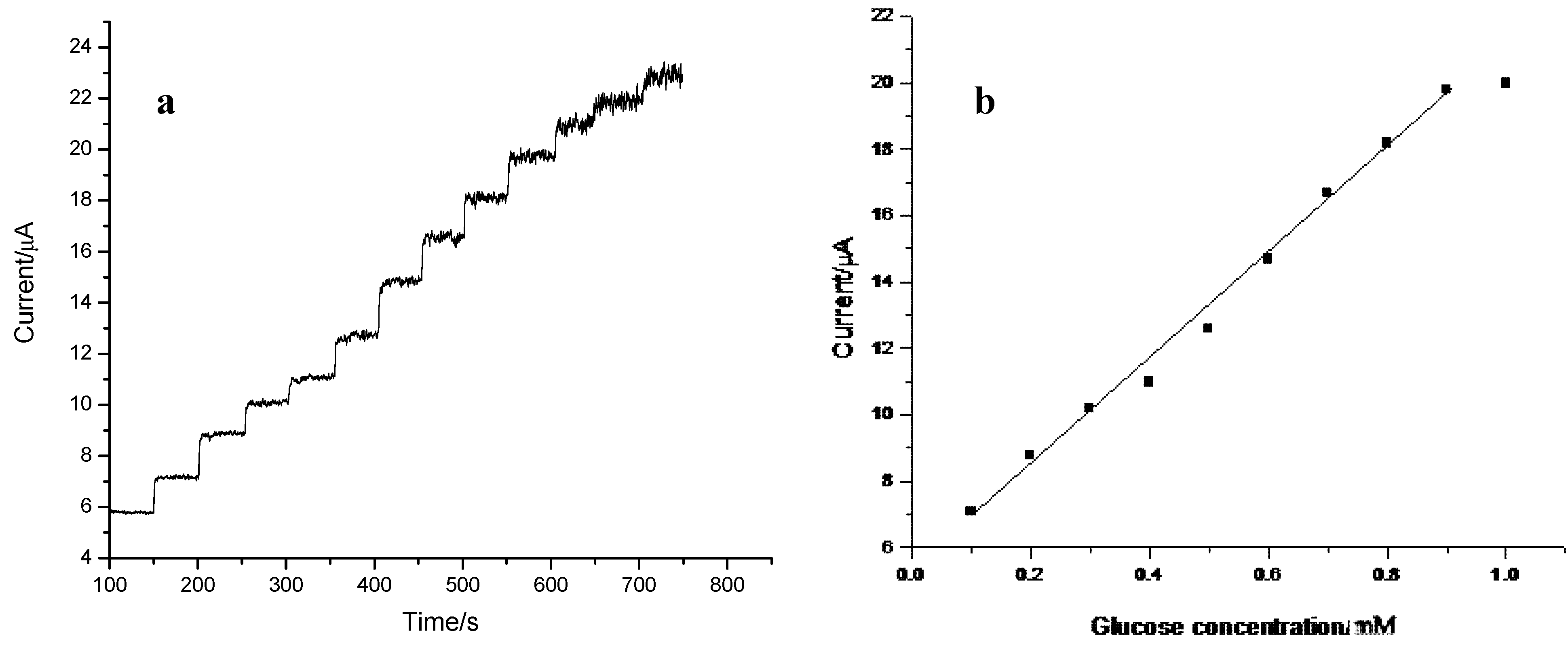
| Electrode | Linear range (μM) | Detection limit (μM) | Sensitivity (mA M−1 cm−2) |
|---|---|---|---|
| GC/CNT/PB/PPD-GOD [this work] | 100–900 | 10 | 102 |
| GC/Pt-CNT-GOD-Nafion [61] | 160–11500 | 55 | ------ |
| GC/CNT/CS/Cu [62] | 50–1200 | 20 | ----- |
| GC/Au-MWNT/GOD/Nafion [63] | 50–2200 | 20 | 5.66 |
| Pt/PB/GA-GOD [64] | 5–1100 | 5 | 43 |
| GC/PB-CS/GOD [65] | 2–400 | 0.397 | ----- |
| GC/MWNTs/PB/PDAB/GOD [45] | 10–2500 | 5 | ----- |
| GC/CS/MWNTs/PB/GOD [66] | 4–2000 | 2.5 | 7.84 |
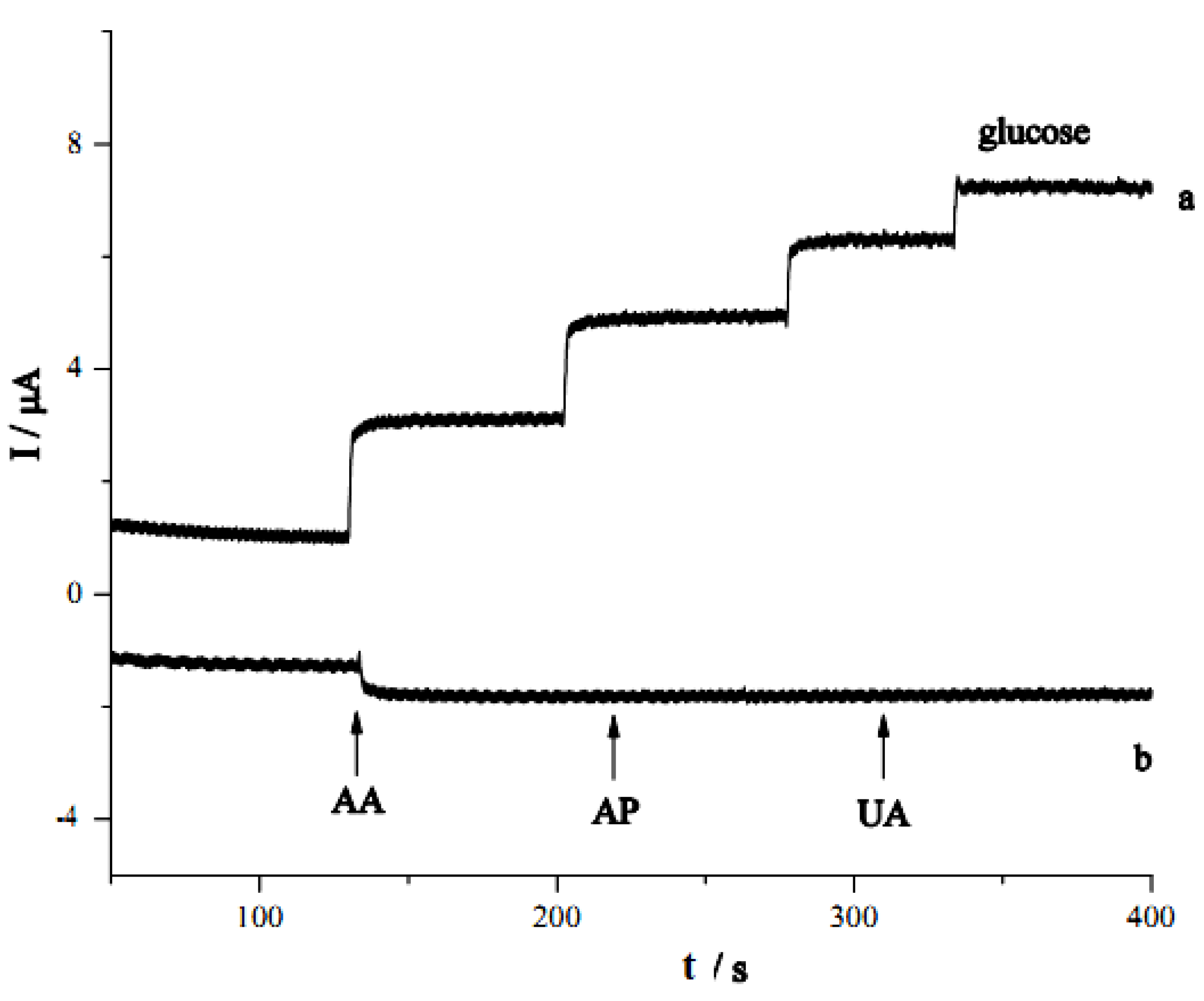
3. Experimental Section
3.1. Chemicals and Instruments
3.2. Preparation of CNT/PB Modified Electrode
3.3. Spectroscopic Characterization of Electrode Substrates
3.4. Preparation of CNT/PB Modified Glucose Biosensor
3.5. Amperometric Determination
4. Conclusions
Acknowledgments
References
- Yang, R.; Qian, Z.; Deng, J. Electrochemical deposition of Prussian blue from a single ferricyanide solution. J. Electrochem. Soc. 1998, 145, 2231–2236. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, K.; Sun, D.C.; Xia, X.H.; Chen, H.Y. Ultrathin layers of densely packed Prussian blue nanoclusters prepared from a ferricyanide solution. Chem. Mater. 2003, 15, 4163–4165. [Google Scholar] [CrossRef]
- Nossol, E.; Zarbin, A.J.G. A Simple and innovative route to prepare a novel carbon nanotube/Prussian blue electrode and its utilization as a highly sensitive H2O2 amperometric sensor. Adv. Funct. Mater. 2009, 19, 3980–3986. [Google Scholar] [CrossRef]
- Karyakin, A.A. Prussian blue and its analogues: Electrochemistry and analytical applications. Electroanalysis 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, K.; Sun, D.C.; Xia, X.H.; Chen, H.Y. Potentiodynamic deposition of Prussian blue from a solution containing single component of ferricyanide and its mechanism investigation. J. Solid State Electrochem. 2003, 7, 561–566. [Google Scholar] [CrossRef]
- Ellis, D.; Eckhoff, M.; Neff, V.D. Electrochromism in the mixed-valence hexacyanides. 1. Voltammetric and spectral studies of the oxidation and reduction of thin films of Prussian blue. J. Chem. Phys. 1981, 85, 1225–1231. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Saturnino, P.G.; Lobo, R.F.M.; Sequeira, C.A.C. Direct borohydride/peroxide fuel cells using Prussian blue cathodes. J. Power Sources 2012, 208, 131–137. [Google Scholar] [CrossRef]
- Fu, L.; You, S.J.; Zhang, G.Q.; Yang, F.L.; Fang, X.H.; Gong, Z. PB/PANI-modified electrode used as a novel oxygen reduction cathode in microbial fuel cell. Biosen. Bioelectron. 2011, 26, 1975–1979. [Google Scholar] [CrossRef]
- Somani, P.; Mandale, A.B.; Radhakrishnan, S. Study and development of conducting polymer-based electrochromic display devices. Acta Mater. 2000, 48, 2859–2871. [Google Scholar] [CrossRef]
- Zhou, P.; Xue, D.; Luo, H.; Chen, X. Fabrication, structure, and magnetic properties of highly ordered Prussian blue nanowire arrays. Nano Lett. 2002, 2, 845–847. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Varley, T.S. In situ spectroelectrochemistry and colour measurement of a complementary electrochromic device based on surface-confined Prussian blue and aqueous solution-phase methyl viologen. Solar Energy Mater. Solar Cells 2012, 99, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.C.; Hsu, C.Y.; Hu, C.W.; Ho, K.C. A complementary electrochromic device based on Prussian blue and poly(ProDOT-Et2) with high contrast and high coloration efficiency. Solar Energy Mater.Solar Cells 2011, 95, 2238–2245. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karykina, E.E. Prussian blue-based “artifical peroxidase” as a transducer for hydrogen peroxide detection. Application to biosensors. Sens. Actuators B 1999, 57, 268–273. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Yuan, Y. Synthesis of magnetic nanoparticles composed by Prussian blue and glucose oxidase for preparing highly sensitive and selective glucose biosensor. Sens. Actuators B 2009, 139, 400–406. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; López-Muñoz, A.; Repetto, D.; Catala, L.; Mallah, T. Patterning of magnetic bimetallic coordination nanoparticles of Prussian blue derivatives by the Langmuir–Blodgett Technique. Langmuir 2012, 28, 4525–4533. [Google Scholar]
- Chi, Q.; Dong, S. Amperometric biosensors based on the immobilization of oxidases in a Prussian blue film by electrochemical codeposition. Anal. Chim. Acta 1995, 310, 429–436. [Google Scholar] [CrossRef]
- Chu, Z.; Shi, L.; Zhang, Y.; Jin, W.; Xu, N. Hierarchical self-assembly of double structured Prussian blue film for highly sensitive biosensors. J. Mater. Chem. 2011, 21, 11968–11972. [Google Scholar] [CrossRef]
- Du, D.; Wang, M.; Qin, Y.; Lin, Y. One-step electrochemical deposition of Prussian blue–multiwalled carbon nanotube nanocomposite thin-film: Preparation, characterization and evaluation for H2O2 sensing. J. Mater. Chem. 2010, 20, 1532–1537. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimisation and applications of Prussian blue modified electrodes. Biosen. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef]
- Itaya, K.; Shoji, N.; Uchida, I. Catalysis of the reduction of molecular oxygen to water at Prussian blue modified electrodes. J. Am. Chem. Soc. 1984, 106, 3423–3429. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. Amperometric biosensor for glutamate using Prussian blue-based “Artificial Peroxidase” as a transducer for hydrogen peroxide. Anal. Chem. 2000, 72, 1720–1723. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G.; Yigzaw, Y.; Gorton, L.; Ruzgas, T.; Karyakin, E.E. Investigation of the effect of different glassy carbon materials on the performance of Prussian blue based sensors for hydrogen peroxide. Electroanalysis 2003, 15, 175–182. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. A high-sensitive glucose amperometric biosensor based on Prussian blue modified electrodes. Anal. Lett. 1994, 27, 2861–2869. [Google Scholar] [CrossRef]
- Moscone, D.; D’Ottavi, D.; Compagnone, D.; Palleschi, G.; Amine, A. Construction and analytical characterization of Prussian blue-based carbon paste electrodes and their assembly as oxidase enzyme sensors. Anal. Chem. 2001, 73, 2529–2535. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, K.; Yao, Y.L.; Xia, X.H.; Chen, H.Y. Multilayer assembly of Prussian blue nanoclusters and enzyme-immobilized poly(toluidine blue) films and its application in glucose biosensor construction. Langmuir 2004, 20, 7303–7307. [Google Scholar] [CrossRef]
- Cox, J.A.; Jaworski, R.K.; Kulesza, P.J. Electroanalysis with electrodes modified by inorganic films. Electroanalysis 1991, 3, 869–877. [Google Scholar] [CrossRef]
- Neff, V.D. Electrochemical oxidation and reduction of thin films of Prussian blue. J. Electrochem. Soc. 1978, 125, 886–887. [Google Scholar] [CrossRef]
- Garjonyte, R.; Malinauskas, A. Electrocatalytic reactions of hydrogen peroxide at carbon paste electrodes modified by some metal hexacyanoferrates. Sens. Actuators B 1998, 46, 236–241. [Google Scholar] [CrossRef]
- Hu, Y.L.; Yuan, J.H.; Chen, W.; Wang, K.; Xia, X.H. Photochemical synthesis of Prussian blue film from an acidic ferricyanide solution and application. Electrochem. Commun. 2005, 7, 1252–1256. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M. Carbon nanotube/teflon composite electrochemical sensors and biosensors. Anal. Chem. 2003, 75, 2075–2079. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Sherrell, P.; Razal, J.M.; Huang, X.F.; Minett, A.L.; Chen, J. Carbon nanotube nanoweb–bioelectrode for highly selective dopamine sensing. ACS Appl. Mater. Interfaces 2011, 4, 44–48. [Google Scholar]
- Baldrich, E.; Gómez, R.; Gabriel, G.; Muñoz, F.X. Magnetic entrapment for fast, simple and reversivle electrode modification with carbon nanotubes: Application to dopamine detection. Biosens. Bioelectron. 2011, 26, 1876–1882. [Google Scholar] [CrossRef]
- Wooten, M.; Gorski, W. Facilitation of NADH electro-oxidation at treated carbon nanotubes. Anal.Chem. 2010, 82, 1299–1304. [Google Scholar] [CrossRef]
- Filip, J.; Šefčovičová, J.; Tomčík, P.; Gemeiner, P.; Tkac, J. A hyaluronic acid dispersed carbon nanotube electrode used for a mediatorless NADH sensing and biosensing. Talanta 2011, 84, 355–361. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Sun, J.J.; Song, J.; Yang, Q.Z. Direct electron teansfer and conformational change of glucose oxidase on carbon nanotube-based electrodes. Carbon 2010, 48, 1508–1514. [Google Scholar] [CrossRef]
- Qiu, J.D.; Zhou, W.M.; Guo, J.; Wang, R.; Liang, R.P. Amperometric sensor based on ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal. Biochem. 2009, 385, 264–269. [Google Scholar] [CrossRef]
- Shobha Jeykumari, D.R.; Ramaprabhu, S.; Sriman Narayanan, S. A thionine functionalized multiwalled carbon nanotube modified electrode for the determination of hydrogen peroxide. Carbon 2007, 45, 1340–1353. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Li, W.; Chen, K.; Nie, L.; Yao, S. Improved electrochemical properties of prussian blue by multi-walled carbon nanotubes. J. Electroanal. Chem. 2007, 603, 59–66. [Google Scholar] [CrossRef]
- Li, J.; Qiu, J.D.; Xu, J.J.; Chen, H.Y.; Xia, X.H. The synergistic effect of Prussian-blue-grafted carbon nanotube/poly(4-vinylpyridine) composites for amperometric sensing. Adv. Funct. Mater. 2007, 17, 1574–1580. [Google Scholar] [CrossRef]
- Gong, K.; Zhu, X.; Zhao, R.; Xiong, S.; Mao, L.; Chen, C. Rational attachment of synthetic triptycene orthoquinone onto carbon nanotubes for electrocatalysis and sensitive detection of thiols. Anal. Chem. 2005, 77, 8158–8165. [Google Scholar] [CrossRef]
- Luo, H.; Shi, Z.; Li, N.; Gu, Z.; Zhuang, Q. Investigation of the electrochemical and electrocatalytic behavior of single-wall carbon nanotube film on a glassy carbon electrode. Anal. Chem. 2001, 73, 915–920. [Google Scholar] [CrossRef]
- Zeng, J.; Wei, W.; Liu, X.; Wang, Y.; Luo, G. A simple method to fabricate a Prussian blue nanoparticles/carbon nanotubes/poly(1,2-diaminobenzene) based glucose biosensor. Microchim. Acta 2008, 160, 261–267. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution properties of single-walled carbon nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef]
- Sha, Y.; Qian, L.; Ma, Y.; Bai, H.; Yang, X. Multilayer films of carbon nanotubes and redox polymer on screen-printed carbon electrodes for electrocatalysis of ascorbic acid. Talanta 2006, 70, 556–560. [Google Scholar] [CrossRef]
- Itaya, K.; Uchida, I.; Neff, V.D. Electrochemistry of polynuclear transition metal cyanides: Prussian blue and its analogues. Acc. Chem. Res. 1986, 19, 162–168. [Google Scholar] [CrossRef]
- Pyrasch, M.; Toutianoush, A.; Jin, W.; Schnepf, J.; Tieke, B. Self-assembled films of Prussian blue and analogues: Optical and electrochemical properties and application as ion-sieving membranes. Chem. Mater. 2003, 15, 245–254. [Google Scholar] [CrossRef]
- Choi, H.C.; Shim, M.; Bangsaruntip, S.; Dai, H. Spontaneous reduction of metal ions on the sidewalls of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 9058–9059. [Google Scholar] [CrossRef]
- Qu, L.; Dai, L. Substrate-enhanced electroless deposition of metal nanoparticles on carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 10806–10807. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Shi, Z.; Li, N.; Gu, Z. Direct electrochemistry of cytochrome c at a glassy carbon electrode modified with single-wall carbon nanotubes. Anal. Chem. 2002, 74, 1993–1997. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Shiu, K.K. Layer-by-layer assembly of Prussian blue and carbon nanotube composites for the sensitive detection of hydrogen peroxide. Anal. Sci. 2010, 26, 431–435. [Google Scholar] [CrossRef]
- Zhou, D.M.; Dai, Y.Q.; Shiu, K.K. Poly(phenylenediamine) film for the construction of glucose biosensors based on platinized glassy carbon electrode. J. Appl. Electrochem. 2010, 40, 1997–2003. [Google Scholar] [CrossRef]
- Sasso, S.V.; Pierce, R.J.; Walla, R.; Yacynch, A.M. Electropolymerized 1,2-diaminobenzene as a means to prevent interferences and fouling and to stabilize immobilized enzyme in electrochemical biosensors. Anal. Chem. 1990, 62, 1111–1117. [Google Scholar] [CrossRef]
- Dai, Y.Q.; Shiu, K.K. Glucose biosensor based on multi-walled carbon nanotube modified glassy carbon electrode. Electroanalysis 2004, 16, 1697–1703. [Google Scholar] [CrossRef]
- Yao, Y.; Shiu, K.K. Electron transfer properties of different carbon nanotube materials and their applications in glucose biosensors. Anal. Bioanal. Chem. 2007, 387, 303–309. [Google Scholar]
- Dai, Y.Q.; Zhou, D.M.; Shiu, K.K. Permeability and permselectivity of polyphenylenediamine films synthesized at a palladium disk electrode. Electrochim. Acta 2006, 52, 297–303. [Google Scholar] [CrossRef]
- Honda, K.; Hayashi, H. Prussian blue containing nafion composite film as rechargeable battery. J. Electrochem. Soc. 1987, 134, 1330–1334. [Google Scholar] [CrossRef]
- Garjonyte, R.; Malinauskas, A. Operational stability of amperometric hydrogen peroxide sensors, based on ferrous and copper hexacyanoferrates. Sens. Actuators B 1998, 56, 93–97. [Google Scholar]
- Hou, W.; Wang, E. Flow-injection amperometric detection of hydrazine by electrocatalytic oxidation at a Prussian blue film-modified electrode. Anal. Chim. Acta 1992, 257, 275–280. [Google Scholar] [CrossRef]
- Gorton, L. Carbon paste electrodes modified with enzymes, tissues, and cells. Electroanalysis 1995, 7, 23–45. [Google Scholar] [CrossRef]
- Wen, Z.; Ci, S.; Li, J. Pt nanoparticles inserting in carbon nanotube arrays: Nanocomposites for glucose biosensors. J. Phys. Chem. C 2009, 113, 13482–13487. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Zeng, J.; Liu, X.; Zeng, X. Fabrication of a copper nanoparticle/chitosan/carbon nanotube-modified glassy carbon electrode for electrochemical sensing of hydrogen peroxide and glucose. Microchim. Acta 2008, 160, 253–260. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Sethupathi, K.; Ramaprabhu, S. A glucose biosensor based on deposition of glucose oxidase onto crystalline gold nanoparticle modified carbon nanotube electrode. J. Phys. Chem. B 2009, 113, 3190–3194. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, Z.; Zhang, Y.; Jin, W. Amperometric glucose biosensor with high sensitivity based on self-assembled Prussian blue modified electrode. Electrochim. Acta 2009, 54, 7490–7494. [Google Scholar] [CrossRef]
- Wang, X.; Gu, H.; Yin, F.; Tu, Y. A glucose biosensor based on Prussian blue/chitosan hydrib film. Biosens. Bioelectron. 2009, 24, 1527–1530. [Google Scholar] [CrossRef]
- Zhai, X.; Wei, W.; Zeng, J.; Liu, X.; Gong, S. New nanocomposite based on Prussian blue nanoparticles/carbon nanotubes/chitosan and its application for assembling of amperometric glucose biosensor. Anal. Lett. 2006, 39, 913–926. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yao, Y.; Bai, X.; Shiu, K.-K. Spontaneous Deposition of Prussian Blue on Multi-Walled Carbon Nanotubes and the Application in an Amperometric Biosensor. Nanomaterials 2012, 2, 428-444. https://doi.org/10.3390/nano2040428
Yao Y, Bai X, Shiu K-K. Spontaneous Deposition of Prussian Blue on Multi-Walled Carbon Nanotubes and the Application in an Amperometric Biosensor. Nanomaterials. 2012; 2(4):428-444. https://doi.org/10.3390/nano2040428
Chicago/Turabian StyleYao, Yanli, Xiaoyun Bai, and Kwok-Keung Shiu. 2012. "Spontaneous Deposition of Prussian Blue on Multi-Walled Carbon Nanotubes and the Application in an Amperometric Biosensor" Nanomaterials 2, no. 4: 428-444. https://doi.org/10.3390/nano2040428




