Magnetism of Amorphous and Nano-Crystallized Dc-Sputter-Deposited MgO Thin Films
Abstract
:1. Introduction
2. Results and Discussion
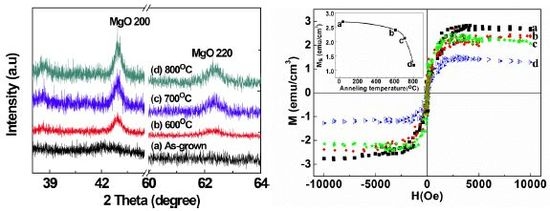
2.1. Structural Properties of Films

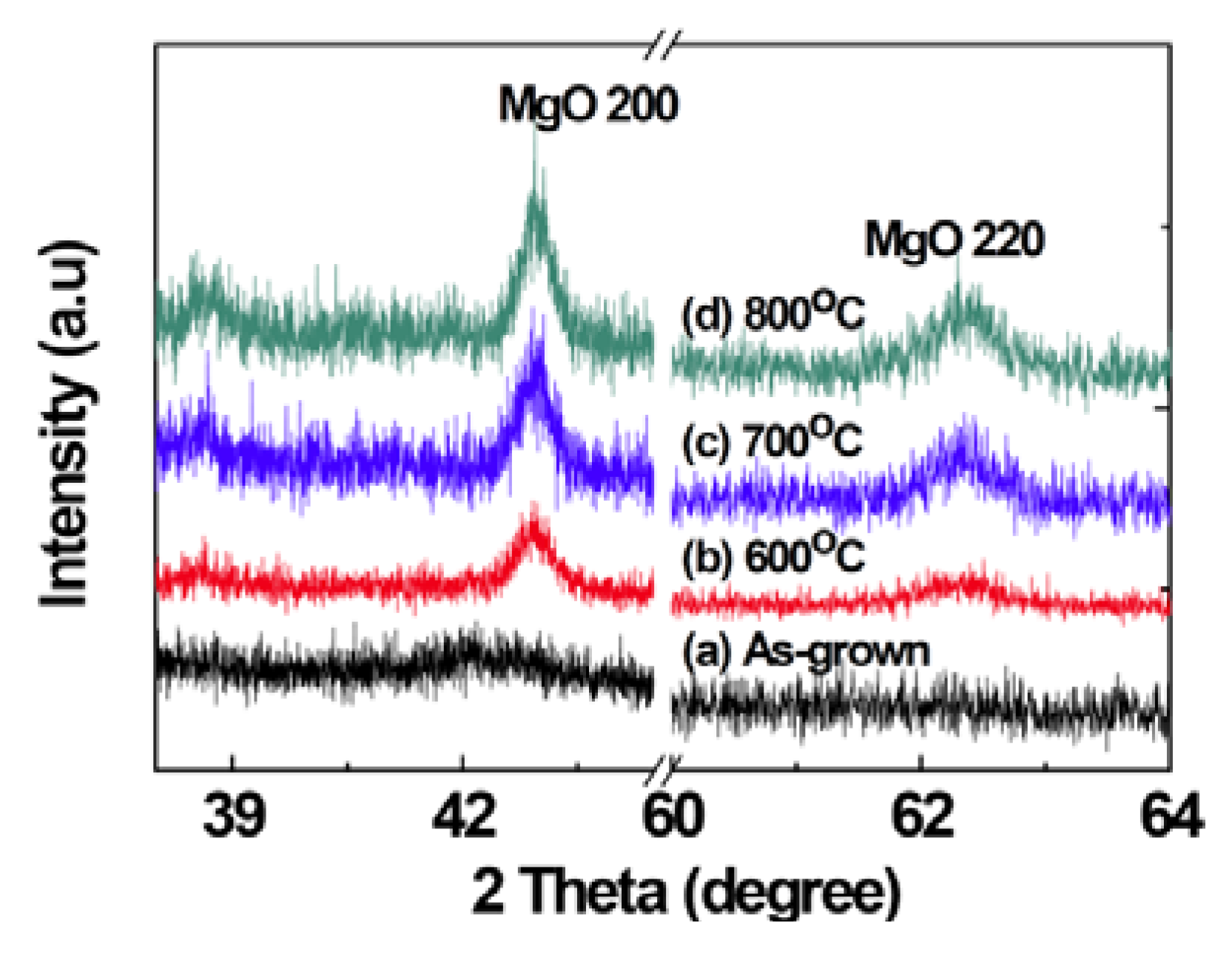
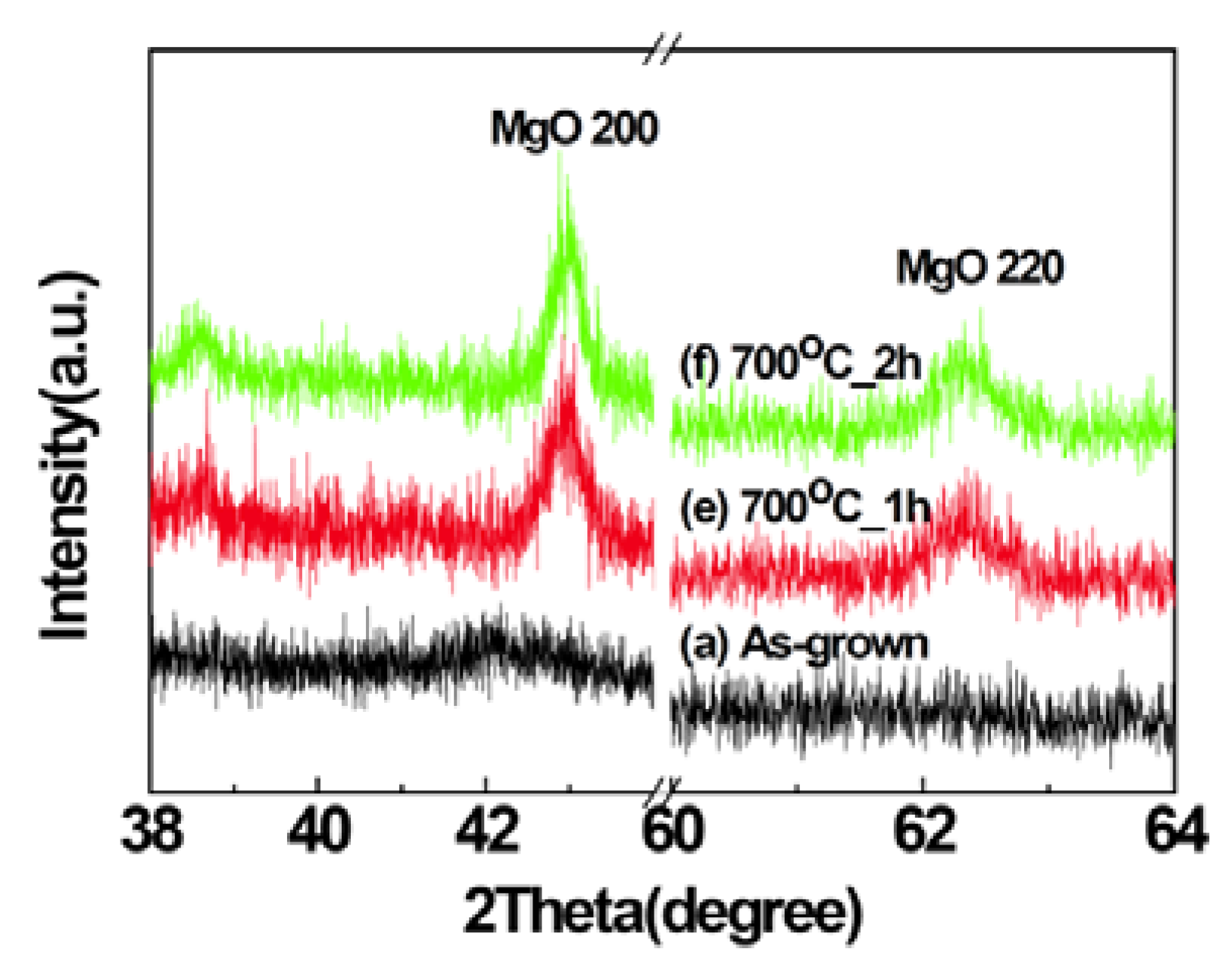
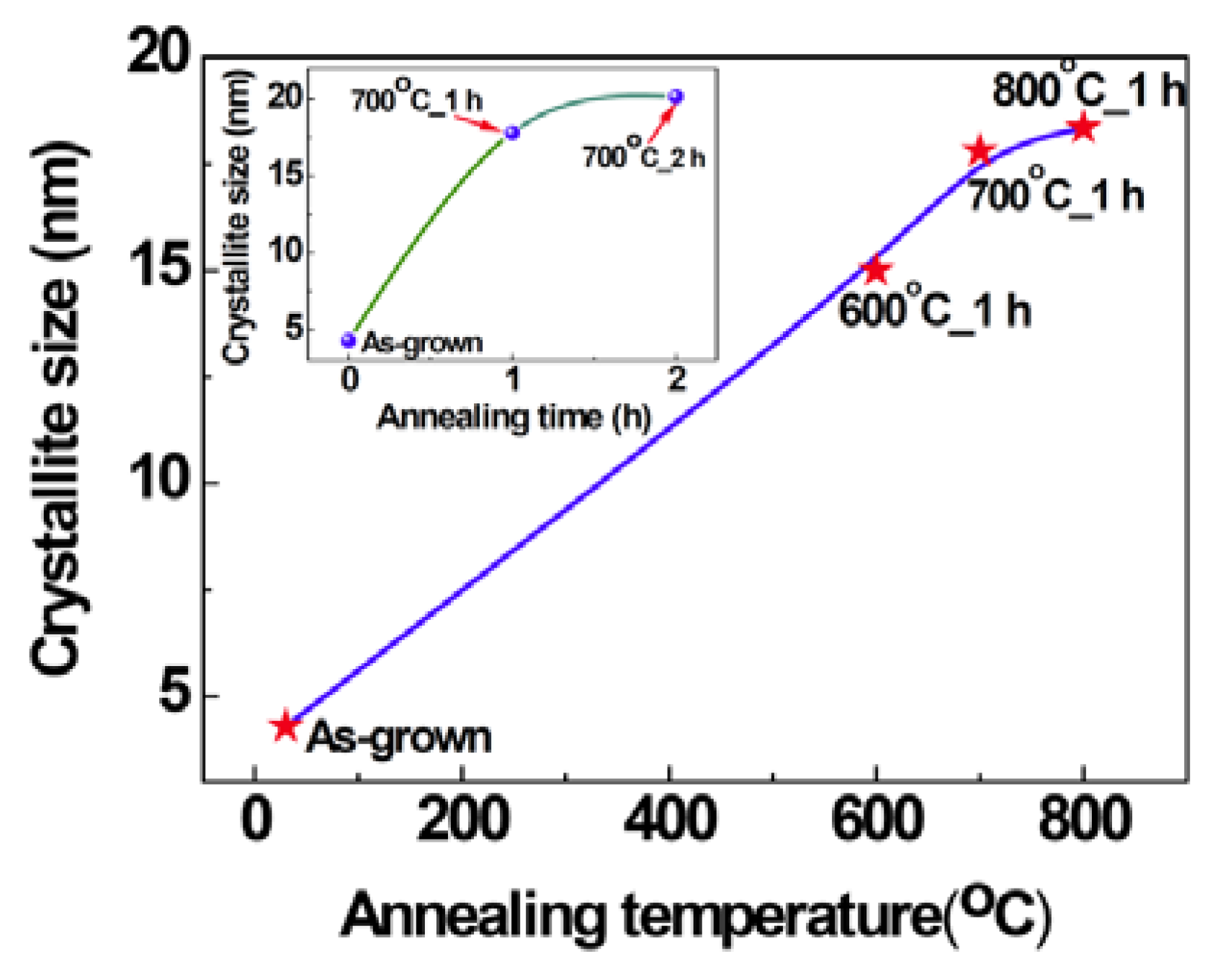
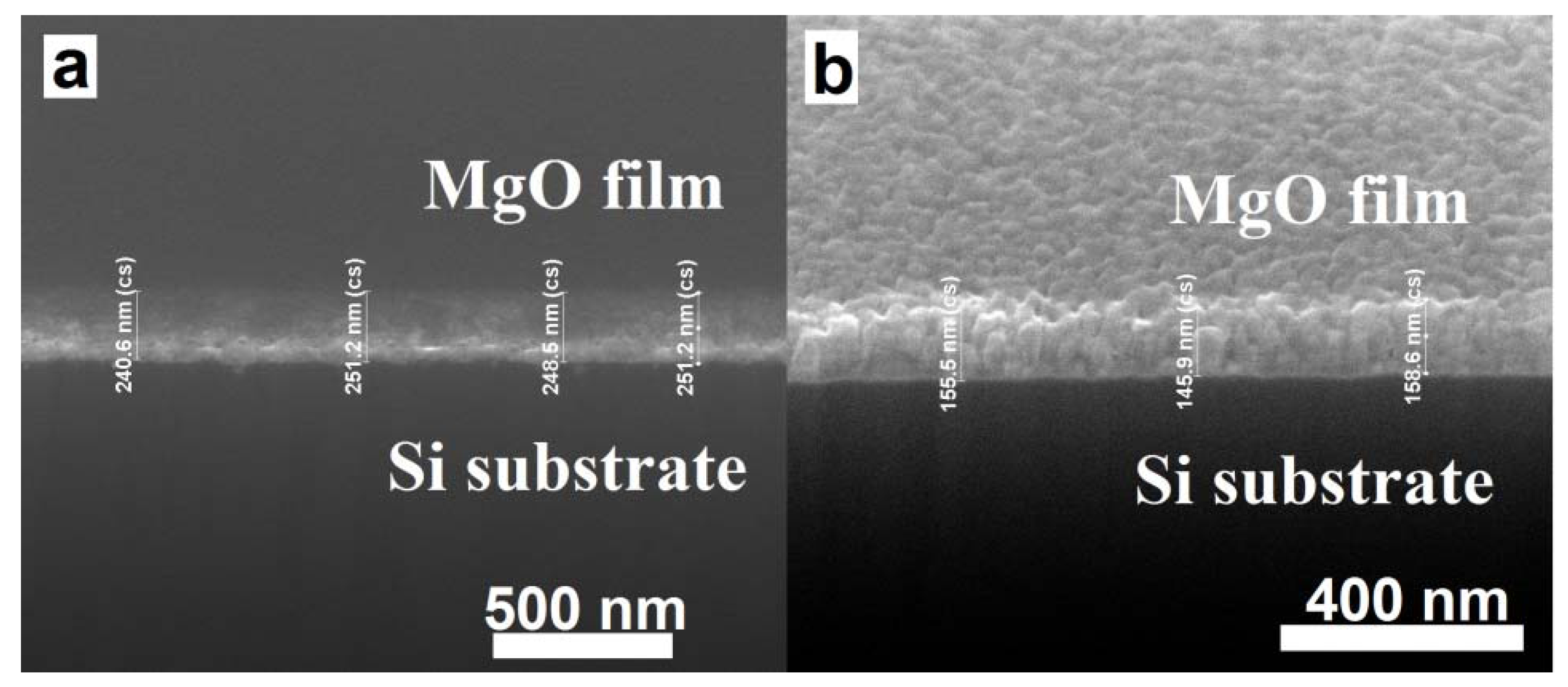
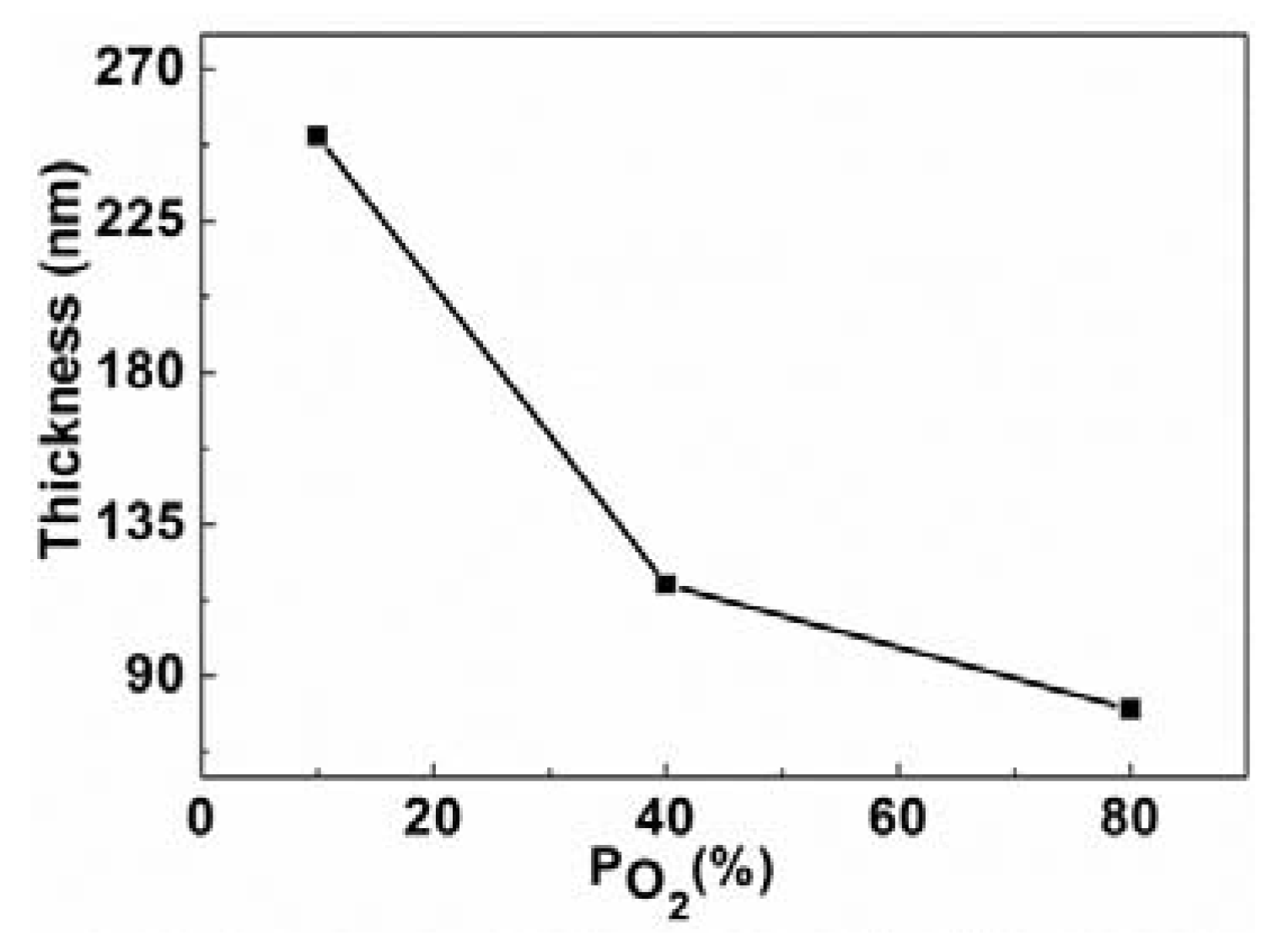
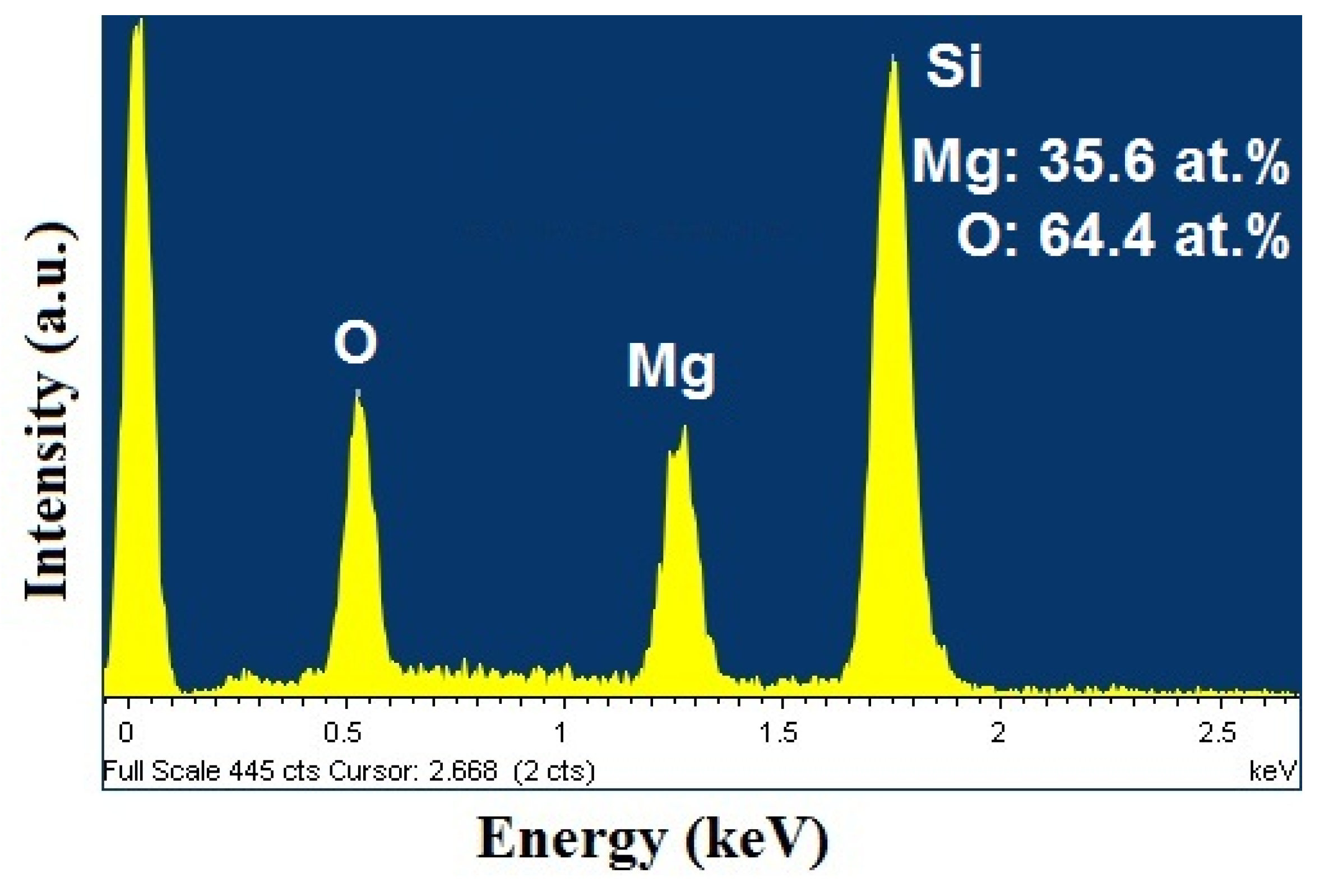
2.2. Scanning Electron Microscopy (SEM)/Focused Ion Beam (FIB) and Energy Dispersive X-Ray Analyses

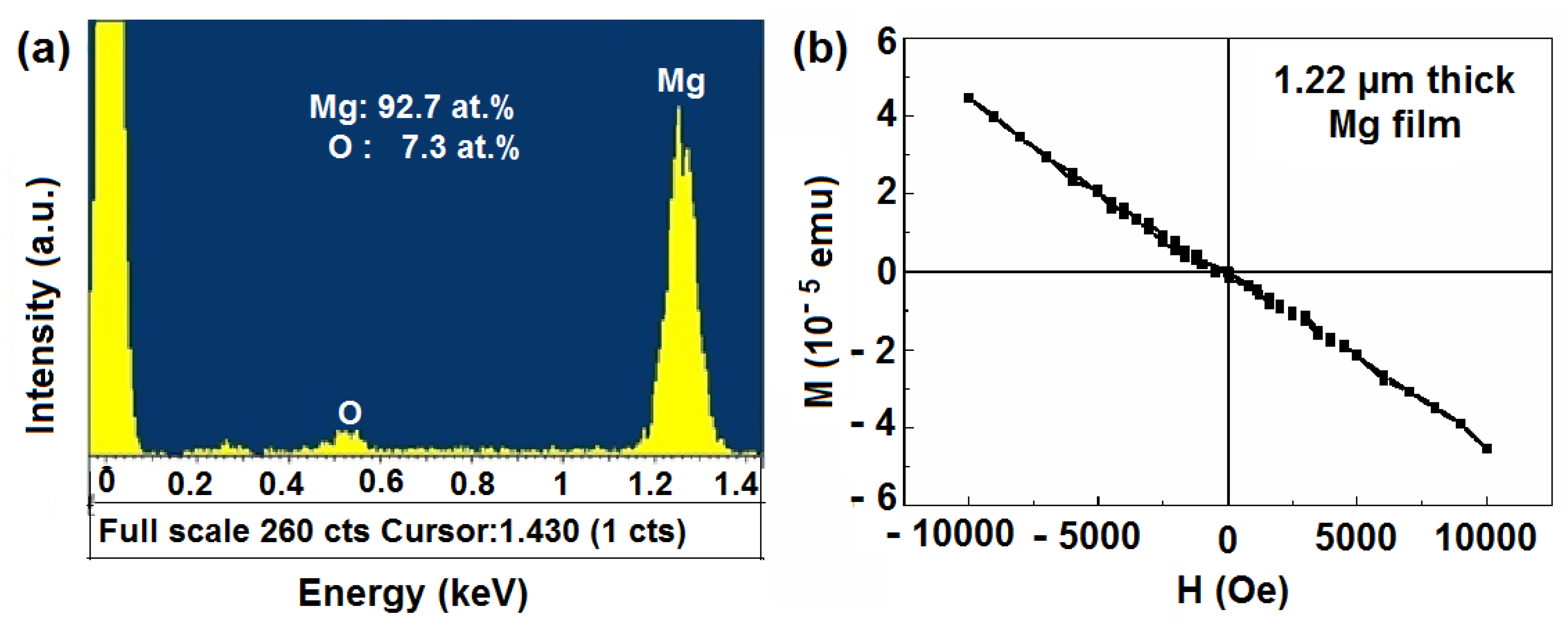
2.3. Magnetic Properties

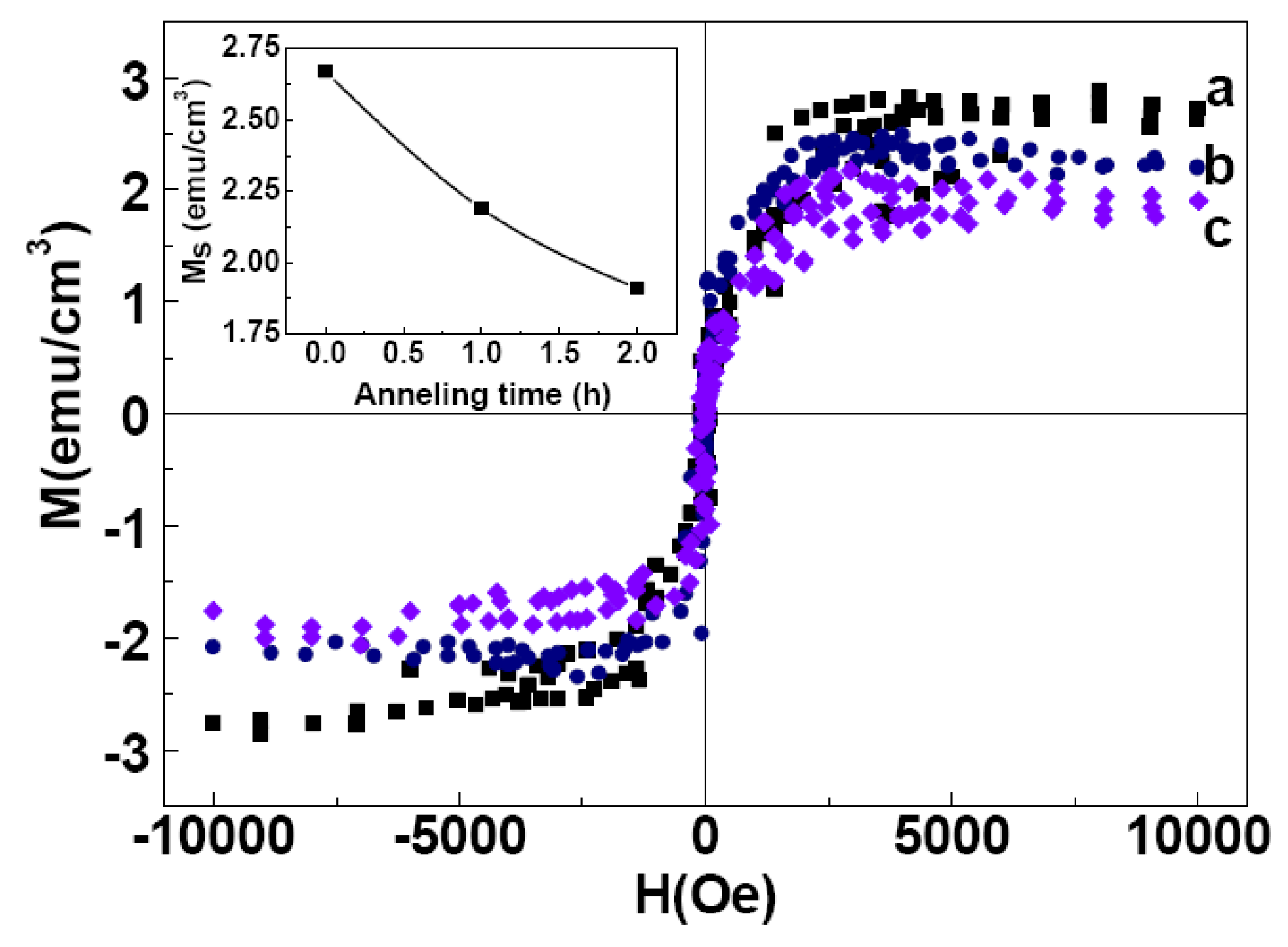
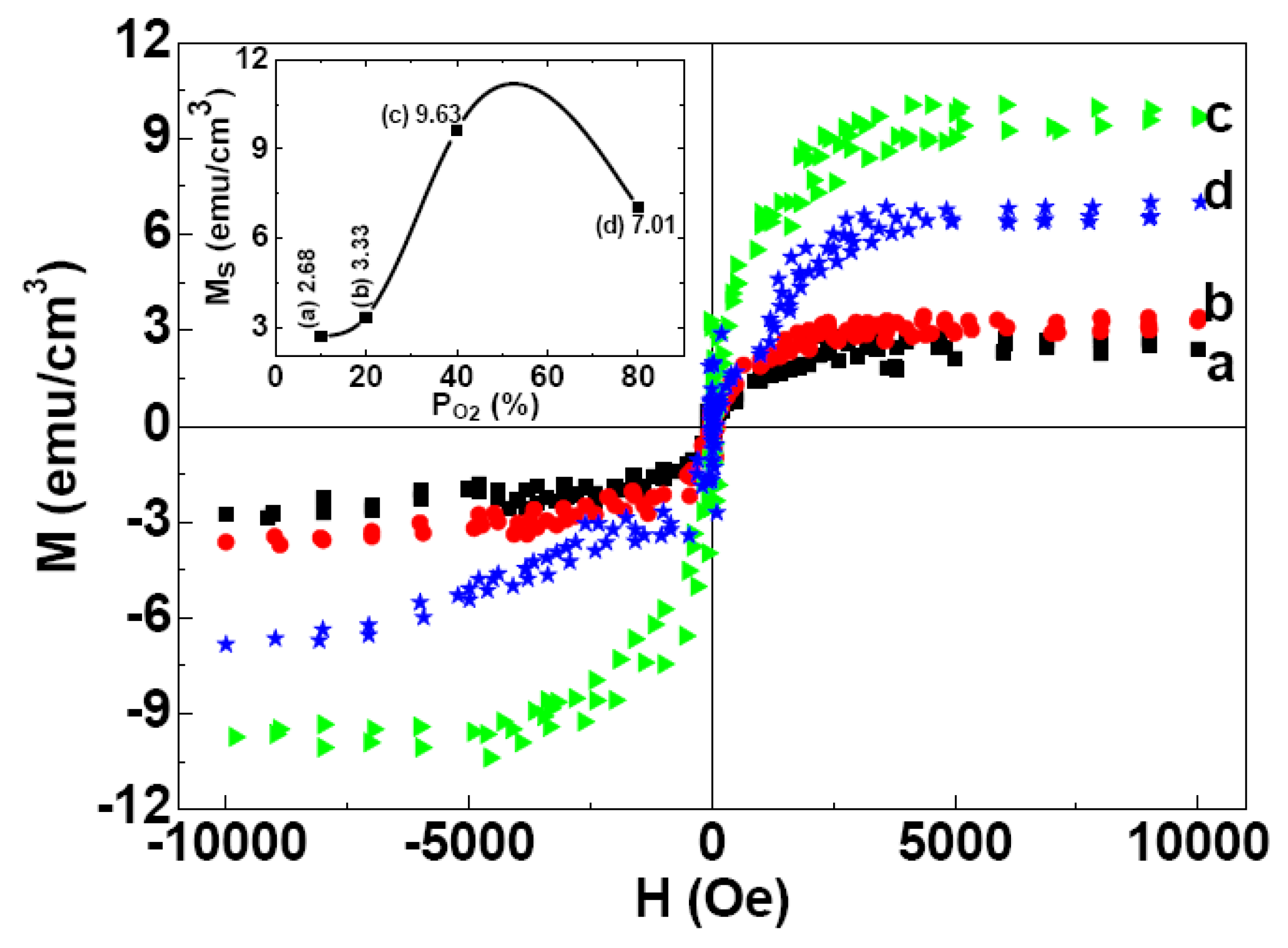
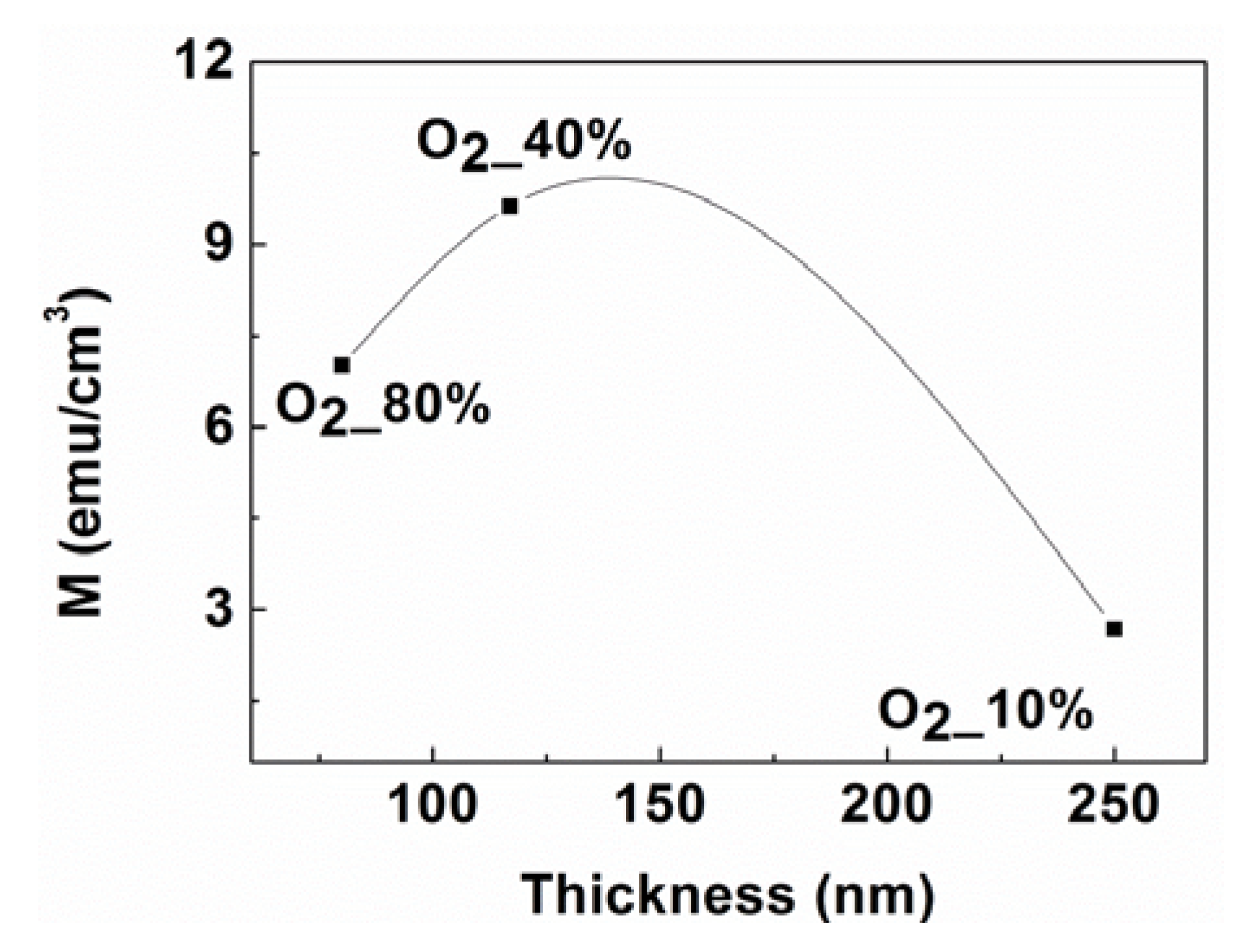
3. Experiment
3.1. Fabrication of MgO Thin Films
3.2. Characterization Techniques
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Renaud, G. Oxide surfaces and metal/oxide interfaces studied by grazing angle incidence X-ray scattering. Surf. Sci. Rep. 1998, 32, 1–90. [Google Scholar] [CrossRef]
- Cáceres, D.; Vergara, I.; González, R. Microstructural characterization of MgO thin films grown by radio-frequency sputtering. Target and substrate-temperature effect. J. Appl. Phys. 2003, 93, 4300–4305. [Google Scholar]
- Balcells, L.I.; Beltrán, J.I.; Martíne-Boubeta, C.; Konstantinović, Z.; Arbiol, J.; Martinez, B. Aging of magnetic properties in MgO films. Appl. Phys. Lett. 2010, 97, 252503:1–252503:3. [Google Scholar]
- Nagashima, K.; Yanagida, T.; Tanaka, H.; Kawai, T. Epitaxial growth of MgO nanowires by pulsed laser deposition. J. Appl. Phys. 2007, 101, 124304:1–124304:4. [Google Scholar]
- Ikeda, S.; Miura, K.; Yamamoto, H.; Mizunuma, K.; Gan, H.D.; Endo, M.; Kanai, S.; Hayakawa, J.; Matsukura, F.; Ohno, H. A perpendicular-anisotropy CoFeB–MgO magnetic tunnel junction. Nat. Mater. 2010, 9, 721–724. [Google Scholar] [CrossRef]
- Parkin, S.S.P.; Kaiser, C.; Panchula, A.; Rice, P.M.; Hughes, B.; Smant, M.; Yang, S.-H. Giant tunnelling magnetoresistance at room temperature with MgO (100) tunnel barriers. Nat. Mater. 2004, 3, 862–867. [Google Scholar] [CrossRef]
- Vuoristo, P.; Mantylä, T.; Kettunen, P. Adhesion and structure of rf sputtered magnesium oxide coatings on various metal substrates. J. Vac. Sci. Technol. A 1986, 4, 2932–2937. [Google Scholar] [CrossRef]
- Costache, M.V.; Moodera, J.S. All magnesium diboride Josephson junctions with MgO and native oxide barriers. Appl. Phys. Lett. 2010, 96, 082508:1–082508:3. [Google Scholar]
- Yamamori, H.; Shoji, A. Improvement of uniformity of NbCN/MgO/NbCN Josephson junctions for large-scale circuit applications. Supercond. Sci. Technol. 1999, 12, 877–879. [Google Scholar] [CrossRef]
- Sugiyama, K.; Akazawa, K.; Oshima, M.; Miura, H.; Matsuda, T.; Nomura, O. Ammonia synthesis by means of plasma over MgO catalyst. Plasma Chem. Plasma Process. 1986, 6, 179–193. [Google Scholar] [CrossRef]
- Nibbelke, R.H.; Scheerová, J.; de Croon, M.H.J.M.; Maroon, G.B. The oxidative coupling of methane over MgO-based catalysts: A steady-state isotope transient kinetic analysis. J. Catal. 1995, 156, 106–119. [Google Scholar] [CrossRef]
- Chen, Y.; Sibley, W.A.; Srygley, F.D.; Weeks, R.A.; Hensley, E.B.; Kroes, R.L. Negative-ion vacancies in irradiated MgO. J. Phys. Chem. Solids 1968, 29, 863–865. [Google Scholar] [CrossRef]
- Freund, M.M.; Freund, F.; Batllo, F. Highly mobile oxygen holes in magnesium oxide. Phys. Rev. Lett. 1989, 63, 2096–2099. [Google Scholar] [CrossRef]
- Martinez Boubeta, C.; Beltrán, J.I.; Balcells, L.I.; Konstantinović, Z.; Valencia, S.; Schmitz, D.; Arbiol, J.; Estrade, S.; Cornil, J.; Martínez, B. Ferromagnetism in transparent thin films of MgO. Phys. Rev. B 2010, 82, 024405:1–024405:7. [Google Scholar]
- Nitesh, K.; Sanyal, D.; Sundaresan, A. Defect induced ferromagnetism in MgO nanoparticles studied by optical and positron annihilation spectroscopy. Chem. Phys. Lett. 2009, 477, 360–364. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Pacchioni, G. Electronic structure of F and V centers on the MgO surface. J. Phys. Chem. 1995, 99, 17010–17018. [Google Scholar] [CrossRef]
- Gu, B.; Bulut, N.; Ziman, T.; Maekawa, S. Possible d° ferromagnetism in MgO doped with nitrogen. Phys. Rev. B 2009, 79. [Google Scholar] [CrossRef]
- Grob, M.; Pratzer, M.; Morgenstern, M.; Lezaic, M. Catalytic growth of N-doped MgO on Mo(001). Phys. Rev. B 2012, 86, 075455. [Google Scholar] [CrossRef]
- Moyses Araujo, C.; Kapilashrami, M.; Xu, J.; Jayakumar, O.D.; Nagar, S.; Wu, Y.; Århammar, C.; Johansson, B.; Belova, L.; Ahuja, R.; et al. Room temperature ferromagnetism in pristine MgO thin films. Appl. Phys. Lett. 2010, 96, 232505:1–232505:3. [Google Scholar]
- Prucnal, S.; Shalimov, A.; Ozerov, M.; Potzger, K.; Skorupa, W. Magnetic and optical properties of virgin arc furnace grown MgO crystals. J. Cryst. Growth 2012, 339, 70–74. [Google Scholar] [CrossRef]
- Gao, F.; Hu, J.F.; Yang, C.L.; Zheng, Y.J.; Qin, H.W.; Sun, L.; Kong, X.W.; Jiang, M.H. First-principles study of magnetism driven by intrinsic defects in MgO. Solid State Commun. 2009, 149, 855–858. [Google Scholar] [CrossRef]
- Beltrán, J.I.; Monty, C.; Balcells, L.I.; Martínez-Boubeta, C. Possible d° ferromagnetism in MgO. Solid State Commun. 2009, 149, 1654–1657. [Google Scholar] [CrossRef]
- Yu, H.K.; Lee, J.-L. Growth mechanism of MgO film on Si (100): Domain matching epitaxy, strain relaxation, preferred orientation formation. Cryst. Growth Des. 2010, 10, 5200–5204. [Google Scholar] [CrossRef]
- Martínez Boubeta, C.; Cebollada, A.; Calleja, J.F.; Contreras, C.; Peirό, F.; Cornet, A. Magnetization reversal and magnetic anisotropies in epitaxial Fe/MgO and Fe/MgO/Fe heterostructures grown on Si (001). J. Appl. Phys. 2003, 93, 2126–2134. [Google Scholar] [CrossRef]
- Kapilashrami, M.; Xu, J.; Ström, V.; Rao, K.V.; Belova, L. Transition from ferromagnetism to diamagnetism in undoped ZnO thin films. Appl. Phys. Lett. 2009, 95, 033104:1–033104:3. [Google Scholar]
- Kim, D.Y.; Hong, J.S.; Park, Y.R.; Kim, K.J. The origin of oxygen vacancy induced ferromagnetism in undoped TiO2. J. Phys. Condens. Matter 2009, 21, 195405:1–195405:4. [Google Scholar]
- Hong, N.H.; Sakai, J.; Poirot, N.; Brize, V. Room-temperature ferromagnetism observed in undoped semiconducting and insulating oxide thin films. Phys. Rev. B 2006, 73, 132404:1–132404:4. [Google Scholar]
- Hong, N.H. Magnetism due to defects/oxygen vacancies in HfO2 thin films. Phys. Status Solidi C 2007, 4, 1270–1275. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Li, Y.; Yang, D.; Xu, Y.; Yan, M. Origin of room temperature ferromagnetism in MgO films. Appl. Phys. Lett. 2013, 102, 072406:1–072406:4. [Google Scholar]
- Kapilashrami, M.; Xu, J.; Rao, K.V.; Belova, L.; Carlegrim, E.; Fahlman, M. Experimental evidence for ferromagnetism at room temperature in MgO thin films. J. Phys. Condens. Matter 2010, 22, 345004:1–345004:5. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mahadeva, S.K.; Fan, J.; Biswas, A.; Sreelatha, K.S.; Belova, L.; Rao, K.V. Magnetism of Amorphous and Nano-Crystallized Dc-Sputter-Deposited MgO Thin Films. Nanomaterials 2013, 3, 486-497. https://doi.org/10.3390/nano3030486
Mahadeva SK, Fan J, Biswas A, Sreelatha KS, Belova L, Rao KV. Magnetism of Amorphous and Nano-Crystallized Dc-Sputter-Deposited MgO Thin Films. Nanomaterials. 2013; 3(3):486-497. https://doi.org/10.3390/nano3030486
Chicago/Turabian StyleMahadeva, Sreekanth K., Jincheng Fan, Anis Biswas, K. S. Sreelatha, Lyubov Belova, and K. V. Rao. 2013. "Magnetism of Amorphous and Nano-Crystallized Dc-Sputter-Deposited MgO Thin Films" Nanomaterials 3, no. 3: 486-497. https://doi.org/10.3390/nano3030486