Investigation of Sub-100 nm Gold Nanoparticles for Laser-Induced Thermotherapy of Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Synthesis and Characterization
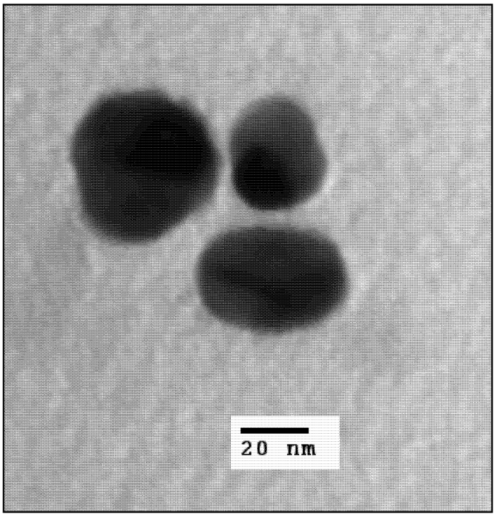
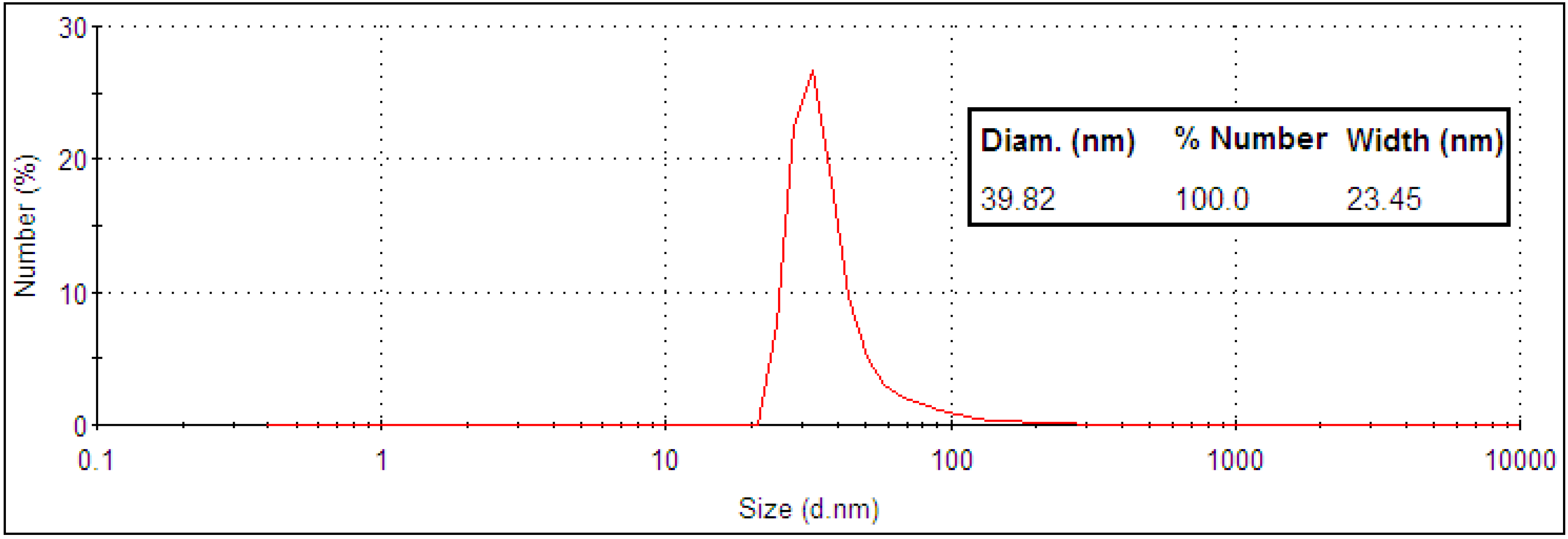
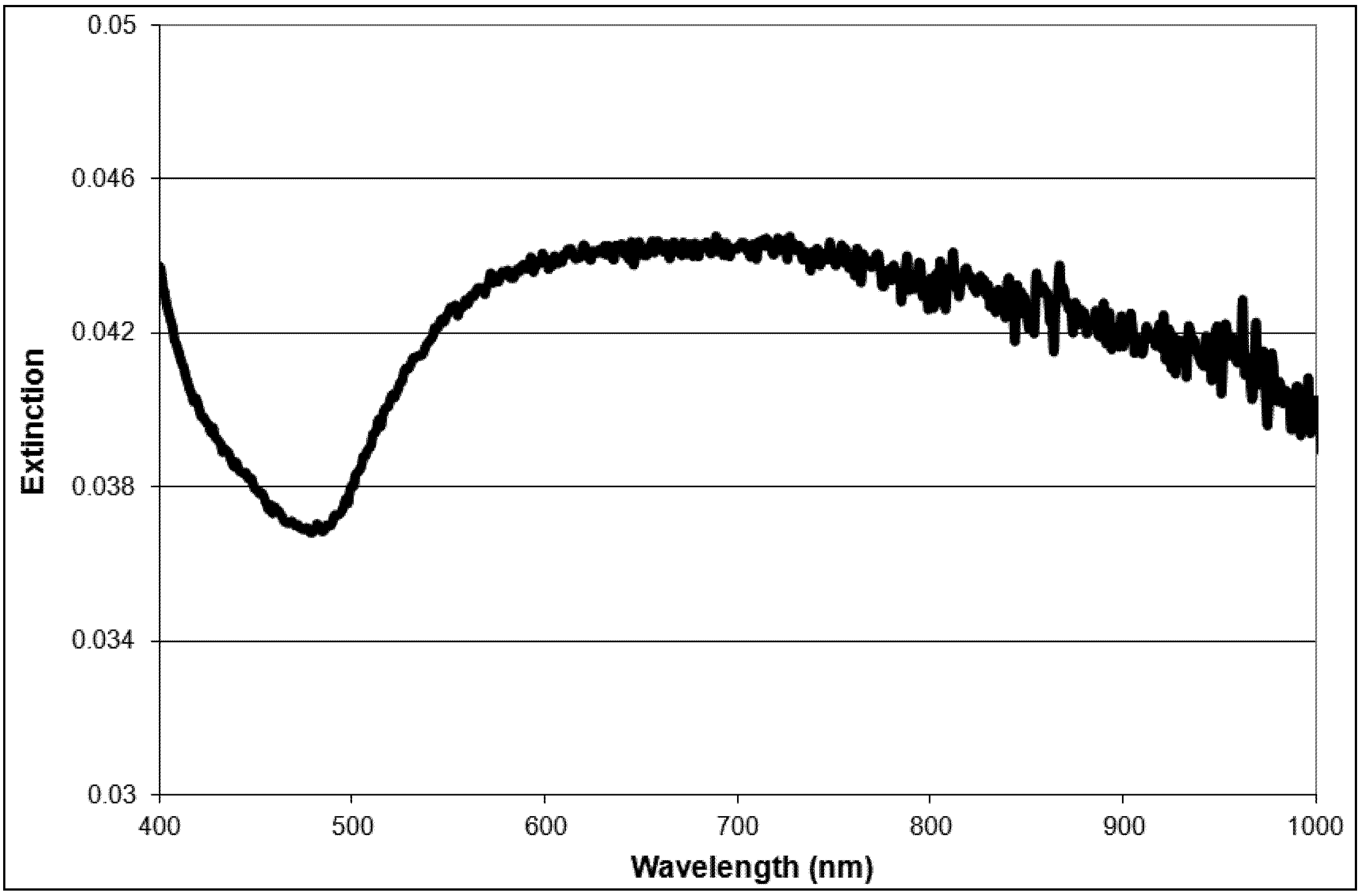
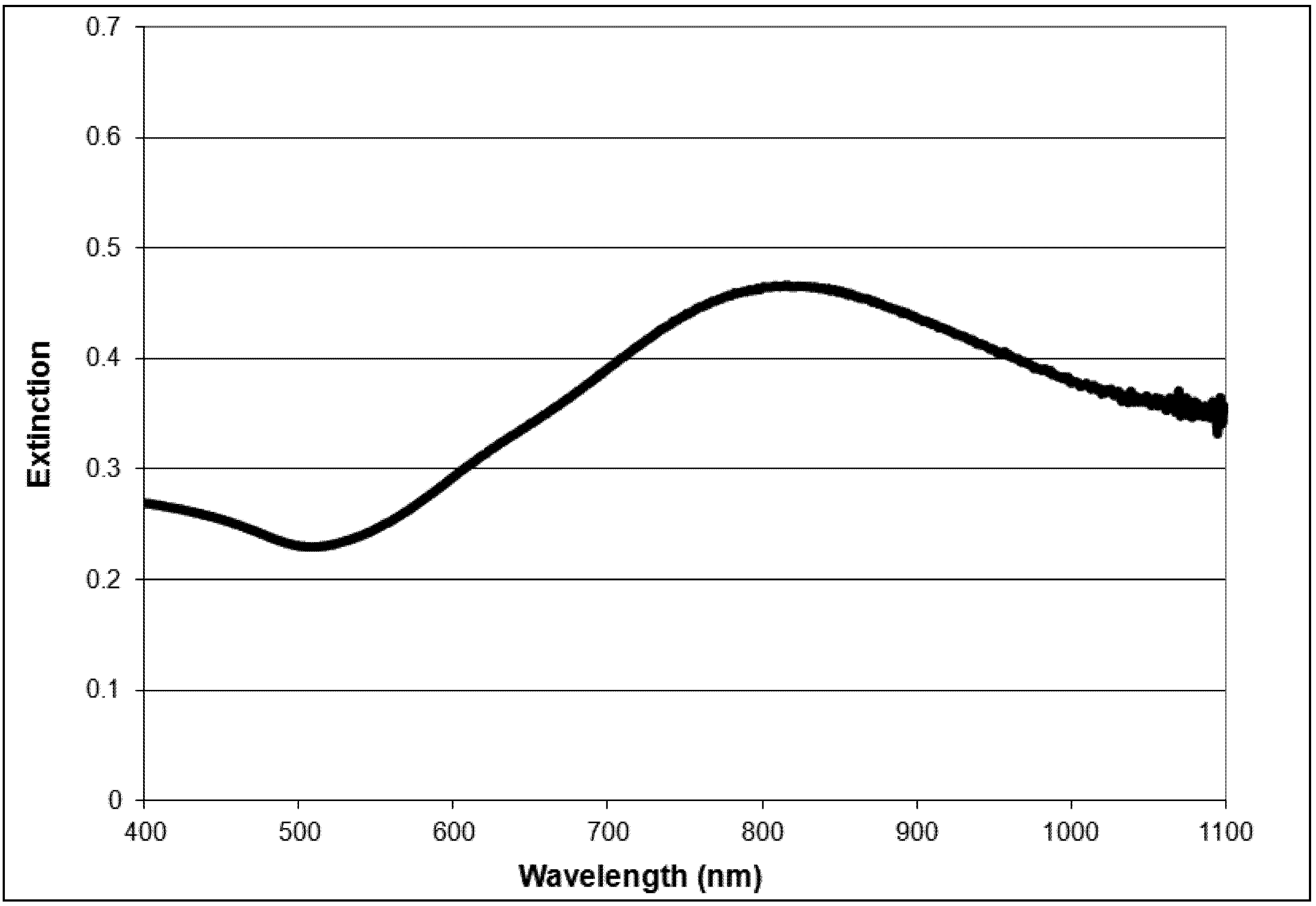
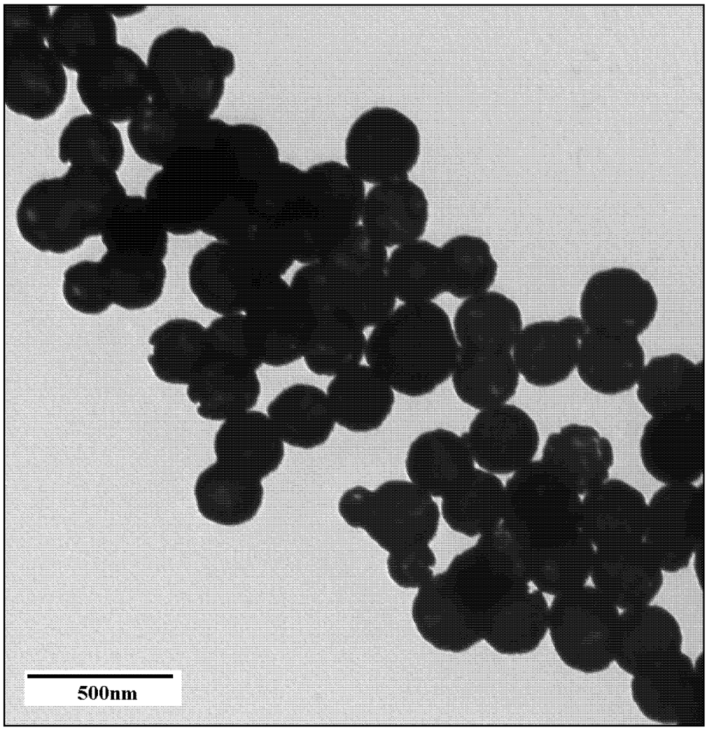
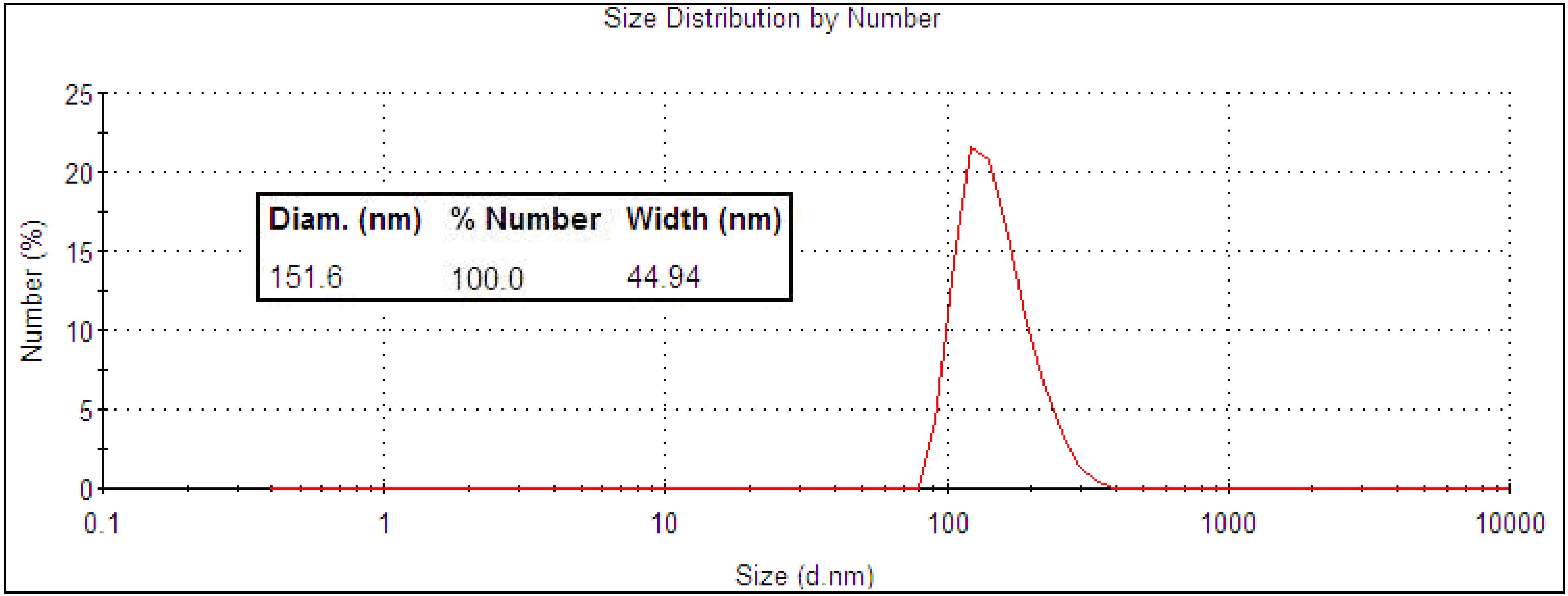
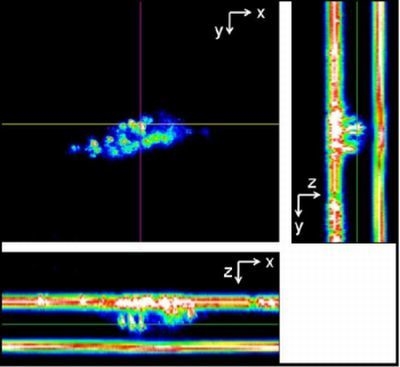
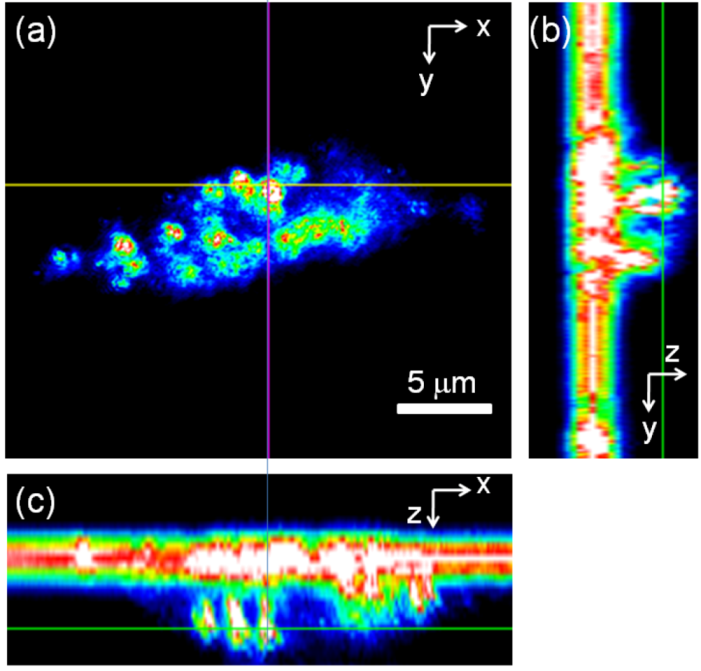
2.2. Light Scattering Microscopy and Cellular Uptake
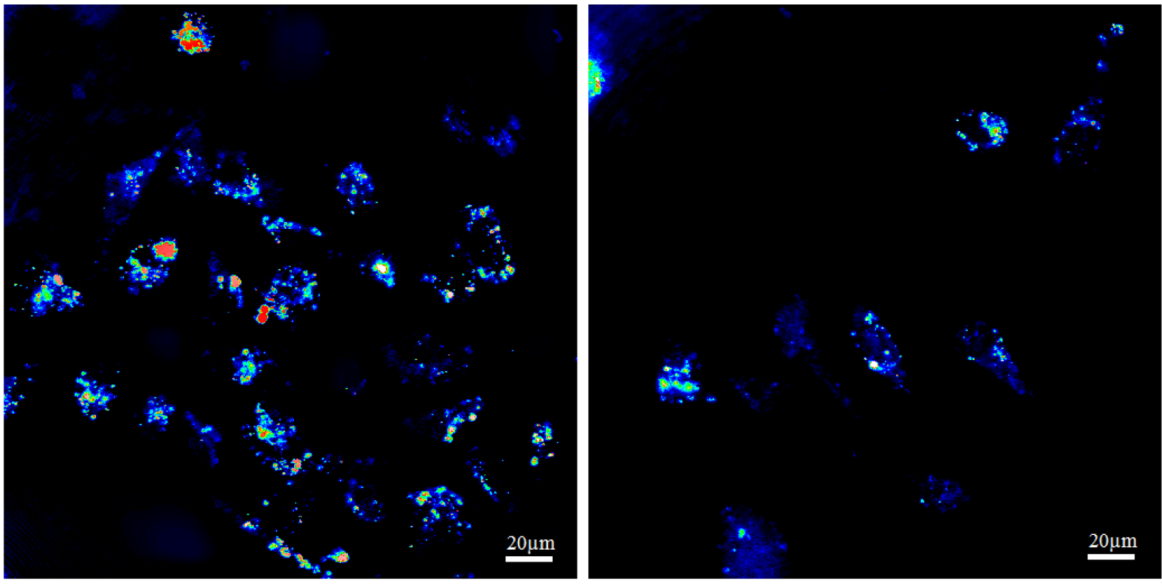
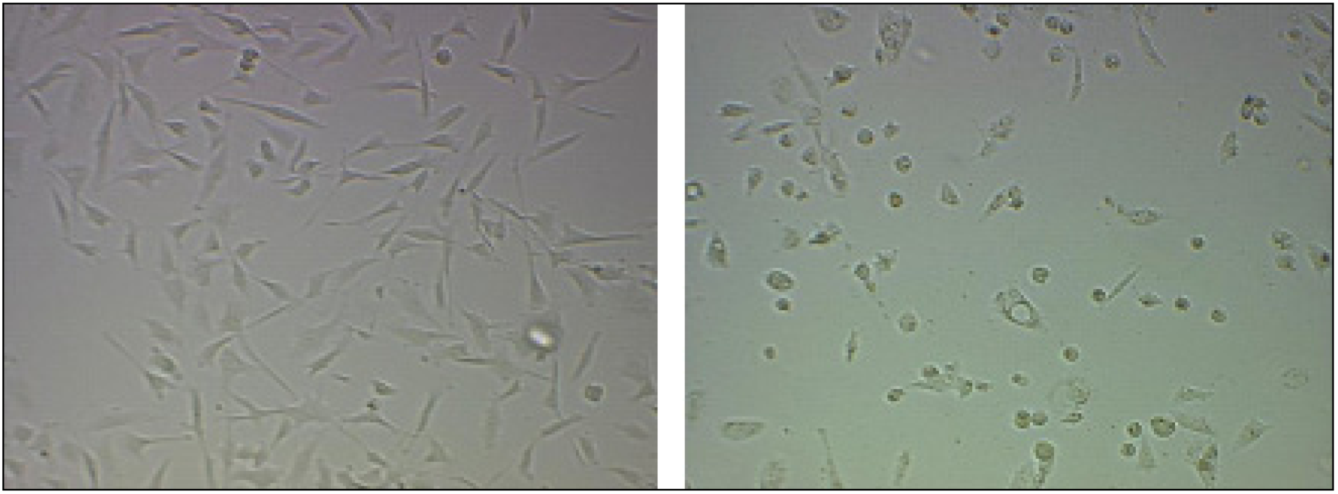
2.3. ASO Gene Therapy

2.4. In Vitro Photothermal Therapy and ASO Gene Therapy
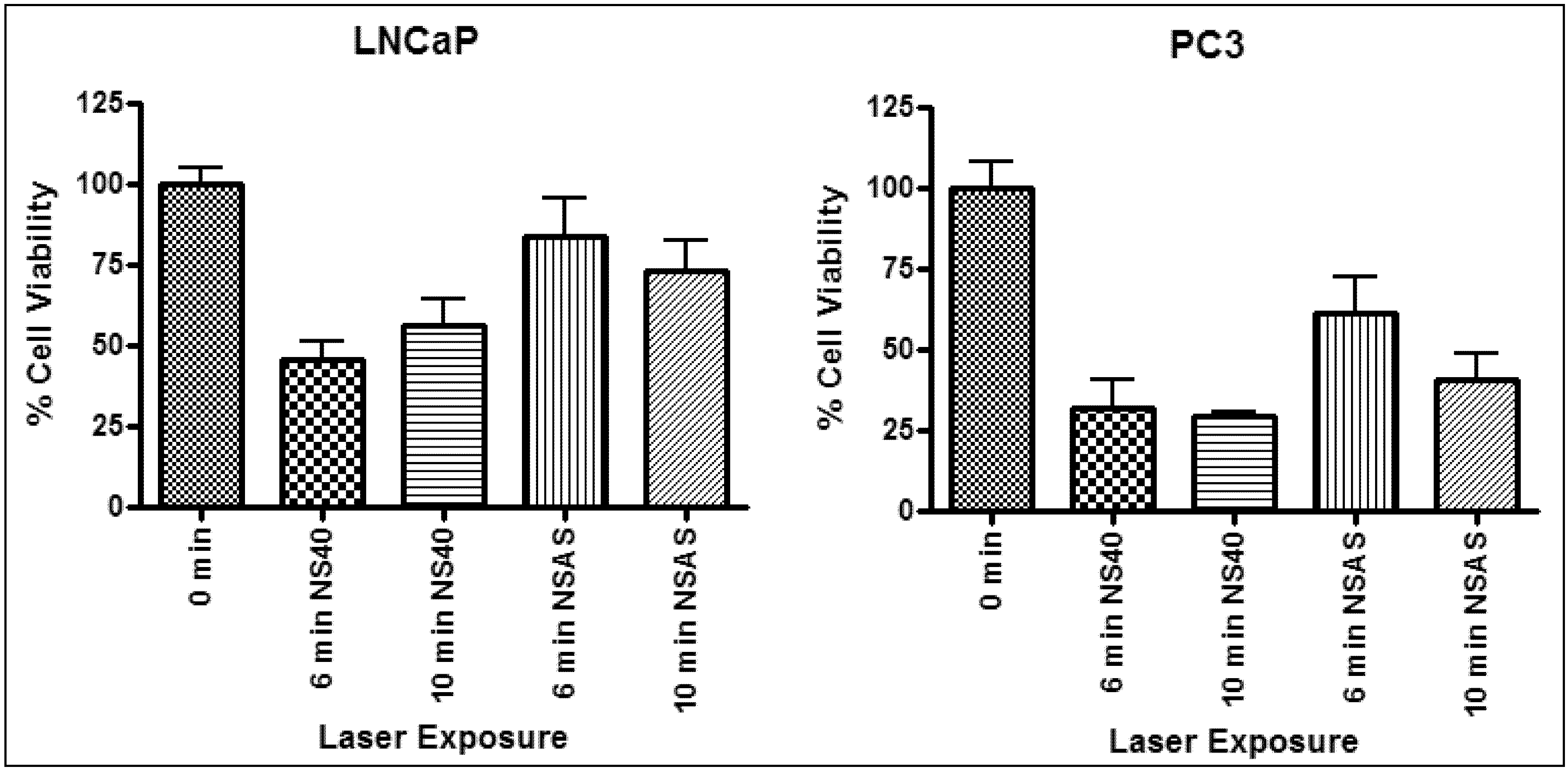
| Laser irradiation (min) | Sample | LNCaP | PC3 | ||||
|---|---|---|---|---|---|---|---|
| Δ Temp (°C) | Δ Cell viability (%) | p value | Δ Temp (°C) | Δ Cell viability (%) | p value | ||
| 6 | NS40 | 12 ± 1 | 54 | <0.05 | 12 ± 1 | 68 | <0.05 |
| NSAS | 8 ± 1 | 27 | <0.05 | 9 ± 1 | 39 | >0.05 | |
| 10 | NS40 | 11 ± 3 | 44 | <0.05 | 12 ± 1 | 71 | <0.05 |
| NSAS | 9 ± 1 | 35 | <0.05 | 9 ± 2 | 60 | <0.05 | |
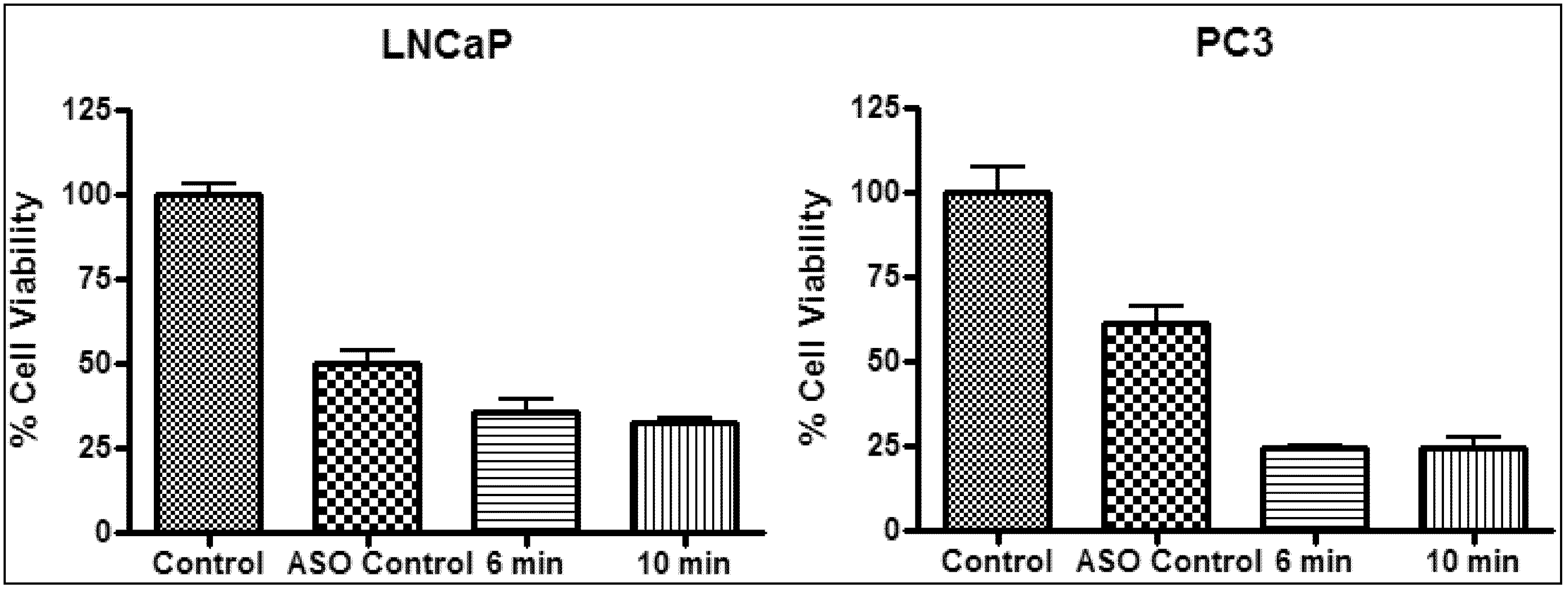
| Sample | Laser irradiation (min) | LNCaP | PC3 | ||||
|---|---|---|---|---|---|---|---|
| Δ Temp (°C) | Δ Cell viability (%) | p value | Δ Temp (°C) | Δ Cell viability (%) | p value | ||
| ASO | - | - | 50 | <0.05 | - | 39 | <0.05 |
| ASO + NS40 | 6 | 11 ± 1 | 64 | <0.05 | 9 ± 1 | 76 | <0.05 |
| ASO + NS40 | 10 | 11 ± 1 | 67 | <0.05 | 10 ± 1 | 76 | <0.05 |
3. Experimental Section
3.1. Materials
3.2. Preparation of Gold Nanoparticles (<100 nm)
3.3. Particle Characterization
3.4. Cell Culture
3.5. Cellular Uptake by Light Scattering Microscopy
3.6. ASO Gene Therapy
3.7. Photothermal Therapy
4. Conclusions
Acknowledgments
Supplementary Files
Supplementary File 1References
- Scher, H.I.; Leiber, S.A.; Fuks, Z.; Cordon-Cardo, C.; Scardino, P.T. Cancer of The Prostate. In Cancer: Principles and Practice of Oncology, 7th; DeVita, V.T., Hellman, S., Rosenberg, S.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 1192–1259. [Google Scholar]
- Zacharakis, E.; Ahmed, H.U.; Ishaq, A.; Scott, R.; Illing, R.; Freeman, A.; Allen, C.; Emberton, M. The feasibility and safety of high-intensity focused ultrasound as salvage therapy for recurrent prostate cancer following external beam radiotherapy. BJU Int. 2008, 102, 786–792. [Google Scholar] [CrossRef]
- Marberger, M.; Carroll, P.R.; Zelefsky, M.J.; Coleman, J.A.; Hricak, H.; Scardino, P.T.; Abenhaim, L.L. New treatments for localized prostate cancer. Urology 2008, 72, 36–43. [Google Scholar]
- Lu, W.; Singh, A.K.; Khan, S.A.; Senapati, D.; Yu, H.; Ray, P.C. Gold nano-popcorn-based targeted diagnosis, nanotherapy treatment, and in situ monitoring of photothermal therapy response of prostate cancer cells using surface-enhanced raman spectroscopy. J. Am. Chem. Soc. 2010, 132, 18103–18114. [Google Scholar]
- Canadian Cancer Society. Prostate Cancer Statistics. Available online: http://www.cancer.ca/British%20Columbia-Yukon/About%20cancer/Cancer%20statistics/Stats%20at%20a%20glance/Prostate%20cancer.aspx?sc_lang=en&r=1 (accessed on 12 October 2010).
- Albertsen, P.C. Treatment of localized prostate cancer: When is active surveillance appropriate? Nat. Rev. Clin. Oncol. 2010, 7, 394–400. [Google Scholar] [CrossRef]
- Kirui, D.K.; Rey, D.A.; Batt, C.A. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology 2010, 21, 105105–105115. [Google Scholar] [CrossRef]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.H.; El-Sayed, I.H.; Chu, H.H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumours under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar]
- Khlebtsov, B.; Zharov, V.; Melnikov, A.; Tuchin, V.; Khlebtsov, N. Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters. Nanotechnology 2006, 17, 5167–5179. [Google Scholar]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and theoretical studies of light-to-heat conversion and collective heating effects in metal nanoparticle solutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar]
- Huang, X.H.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Control. Release 2006, 114, 343–347. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, D.L.; Xi, J.F.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.Y.; Zhang, H.; Xia, Y.N.; Li, X.D. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007, 7, 1318–1322. [Google Scholar] [CrossRef]
- Erickson, T.A.; Tunnel, J.W. Gold Nanoshells in Biomedical Applications. In Mixed Metal Nanomaterials; Kumar, C.S.S.R., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 1–44. [Google Scholar]
- Nanospectra Biosciences Inc. Tumour Ablation Using AuroLase® Therapy. Available online: http://www.nanospectra.com (accessed on 14 August 2010).
- Prevo, B.G.; Esakoff, S.A.; Mikhailovsky, A.; Zasadzinski, J.A. Scalable routes to gold nanoshells with tunable sizes and response to near-infrared pulsed-laser irradiation. Small 2008, 4, 1183–1195. [Google Scholar] [CrossRef]
- Rasch, M.R.; Sokolov, K.V.; Korgel, B.A. Limitations on the optical tunability of small diameter gold nanoshells. Langmuir 2009, 25, 11777–11785. [Google Scholar] [CrossRef]
- Storti, B.; Elisei, F.; Abbruzzetti, S.; Viappiani, C.; Latterini, L. One-pot synthesis of gold nanoshells with high photon-to-heat conversion efficiency. J. Phys. Chem. C 2009, 113, 7516–7521. [Google Scholar]
- Chithrani, B.D.; Chan, W.C.W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar]
- Morino, M.; Tsuzuki, T.; Ishikawa, Y.; Shirakami, T.; Yoshimura, M.; Kiyosuke, Y.I.; Matsunaga, K.; Yoshikumi, C.; Saijo, N. Specific expression of HSP27 in human tumour cell lines in vitro. In Vivo 1997, 11, 179–184. [Google Scholar]
- Sreedhar, A.S.; Csermely, P. Heat shock proteins in the regulation of apoptosis: New strategies in tumour therapy—A comprehensive review. Pharmacol. Ther. 2004, 101, 227–257. [Google Scholar] [CrossRef]
- Kamada, M.; So, A.; Muramaki, M.; Rocchi, P.; Beraldi, E.; Gleave, M. Hsp27 knockdown using nucleotide-based therapies inhibit tumour growth and enhance chemotherapy in human bladder cancer cells. Mol. Cancer Ther. 2007, 6, 299–308. [Google Scholar] [CrossRef]
- Rocchi, P.; Jugpal, P.; So, A.; Sinneman, S.; Ettinger, S.; Fazli, L.; Nelson, C.; Gleave, M. Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro. BJU Int. 2006, 98, 1082–1089. [Google Scholar] [CrossRef]
- Cornford, P.A.; Dodson, A.R.; Parsons, K.F.; Desmond, A.D.; Woolfenden, A.; Fordham, M.; Neoptolemos, J.P.; Ke, Y.Q.; Foster, C.S. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000, 60, 7099–7105. [Google Scholar]
- Dozmorov, M.G.; Hurst, R.E.; Culkin, D.J.; Kropp, B.P.; Frank, M.B.; Osban, J.; Penning, T.M.; Lin, H.K. Unique patterns of molecular profiling between human prostate cancer LNCaP and PC-3 cells. Prostate 2009, 69, 1077–1090. [Google Scholar]
- Tate, A.; Isotani, S.; Bradley, M.J.; Sikes, R.A.; Davis, R.; Chung, L.W.K.; Edlund, M. Met-independent hepatocyte growth factor-mediated regulation of cell adhesion in human prostate cancer cells. BMC Cancer 2006, 6, 197–212. [Google Scholar] [CrossRef]
- Gleave, M.; Miyake, H.; Chi, K. Beyond simple castration: targeting the molecular basis of treatment resistance in advanced prostate cancer. Cancer Chemother. Pharmacol. 2005, 56, S47–S57. [Google Scholar] [CrossRef]
- Liu, Y.L.; Franzen, S. Factors determining the efficacy of nuclear delivery of antisense oligonucleotides by gold nanoparticles. Bioconjug. Chem. 2008, 19, 1009–1016. [Google Scholar] [CrossRef]
- Bilanges, B.; Stokoe, D. Direct comparison of the specificity of gene silencing using antisense oligonucleotides and RNAi. Biochem. J. 2005, 388, 573–583. [Google Scholar] [CrossRef]
- Gabai, V.L.; Budagova, K.R.; Sherman, M.Y. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene 2005, 24, 3328–3338. [Google Scholar] [CrossRef]
- So, A.; Rocchi, P.; Gleave, M. Antisense oligonucleotide therapy in the management of bladder cancer. Curr. Opin. Urol. 2005, 15, 320–327. [Google Scholar] [CrossRef]
- Hadchity, E.; Aloy, M.T.; Paulin, C.; Armandy, E.; Watkin, E.; Rousson, R.; Gleave, M.; Chapet, O.; Rodriguez-Lafrasse, C. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol. Ther. 2009, 17, 1387–1394. [Google Scholar] [CrossRef]
- Rossi, A.; Ciafre, S.; Balsamo, M.; Pierimarchi, P.; Santoro, M.G. Targeting the heat shock factor 1 by RNA interference: A potent tool to enhance hyperthermochemotherapy efficacy in cervical cancer. Cancer Res. 2006, 66, 7678–7685. [Google Scholar] [CrossRef]
- Huang, H.C.; Barua, S.; Kay, D.B.; Rege, K. Simultaneous enhancement of photothermal stability and gene delivery efficacy of gold nanorods using polyelectrolytes. ACS Nano 2009, 3, 2941–2952. [Google Scholar] [CrossRef]
- Jain, P.K.; Ei-Sayed, M.A. Surface plasmon resonance sensitivity of metal nanostructures: Physical basis and universal scaling in metal nanoshells. J. Phys. Chem. C 2007, 111, 17451–17454. [Google Scholar] [CrossRef]
- Preston, T.C.; Signorell, R. Preparation and optical properties of metallodielectric core-shell-corona particles. J. Phys. Chem. C 2008, 112, 17844–17848. [Google Scholar]
- Zhai, Y.M.; Zhai, J.F.; Wang, Y.L.; Guo, S.J.; Ren, W.; Dong, S.J. Fabrication of iron oxide core/gold shell submicrometer spheres with nanoscale surface roughness for efficient surface-enhanced raman scattering. J. Phys. Chem. C 2009, 113, 7009–7014. [Google Scholar]
- Perez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar]
- Alberts, B. Molecular Biology of the Cell, 4th ed; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Arnida, A.; Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217. [Google Scholar]
- Niidome, T.; Akiyama, Y.; Yamagata, M.; Kawano, T.; Mori, T.; Niidome, Y.; Katayama, Y. Poly(ethylene glycol)-modified gold nanorods as a photothermal nanodevice for hyperthermia. J. Biomater. Sci. 2009, 20, 1203–1215. [Google Scholar]
- Hamblin, M.R.; Miller, J.L.; Rizvi, I.; Loew, H.G.; Hasan, T. Pegylation of charged polymer-photosensitiser conjugates: Effects on photodynamic efficacy. Br. J. Cancer 2003, 89, 937–943. [Google Scholar]
- Calderwood, S.K.; Asea, A. Targeting HSP70-induced thermotolerance for design of thermal sensitizers. Int. J. Hyperth. 2002, 18, 597–608. [Google Scholar] [CrossRef]
- Stern, J.M.; Cadeddu, J.A. Emerging use of nanoparticles for the therapeutic ablation of urologic malignancies. Urol. Oncol.-Semin. Orig. Investig. 2008, 26, 93–96. [Google Scholar]
- Cheng, F.Y.; Chen, C.T.; Yeh, C.S. Comparative efficiencies of photothermal destruction of malignant cells using antibody-coated silica@Au nanoshells, hollow Au/Ag nanospheres and Au nanorods. Nanotechnology 2009, 20, 425104–425113. [Google Scholar] [CrossRef]
- Takahashi, H.; Niidome, T.; Nariai, A.; Niidome, Y.; Yamada, S. Photothermal reshaping of gold nanorods prevents further cell death. Nanotechnology 2006, 17, 4431–4435. [Google Scholar] [CrossRef]
- Stauffer, P.R.; Goldberg, S.N. Introduction: Thermal ablation therapy. Int. J. Hyperth. 2004, 20, 671–677. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar]
- Sun, Y.Y.; Yan, F.; Yang, W.W.; Zhao, S.A.; Yang, W.S.; Sun, C.Q. Effect of silica nanoparticles with different sizes on the catalytic activity of glucose oxidase. Anal. Bioanal. Chem. 2007, 387, 1565–1572. [Google Scholar] [CrossRef]
- Duff, D.G.; Baiker, A.; Edwards, P.P. A new hydrosol of gold clusters 1. Formation and particle size variation. Langmuir 1993, 9, 2301–2309. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Lee, R.; Halas, N.J.; West, J.L. Whole-Blood Immunoassay Facilitated by Gold Nanoshell-Conjugate Antibodies. In Nanobiotechnology Protocols; Rosenthal, S.J., Wright, D.W., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 101–111. [Google Scholar]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Au, L.; Zheng, D.S.; Zhou, F.; Li, Z.Y.; Li, X.D.; Xia, Y.N. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano 2008, 2, 1645–1652. [Google Scholar]
- Liu, Y.L.; Shipton, M.K.; Ryan, J.; Kaufman, E.D.; Franzen, S.; Feldheim, D.L. Synthesis, stability, and cellular internalization of gold nanoparticles containing mixed peptide-poly(ethylene glycol) monolayers. Anal. Chem. 2007, 79, 2221–2229. [Google Scholar]
- Weiss, A.; Preston, T.C.; Popov, J.; Li, Q.F.; Wu, S.; Chou, K.C.; Burt, H.M.; Bally, M.B.; Signorell, R. Selective recognition of rituximab-functionalized gold nanoparticles by lymphoma cells studied with 3D Imaging. J. Phys. Chem. C 2009, 113, 20252–20258. [Google Scholar]
- Leung, J.P. Photothermal Therapy of Prostate Cancer using Gold Nanoparticles. M.Sc. Thesis, University of British Columbia, Vancouver, Canada, November 2010. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leung, J.P.; Wu, S.; Chou, K.C.; Signorell, R. Investigation of Sub-100 nm Gold Nanoparticles for Laser-Induced Thermotherapy of Cancer. Nanomaterials 2013, 3, 86-106. https://doi.org/10.3390/nano3010086
Leung JP, Wu S, Chou KC, Signorell R. Investigation of Sub-100 nm Gold Nanoparticles for Laser-Induced Thermotherapy of Cancer. Nanomaterials. 2013; 3(1):86-106. https://doi.org/10.3390/nano3010086
Chicago/Turabian StyleLeung, Jennifer P., Sherry Wu, Keng C. Chou, and Ruth Signorell. 2013. "Investigation of Sub-100 nm Gold Nanoparticles for Laser-Induced Thermotherapy of Cancer" Nanomaterials 3, no. 1: 86-106. https://doi.org/10.3390/nano3010086