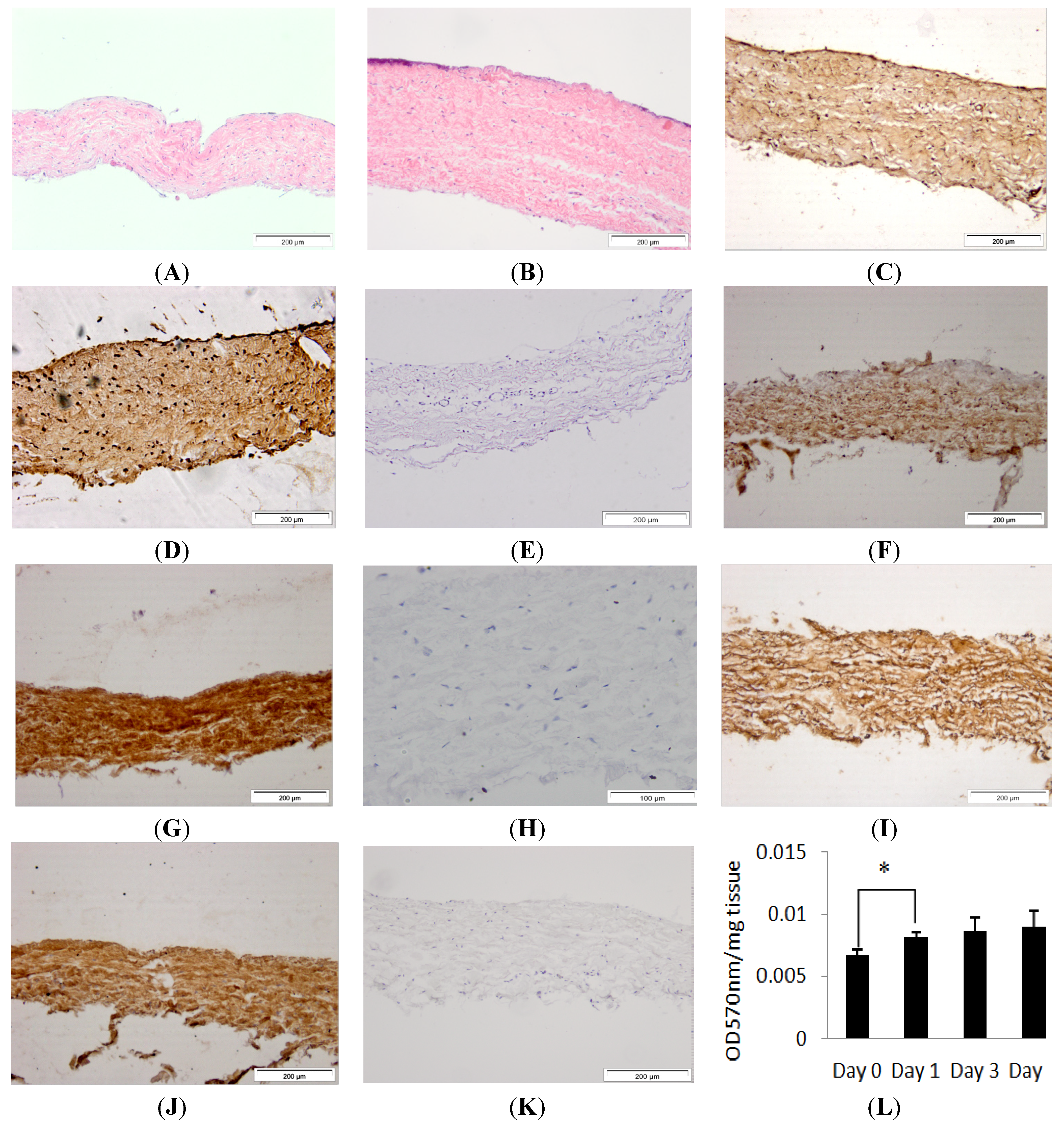
2.1. Histological Characterisation and Viability Assessment of Dura Mater in Organ Culture
Porcine dura mater, freshly isolated from the meninges surrounding the spinal cord was processed for histology and sections were stained using H & E (Hematoxylin & Eosin) and visualised by light microscopy to determine its native characteristics. Native porcine dura mater was composed of an outer epithelial cell layer and an underlying dense collagen I and II matrix with interspersed fibroblast cells (
Figure 1A,C,E,G). A more comprehensive study of the composition of the porcine dura mater has been reported in a previous study by Papageorgiou
et al. [
14]. The structural arrangement agreed with an ultrastructural study of the human meninges that showed that the dura mater was composed of an outermost loosely arranged fibroelastic layer, a central fibrous layer and an innermost cellular layer [
11].
Porcine dura mater, freshly isolated from the meninges surrounding the spinal cord was maintained in organ culture for seven days and then subject to histological and immunohistochemical evaluation. After seven days of
in vitro culture, the histological characteristics of the porcine dura mater remained unchanged (
Figure 1B,D,F,H). There was no alteration in the structure of the dura mater. Porcine dura mater maintained in organ culture over a seven day period was also assessed for tissue viability using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and there was no loss of viability (
Figure 1K). There was a significant (
p < 0.05) increase in MTT conversion after the first day of organ culture which was most probably due to the recovery of the tissue after the initial dissection process. MTT assay and immunohistochemical staining, have been used previously to assess tissue viability and tissue morphology in placental tissue explant cultures and buccal mucosa [
20,
21].
Figure 1.
Images of histological sections of the isolated porcine dura-mater tissue at Day 0 (A) and after seven days in organ culture (B) stained with H&E. Immunohistochemical staining of the porcine dura mater with antibodies to fibronectin (C,D), collagen I (F,G), collagen II (I,J) at Day 0 and Day 7 respectively. Isotypes controls for fibronectin (E), collagen I (H) and collagen II (K) antibodies. Tissue viability over a period of 7 days in organ culture as determined by MTT assay (L). Results are presented as OD at 570 nm per mg of wet weight of tissue. Data is presented as the mean (n = 3) ± 95% confidence limits. Data were analysed by one-way analysis of variance and individual differences between group means determined by the T-method. * indicates significant difference (* p < 0.05).
Figure 1.
Images of histological sections of the isolated porcine dura-mater tissue at Day 0 (A) and after seven days in organ culture (B) stained with H&E. Immunohistochemical staining of the porcine dura mater with antibodies to fibronectin (C,D), collagen I (F,G), collagen II (I,J) at Day 0 and Day 7 respectively. Isotypes controls for fibronectin (E), collagen I (H) and collagen II (K) antibodies. Tissue viability over a period of 7 days in organ culture as determined by MTT assay (L). Results are presented as OD at 570 nm per mg of wet weight of tissue. Data is presented as the mean (n = 3) ± 95% confidence limits. Data were analysed by one-way analysis of variance and individual differences between group means determined by the T-method. * indicates significant difference (* p < 0.05).
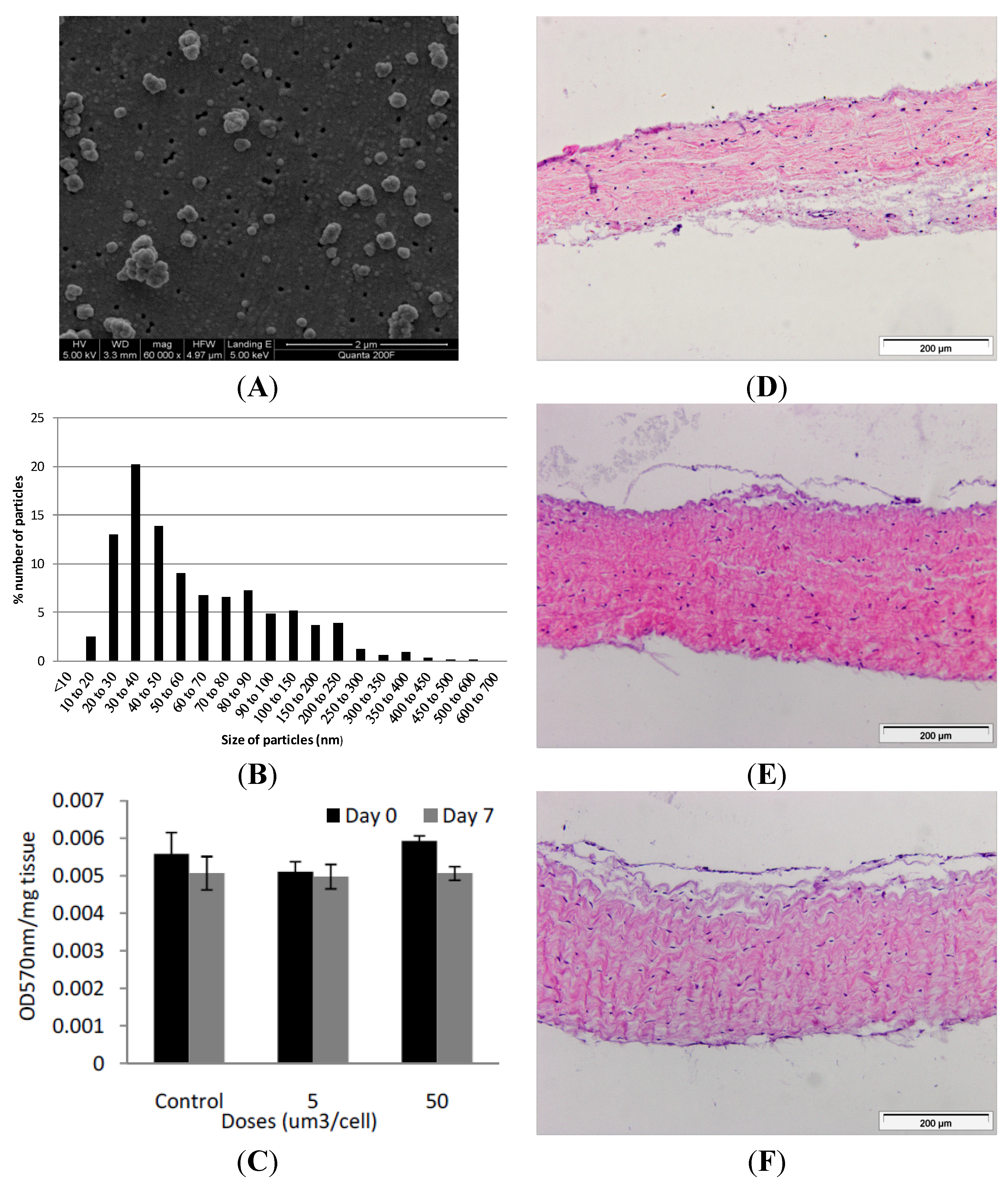
Figure 2.
(A) Representative image of cobalt-chrome nanoparticles that were generated using a pin-on-plate tribometer. Images were captured by FEGSEM; (B) Size distribution of cobalt-chrome nanoparticles. The size distribution of the cobalt-chrome nanoparticles was determined from SEM images taken from different locations on the filter membrane and analysed using Image Pro-Plus imaging software; (C) Effects of CoCr nanoparticles on the viability of dura-mater at 0 and 7 days of culture. Porcine dura mater tissue was cultured in the absence (control) and presence of CoCr nanoparticles at an estimated dose of 5 and 50 µm3 per epithelial cell and viability determined by the MTT assay. Data are expressed as the mean (n = 3) ± 95% confidence limits. The data were analysed by ANOVA which revealed no significant variation between control and CoCr-treated tissues at day 0 or day 7. Images of dura mater tissue exposed to cobalt-chrome nanoparticles at an estimated dosage of 0 (D), 5 (E) and 50 (F) µm3 per epithelial cell for a period of 7 days and stained with H&E. Tissues sections shown are orientated so that the outer epithelial layer is closest to the top of the section.
Figure 2.
(A) Representative image of cobalt-chrome nanoparticles that were generated using a pin-on-plate tribometer. Images were captured by FEGSEM; (B) Size distribution of cobalt-chrome nanoparticles. The size distribution of the cobalt-chrome nanoparticles was determined from SEM images taken from different locations on the filter membrane and analysed using Image Pro-Plus imaging software; (C) Effects of CoCr nanoparticles on the viability of dura-mater at 0 and 7 days of culture. Porcine dura mater tissue was cultured in the absence (control) and presence of CoCr nanoparticles at an estimated dose of 5 and 50 µm3 per epithelial cell and viability determined by the MTT assay. Data are expressed as the mean (n = 3) ± 95% confidence limits. The data were analysed by ANOVA which revealed no significant variation between control and CoCr-treated tissues at day 0 or day 7. Images of dura mater tissue exposed to cobalt-chrome nanoparticles at an estimated dosage of 0 (D), 5 (E) and 50 (F) µm3 per epithelial cell for a period of 7 days and stained with H&E. Tissues sections shown are orientated so that the outer epithelial layer is closest to the top of the section.
![Nanomaterials 04 00485 g002]()
2.2. Cobalt-Chrome Alloy Nanoparticle Generation and Characterisation
Nanometre-sized cobalt-chrome particles were generated using a pin-on-plate tribometer in distilled water and characterised using field emission gun scanning electron microscopy (FEGSEM) for size and shape. Six batches of particles were prepared and pooled. The particles were sonicated for 10 min prior to filtration to disperse agglomerates. The particles were round or oval in shape, with a few showing jagged edges (
Figure 2A). Utilising Image Pro-Plus image analysis software, it was found that 68% of the particles were less than 100 nm in size, with 100 particles analysed from 6 randomly chosen regions of the filter. The highest percentile of nanoparticles fell within the 30–40 nm sized range (
Figure 2B). Additional information regarding the properties of these CoCr nanoparticles, such as the sedimentation rate in tissue culture medium and metal ion release can be found in our previous publication [
17].
2.3. Exposure of the Porcine Dura Mater to Cobalt-Chrome Nanoparticles
Samples of porcine dura-mater tissue were incubated with CoCr nanoparticles at an estimated dose of 0, 5 and 50 µm
3∙cell
−1 (0, 0.833 and 8.33 µg∙mm
−3 of tissue). Despite sonication of the particles immediately prior to tissue exposure, re-agglomeration of the particles was unavoidable [
22,
23]. Particle agglomeration would also likely occur
in vivo. All exposures were repeated in triplicate at each time point and at each dose. The effect of nanoparticle exposure on tissue viability was determined immediately following exposure (day 0 of exposure) and after seven days of exposure (day 7) by MTT assay (
Figure 2C). Optical densities for the MTT assays were corrected against tissue only controls without MTT. At day 0, exposure of the dura mater to 5 or 50 µm
3∙cell
−1 of CoCr particles had no significant effect on tissue viability (
p > 0.05) compared to the control tissue which had not been exposed to particles. After seven days of exposure to CoCr nanoparticles at 5 or 50 µm
3∙cell
−1, there was no significant difference in tissue viability compared to the control tissues. Both the day 0 and day 7 tissue samples across control and experimental groups showed consistent levels of viability (0.005–0.006 OD 570 nm∙mg
−1 tissue) and there were no significant differences between any groups (
p > 0.05).
The results for tissue viability following CoCr nanoparticle exposure were not entirely inconsistent with the results obtained in our previous study in which dural epithelial cells, but not dural fibroblasts isolated from the porcine dura mater showed a reduction in viability following exposure to CoCr nanoparticles at the same doses [
17]. The differences between the results of the toxicity of CoCr nanoparticles obtained in organ culture and monolayer cell culture may be explained by differences in the behaviour of the epithelial cells in the two systems. With some toxic chemicals, the functional status of the cell (intrinsic cell characteristics) rather than the cell type determines the extent of its sensitivity to chemical toxicity. Such cellular characteristics may include membrane binding and permeability, intracellular synthetic pathways and adaptive and recovery mechanisms, which may differ in organ culture and monolayer cell culture [
24]. Alternatively the results may simply have been due to the fibroblasts, being the predominant cell type in the dura, contributing the majority of MTT conversion in the assay of the tissue, with the assay not being sensitive enough to detect toxic effects of the particles on the epithelial cells. Further studies using live/dead cell staining to determine any differential effects of the CoCr nanoparticles on the epithelial cells and fibroblasts in the organ culture would provide further insight.
When the dura-mater tissue was cultured for seven days with 5 µm
3 per epithelial cell of CoCr nanoparticles there were structural distortions to the epithelial layer compared to the control tissue observed in histological sections (
Figure 2D,E). The epithelial layer detached from the outer edges of the tissue across an estimated 40% of the tissue sections. The remainder of the tissue appeared similar in structure to control tissues. Porcine dura-mater tissue incubated for seven days with 50 µm
3∙cell
−1 of CoCr nanoparticles showed similar loosening of the collagen layer near the epithelium as well as the detachment of epithelial layer across the majority of the tissue section (
Figure 2F). H & E staining of tissue sections also showed some loosening of the collagen layers immediately adjacent to the detached epithelial layer in small areas across the tissue in all replicates.
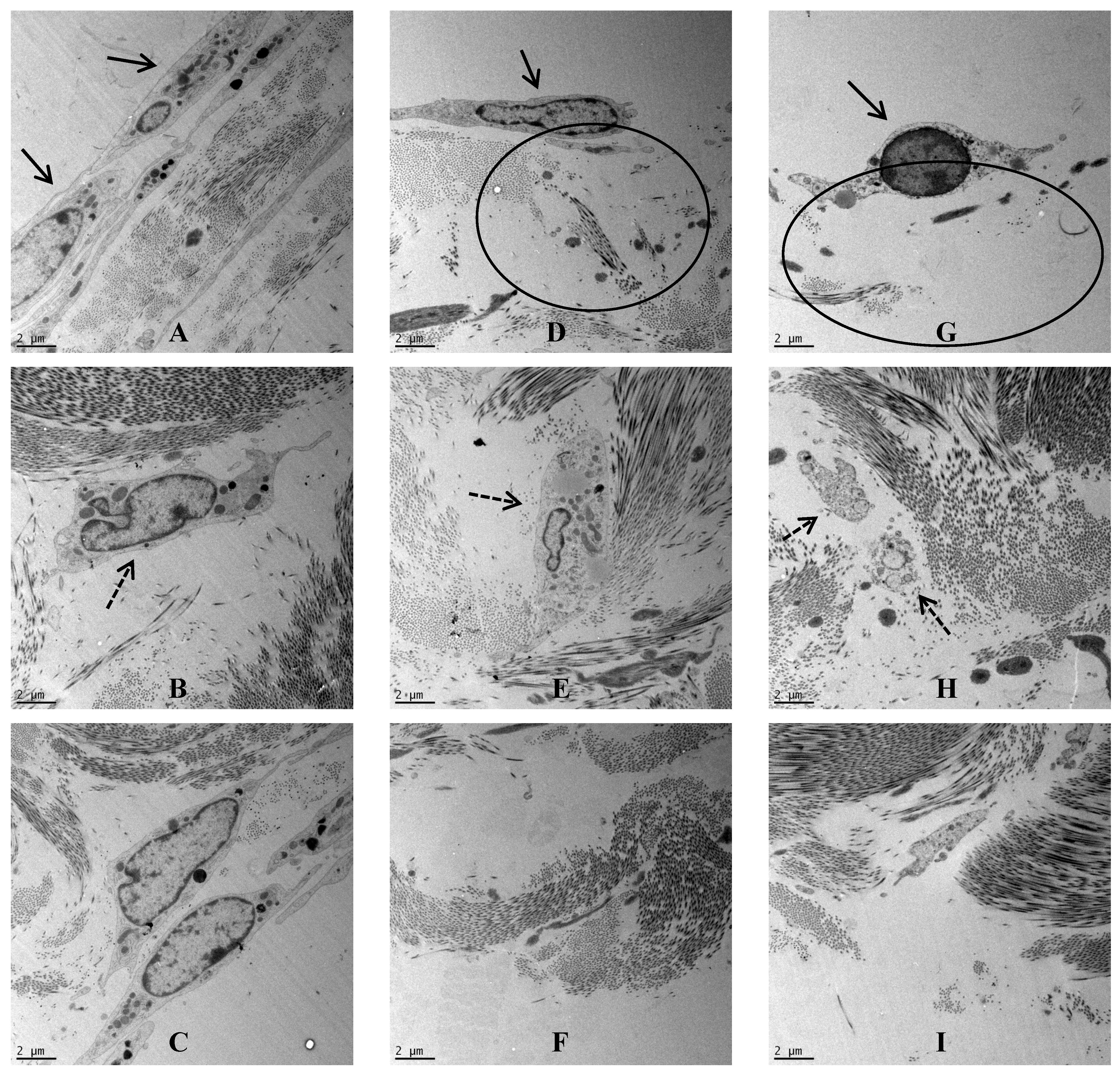
Porcine dura-mater was visualised using transmission electron microscopy to determine the ultrastructural characteristics of the native tissue and to determine any possible structural defects following exposure to nanoparticles. In the control tissue after 7 days of organ culture, the epithelial cell-layer was observed surrounding the dura-mater that was mainly composed of a network of highly ordered collagen fibers (
Figure 3A). Interspersed with the collagen bundles were regularly arranged fibroblasts (
Figure 3B). This tight structure appeared to be disrupted when the dura mater was exposed to CoCr nanoparticles for 7 days. In the low dose organ culture (5 µm
3∙cell
−1), the epithelial layer was disrupted on the apical side (
Figure 3D), the fibroblasts appeared vacuolated (
Figure 3E) and the basal layer of collagen appeared loosened (
Figure 3F). Similar structural changes were observed in the tissues treated with the highest dose of CoCr particles (50 µm
3∙cell
−1). In addition to disruption at the surface, the collagen fibres on the apical side were also disrupted (
Figure 3G). The fibroblasts deep in the dura mater also showed signs of cellular degradation (
Figure 3H), and in addition the basal layer of collagen also appeared loosened (
Figure 3I).
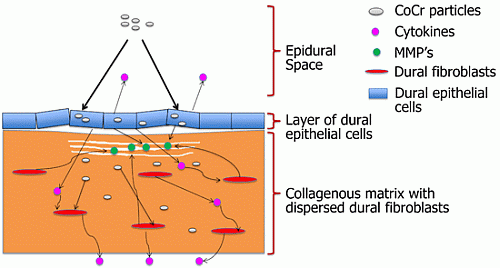
These preliminary histological and TEM observations suggested that there were structural alterations in the dura mater following exposure to CoCr nanoparticles for a period of seven days. In future studies it will be important to quantify this phenomenon further for example by: measurement of the transepithelial electrical resistance of the membrane, assessment of epithelial integrity through ZO-1 and E-cadherin staining, determination of total collagen and glycosaminoglycan content and biomechanical tests to determine any functional changes in the properties of the dura mater. In addition, the orientation of the collagen fibers could be quantified using atomic force microscopy [
25] to determine any effects of CoCr nanoparticle exposure on the packing of the collagen fibers.
Figure 3.
TEM images across the dura mater tissue that was exposed to CoCr nanoparticlesfor a period of seven days. (A–C) Images of the control dura mater from the epithelial region (A), inner collagen region (B) and the basal side of the dura mater close to arachnoid mater (C); (D–F) Images of the dura mater exposed to an estimated dose of 5 µm3 of CoCr particles per epithelial cell, from the epithelial region (D), inner collagen region (E), and the basal side of the dura mater close to arachnoid mater (F); (G–I) Images of the dura mater exposed to an estimated dose of 50 µm3 of CoCr particles per epithelial cell, from the epithelial region (G), inner collagen region (H), and the basal side of the dura mater close to arachnoid mater (I). Single continuous black arrows indicate the dural epithelial cells. Single dotted black arrow indicated the dural fibroblast cells. Black circles indicated disruption of the collagen layer.
Figure 3.
TEM images across the dura mater tissue that was exposed to CoCr nanoparticlesfor a period of seven days. (A–C) Images of the control dura mater from the epithelial region (A), inner collagen region (B) and the basal side of the dura mater close to arachnoid mater (C); (D–F) Images of the dura mater exposed to an estimated dose of 5 µm3 of CoCr particles per epithelial cell, from the epithelial region (D), inner collagen region (E), and the basal side of the dura mater close to arachnoid mater (F); (G–I) Images of the dura mater exposed to an estimated dose of 50 µm3 of CoCr particles per epithelial cell, from the epithelial region (G), inner collagen region (H), and the basal side of the dura mater close to arachnoid mater (I). Single continuous black arrows indicate the dural epithelial cells. Single dotted black arrow indicated the dural fibroblast cells. Black circles indicated disruption of the collagen layer.
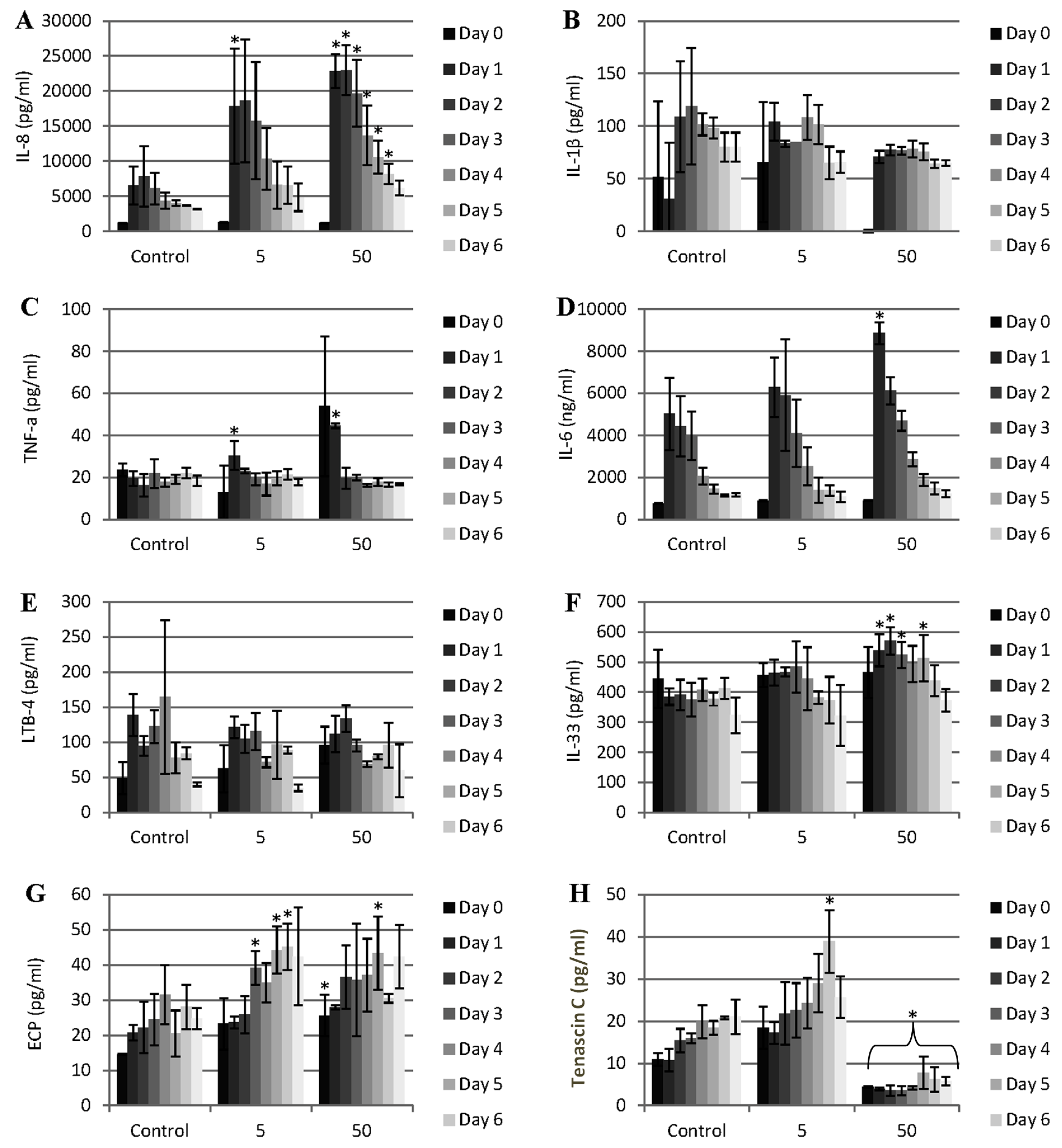
Figure 4.
Pattern of cytokine and other mediator release during the exposure of porcine dura mater to CoCr nanoparticles for a period of 0–7 days. The dura mater organ culture was exposed to estimated doses of 0 (control), 5 and 50 µm3 of CoCr nanoparticles per epithelial cell. The factors that were investigated were IL-8 (A), IL-1β (B), TNF-α (C), IL-6 (D), LBT-4 (leukotriene B4) (E), IL-33 (F), ECP (eosinophil chemotactic protein, eotaxin, CCL-11) (G), and tenascin C (H). Data is expressed as the mean (n = 3) ±95% confidence limits. Data were analysed by one-way analysis of variance and individual differences between group means determined by the T-method. * indicates significantly difference between control and CoCr-treated tissue (* p < 0.05).
Figure 4.
Pattern of cytokine and other mediator release during the exposure of porcine dura mater to CoCr nanoparticles for a period of 0–7 days. The dura mater organ culture was exposed to estimated doses of 0 (control), 5 and 50 µm3 of CoCr nanoparticles per epithelial cell. The factors that were investigated were IL-8 (A), IL-1β (B), TNF-α (C), IL-6 (D), LBT-4 (leukotriene B4) (E), IL-33 (F), ECP (eosinophil chemotactic protein, eotaxin, CCL-11) (G), and tenascin C (H). Data is expressed as the mean (n = 3) ±95% confidence limits. Data were analysed by one-way analysis of variance and individual differences between group means determined by the T-method. * indicates significantly difference between control and CoCr-treated tissue (* p < 0.05).
2.4. Proinflammatory Cytokine Response of the Dura Mater Exposed to Cobalt-Chrome Nanoparticles
In order to determine if the CoCr nanoparticles had any effect on pro-inflammatory mediator release from the dura mater, the release of interleukin 8 (IL-8), tumour necrosis factor-α (TNF-α), IL-6, IL-1β, and leukotriene B-4 (LTB-4) was measured daily in the organ culture conditioned medium for a period of seven days after the exposure of the organ culture to 5 and 50 µm
3 CoCr nanoparticles per epithelial cell. There was significant release of IL-8 associated with both doses of CoCr nanoparticles compared to the control (
Figure 4A). At the highest particle dose, the pattern of IL-8 release was continuous for the whole period of treatment (seven days). This IL-8 release was in agreement with the results of the previous study by Behl
et al. [
17], in which it was shown that dural fibroblasts and dural epithelial cells exposed to CoCr nanoparticles released significant amounts of IL-8. IL-8 release is a sign of a pro-inflammatory response of the tissue. IL-8 has a role in the recruitment and activation of neutrophils and T-lymphocytes [
26,
27]. In addition to its action as a chemokine, IL-8 induces neutrophils to release lysosomal enzymes and down-regulates collagen expression by human fibroblasts [
26]. It might be hypothesized that
in vivo, exposure of the dura mater to CoCr nanoparticles could stimulate the release of IL-8 resulting in the recruitment of neutrophils and release of lysosomal enzymes that would alter the structural integrity of the dura mater.
The pattern of TNF-α and IL-6 release was transient (
Figure 4C,D). TNF-α was released only during the first 24 h of exposure to nanoparticles in a dose dependent manner. According to Harada
et al. [
26] the production of IL-8 occurs in the presence of inflammatory stimuli such as IL-1 and TNF-α. Hence, IL-8 release could have been initiated by the transient TNF-α response of the tissue. IL-6 was also was significantly elevated in the first day of exposure to CoCr nanoparticle but only at the highest particle dose (50 µm
3∙cell
−1). IL-6 release from the organ cultures exposed to CoCr nanoparticles was not consistent with the results from our previous study in which no significant IL-6 release was observed over a period of 4 days at a range of particle doses for both dural fibroblasts and dural epithelial cells [
17]. Similar results were also observed in a study by Papageorgiou
et al. [
28], in which exposure of dermal fibroblasts to CoCr particles did not cause the release of significantly elevated levels of IL-6. The IL-6 release that was observed in the organ culture exposed to CoCr nanoparticles could have been the result of intercellular signaling between dural epithelial cells and dural fibroblasts. Such epithelial cell/fibroblast interactions have been documented in the lung airway wall [
29] and involves cytokines such as IL-6 which can lead to [
30] remodeling of the airway wall. Similarly, it can be hypothesized that the dural epithelial layer activates the underlying fibroblasts which in turns activate the epithelial layer further leading to an enhanced cytokine response.
There was no IL-1β or LTB-4 (leukotriene B4) released at any time or dose of exposure to the nanoparticles (
Figure 4B,E).
IL-33 is a member of the IL-1 cytokine family and is released by stressed epithelial cells and fibroblasts. Significant amounts of IL-33 were released between day 2 and day 5 at the highest CoCr nanoparticle treatment dose of 50 µm
3∙cell
−1 (
Figure 4F). This cytokine specifically modulates Th2-type proinflamatory signals after its release from necrotic cells that may promote eosinophilia [
31]. A similar phenomenon occurred with ECP release at the low particle treatment dose of 5 µm
3∙cell
−1 (
Figure 4G). Nearly double the amount of ECP, relative to the control levels, was released from the treated dura mater between day 3 and day 6 of exposure to CoCr nanoparticles. Since ECP is documented to promote eosinophilia [
31], it might be hypothesized that CoCr nanoparticle exposure of the dura mater
in vivo could stimulate the release of IL-33 and ECP that would in turn promote eosinophil recruitment to the site of CoCr nanoparticle exposure.
Tenascin C in an extracellular matrix glycoprotein that has been reported to be expressed in response to tissue injury and infection [
32,
33]. Its expression is linked with cytokines associated with inflammation such as TNF-α [
34]. There was a significant release of tenascin C when the organ culture was exposed to the low particle dose (5 µm
3∙cell
−1) after six days. However, for the high particle dose (50 µm
3∙cell
−1) significantly lower amounts of tenascin C were released at all-time points, compared to the control values. It is possible that TNF-α release in response to the low dose of CoCr nanoparticles caused the induction the tenascin expression. However, at the high dose of CoCr nanoparticles the drop of tenanscin C expression (even below the control tissue levels) could be attributed to the activation of MMP’s and the cleavage of tenascin-C. There is a feedback loop between induction of metalloproteases by tenascin-C and cleavage of tenascin-C by these enzymes [
32]. Chiquet-Ehrisman
et al. [
35] suggested that the production of tenascin C in inflamed tissues belongs to a set of mechanisms required to control the spread of inflammation.
2.5. MMP and TIMP-1 Expression in Dura Mater after Exposure to Cobalt-Chrome Nanoparticles
Generally, MMP’s are not expressed in healthy tissues. However, MMP expression can be detected in inflamed tissues and/or in tissues under repair and remodelling. Metalloproteinases control the degradation of the extracellular matrix (as their name implies), they modulate chemokine activity of the inflamed region and they establish different chemokine gradients to modulate the influx of inflammatory cells [
36]. The expression of MMPs was therefore investigated following exposure of the dural tissue in organ culture to CoCr nanoparticles.
The tissue were investigated for the expression of MMP-1, -3, -9, and -13 since these are the MMPs implicated in collagen, and fibronectin degradation as well as disruption of the basement membrane and general remodelling of inflamed tissues [
36,
37]. The staining pattern of the CoCr-treated tissue is summarised in
Table 1.
Table 1.
Expression pattern of different matrix metalloproteinases in dural epithelial and fibroblast cells as well as in the extracellular matrix exposed to CoCr wear nanoparticles of different doses. Control tissues showed no positive staining and are not included. (Intensity of staining was assessed as: −−−: no staining, +/−: very light staining, +: light staining, ++: strong staining, +++: very strong staining).
Table 1.
Expression pattern of different matrix metalloproteinases in dural epithelial and fibroblast cells as well as in the extracellular matrix exposed to CoCr wear nanoparticles of different doses. Control tissues showed no positive staining and are not included. (Intensity of staining was assessed as: −−−: no staining, +/−: very light staining, +: light staining, ++: strong staining, +++: very strong staining).
| | CoCr Nanoparticle Treatment (µm3 per epithelial cell) |
|---|
| 5 µm3 | 50 µm3 |
|---|
| Type of MMP/TIMP | Epithelial cells | Fibroblasts | Extracellular matrix | Epithelial cells | Fibroblasts | Extracellular matrix |
| MMP-1 | ++ | + | −−− | + | + | −−− |
| MMP-3 | ++ | + | + | + | +/− | −−− |
| MMP-9 | +++ | ++ | ++ | +++ | ++ | +++ |
| MMP-13 | + | +/− | +/− | ++ | ++ | ++ |
| TIMP-1 | +++ | +/− | −−− | + | +/− | −−− |
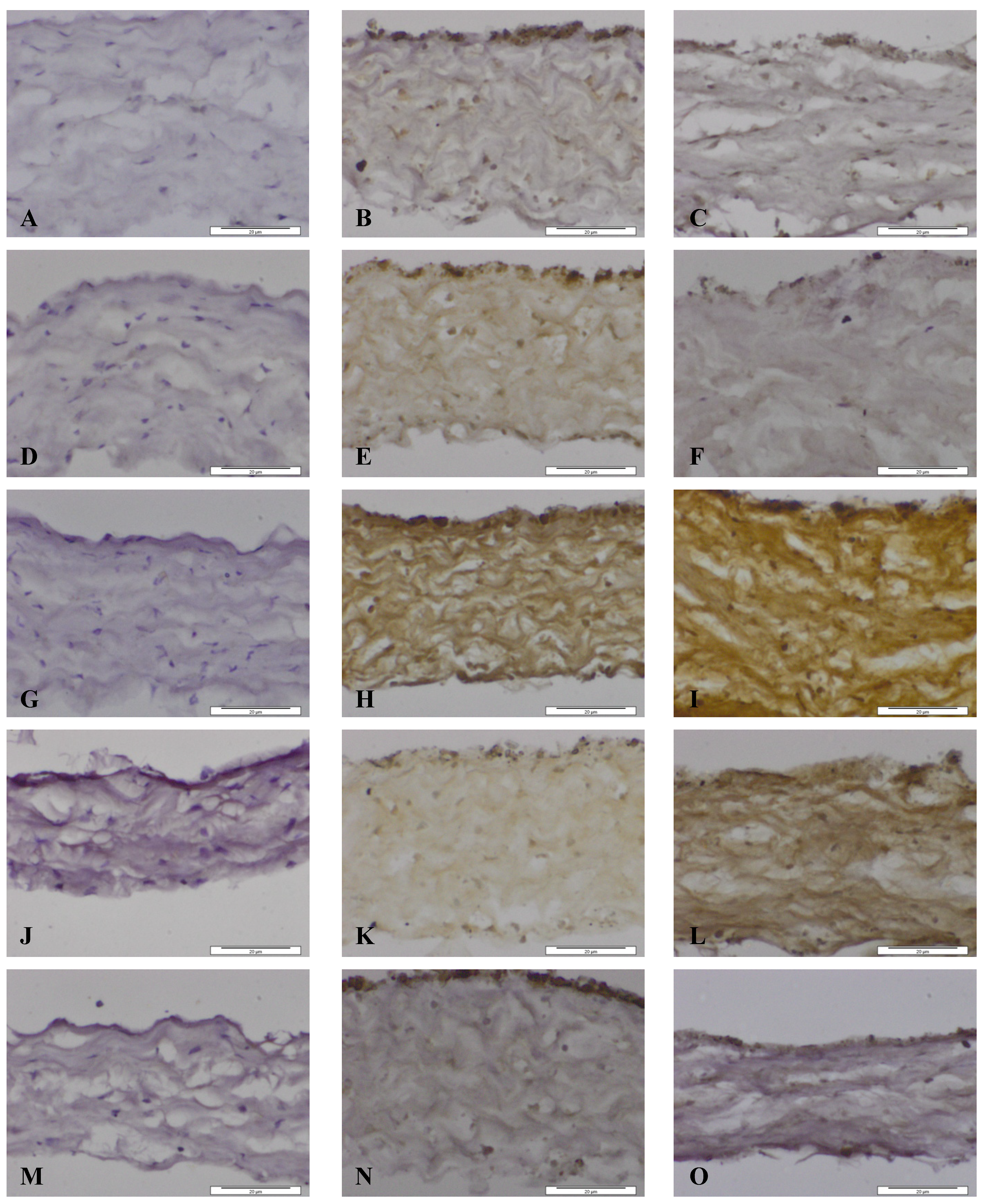
Figure 5.
Images of dura mater exposed for seven days to CoCr nanoparticles and stained for the presence of matrix metalloproteinases and TIMP-1 by immunhistochemistry. Images of section of control dura mater tissue (A,D,G,J,M), dura mater exposed to an estimated dose of 5 µm3 CoCr nanoparticles per epithelial cell (B,E,H,K,N) and dura mater exposed to an estimated dose of 50 µm3 CoCr nanoparticles per epithelial cell (C,F,I,L,O). The tissues were stained for MMP-1 (A–C), MMP-3 (D–F), MMP-9 (G–I), MMP-13 (J–L), TIMP-1 (M–O).
Figure 5.
Images of dura mater exposed for seven days to CoCr nanoparticles and stained for the presence of matrix metalloproteinases and TIMP-1 by immunhistochemistry. Images of section of control dura mater tissue (A,D,G,J,M), dura mater exposed to an estimated dose of 5 µm3 CoCr nanoparticles per epithelial cell (B,E,H,K,N) and dura mater exposed to an estimated dose of 50 µm3 CoCr nanoparticles per epithelial cell (C,F,I,L,O). The tissues were stained for MMP-1 (A–C), MMP-3 (D–F), MMP-9 (G–I), MMP-13 (J–L), TIMP-1 (M–O).
When the dura mater was exposed to both doses of nanoparticles MMP-1 was expressed in the cytoplasm of both dural epithelial and dural fibroblast cells (
Figure 5B,C). The pattern of staining of MMP-3 was, however different. At the low particle dose (5 µm
3∙cell
−1) MMP 3 was expressed by both cell types and was also present in the collagen region (
Figure 5E). At the high particle dose (50 µm
3∙cell
−1), there was only a limited amount of staining of the epithelial cells, but no staining was observed in dural fibroblasts or the collagen matrix (
Figure 5F). According to a study by Chao
et al. [
38] increased levels of eotaxin not only induce MMP-3 gene expression but also promote MMP-3 protein secretion. It may be hypothesized that since eotaxin was released from the tissue during the six days of exposure to the low dose CoCr nanoparticles (
Figure 4G), MMP-3 was produced and secreted from epithelial and fibroblast cells in response to eotaxin.
MMP-9 was present in both dural epithelial and fibroblast cells as well as in the collagen region of the dural tissue that had been exposed to both doses of CoCr nanoparticles (
Figure 5H,I). MMP-9 is reported to digest gelatin [
37] and it may be hypothesized that it contributes to the structural alterations observed in CoCr-treated dura mater.
Epithelial and fibroblast cells were stained positively for MMP-13 (
Figure 5J,L), however, with the low dose treatment (5 µm
3∙cell
−1) the collagen layer did not stain as intensively as with the high dose treatment (50 µm
3∙cell
−1). This metalloproteinase, cleaves collagen I, II, and III [
36] and may have contributed to the structural loosening that was observed in the dura mater treated with CoCr nanoparticles.
MMP activities in tissues are regulated by specific inhibitors such as the tissue inhibitors of metalloproteinases (TIMPs). Four TIMP’s (TIMP-1, -2, -3, -4) have been identified in vertebrates and their expression is regulated in tissue remodelling [
37]. TIMP-1 was chosen for investigation because it is a stromal factor that has a wide spectrum of functions in different tissues and can inhibit all the MMP’s investigated above [
37,
39]. The pattern of staining of TIMP-1 is shown in
Figure 5M–O. At both particle treatment doses the dural epithelial cells were stained intensively; whereas the dural fibroblast cells and the collagen layer around them did not have any evidence of staining. Since the principal physiological role of TIMP-1 is to regulate the degradation of the basement membrane and the extracellular matrix by MMPs [
40], the presence of TIMP-1 in the dural epithelial cells might indicate that this was upregulated in order to limit tissue degradation. It will be important to determine in future studies whether the observed MMP expression in the dura mater following exposure to CoCr nanoparticles correlates with enzyme activity using zymography [
41].