Self-Assembly in Biosilicification and Biotemplated Silica Materials
Abstract
:1. Introduction
2. Natural Self-Assembled Silicified Structures
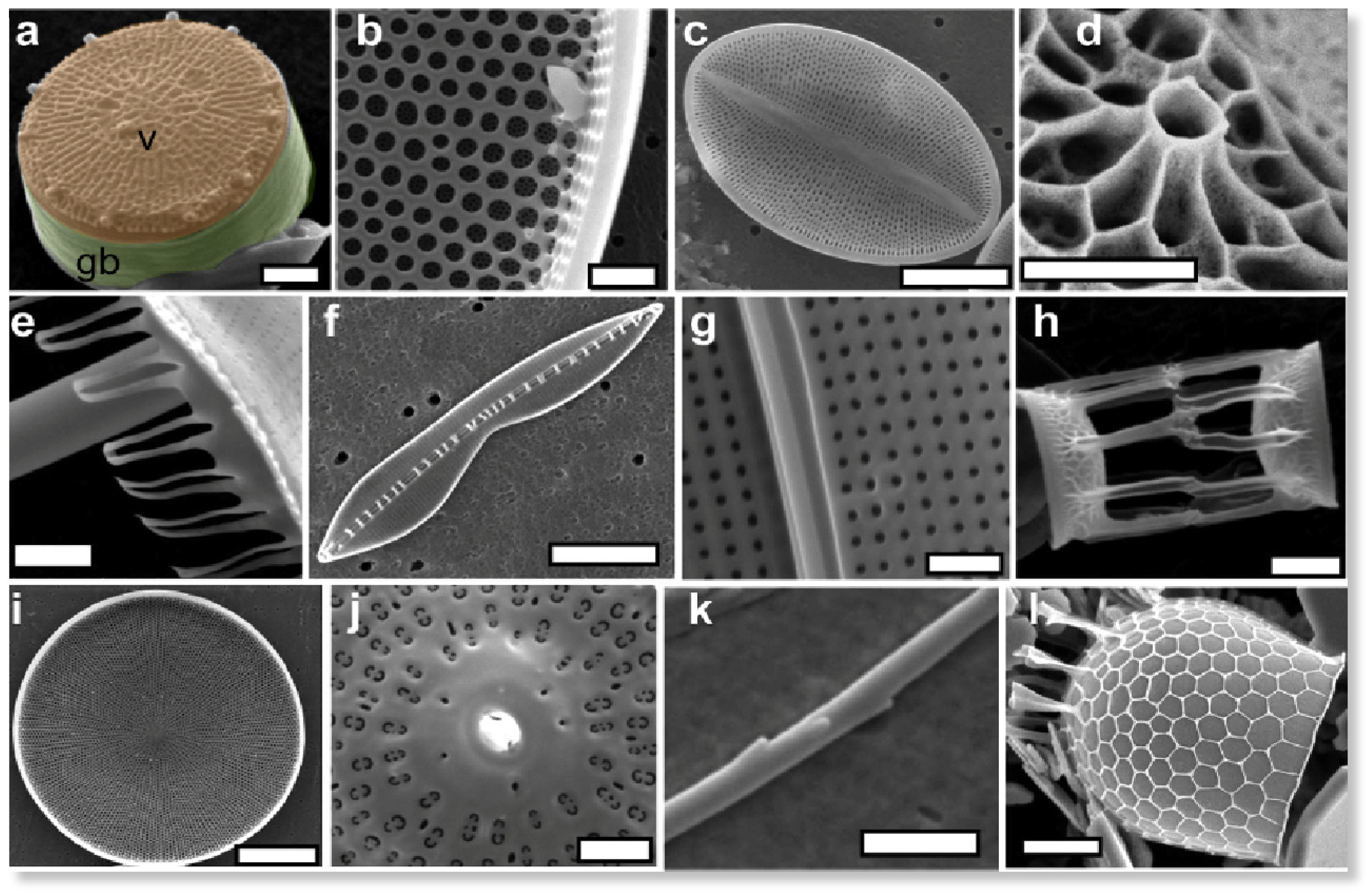
2.1. Diatoms
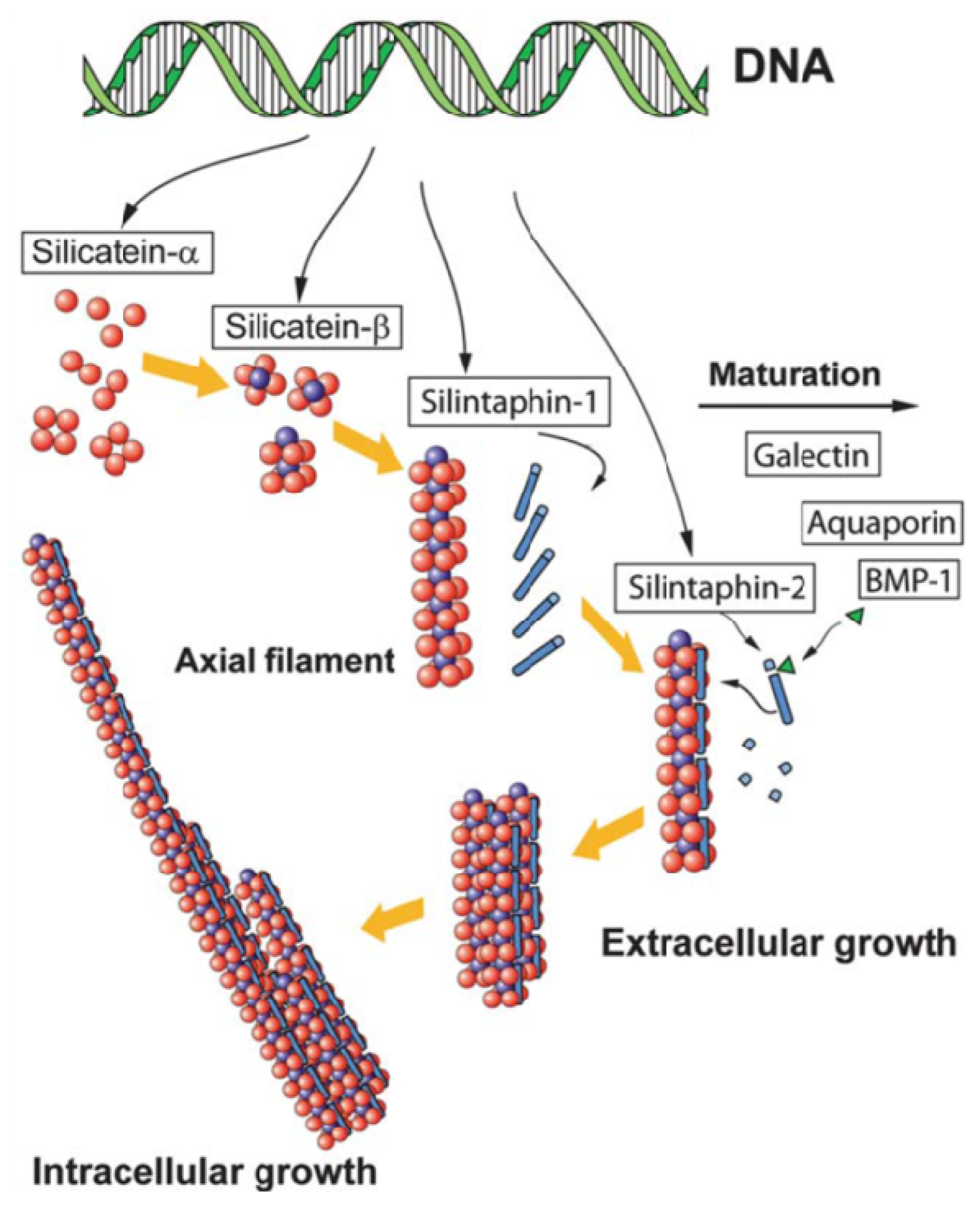
2.2. Sponges
3. Silicification of Self-Assembled Biomolecules
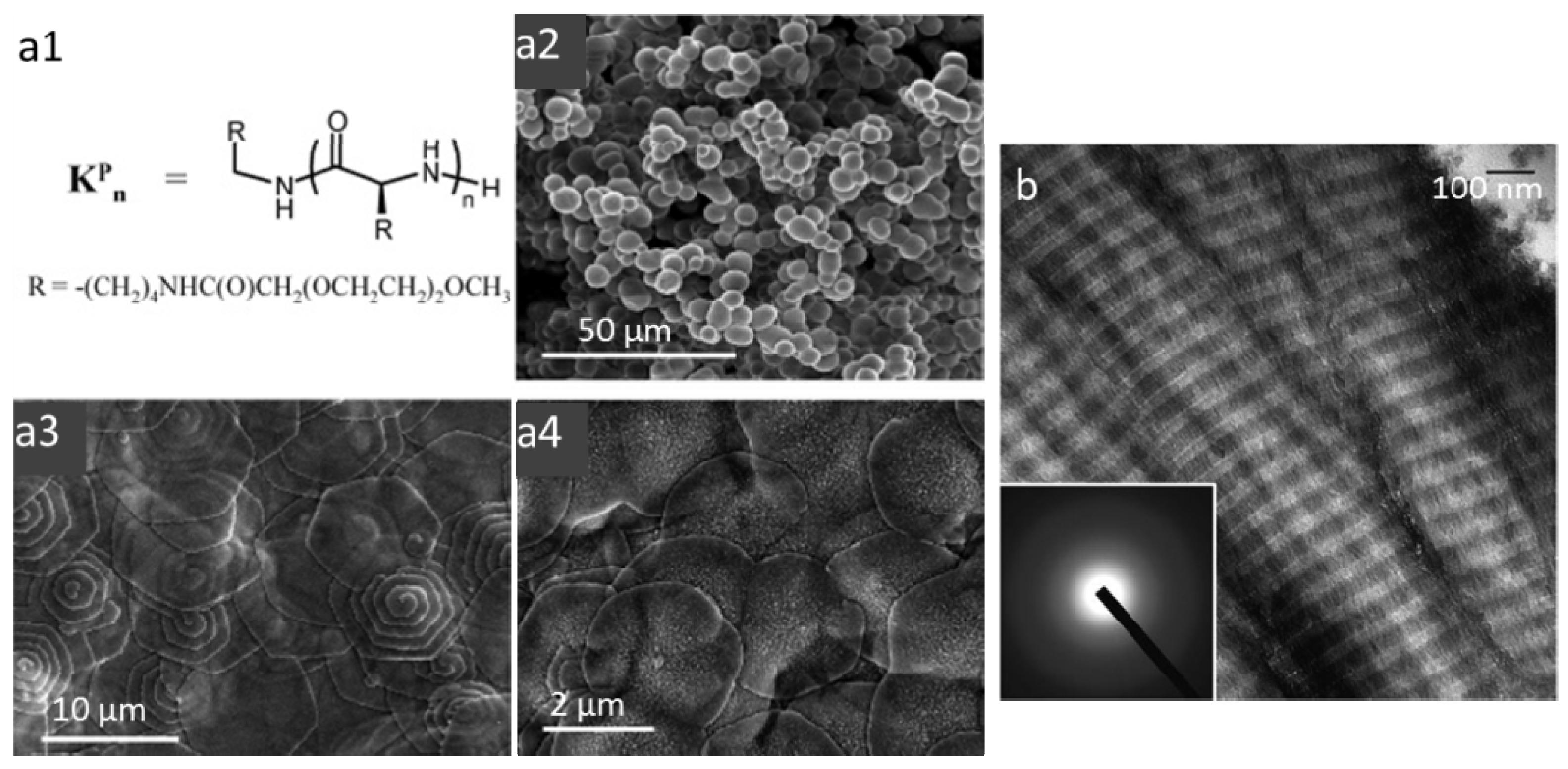
3.1. Self-Assembled Peptides
3.2. Proteins
3.3. Polysaccharides
3.4. Lipids
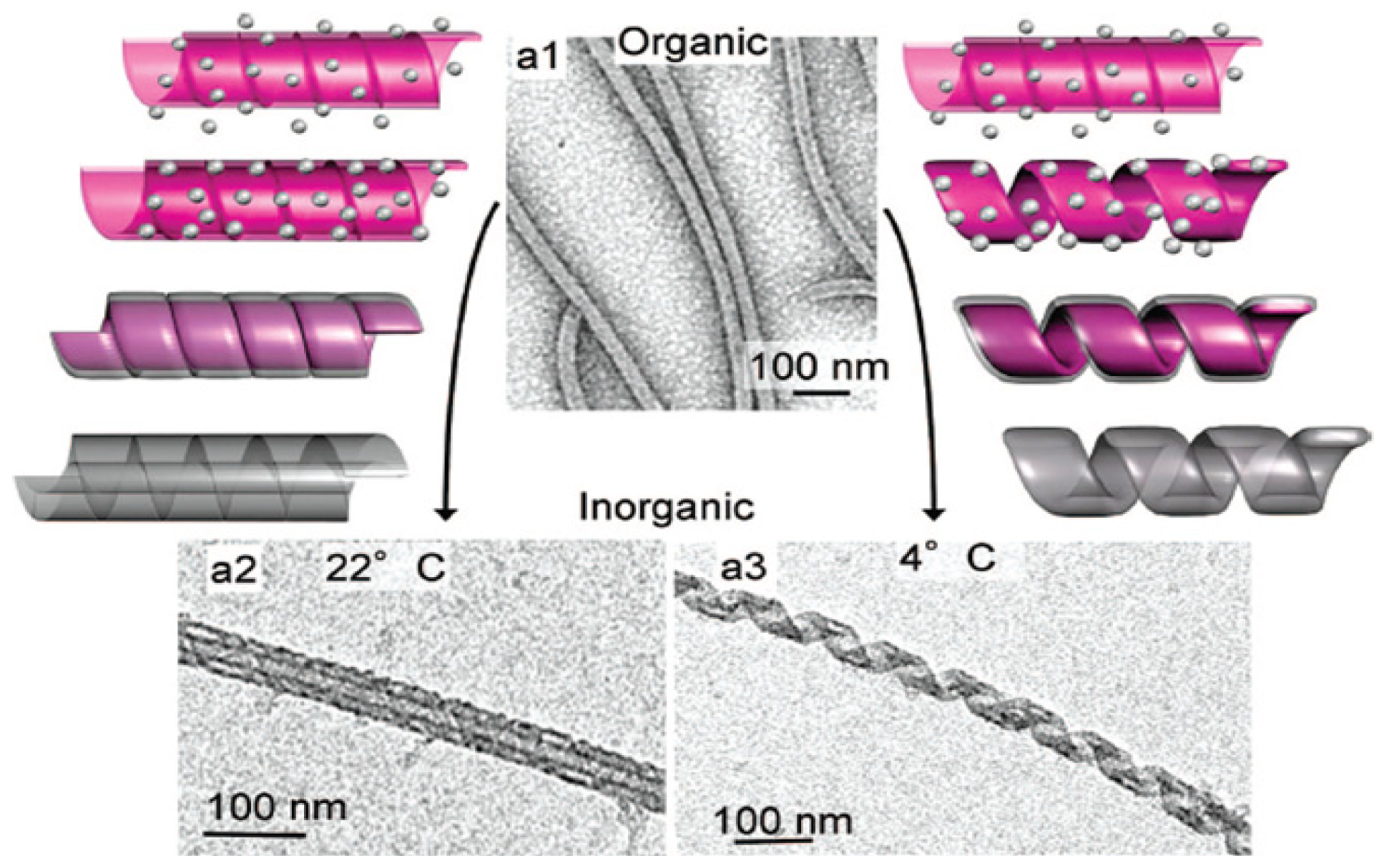
3.5. Virus

4. Biological Self-Assembly of Silica Nanostructures: A Change of Perspective
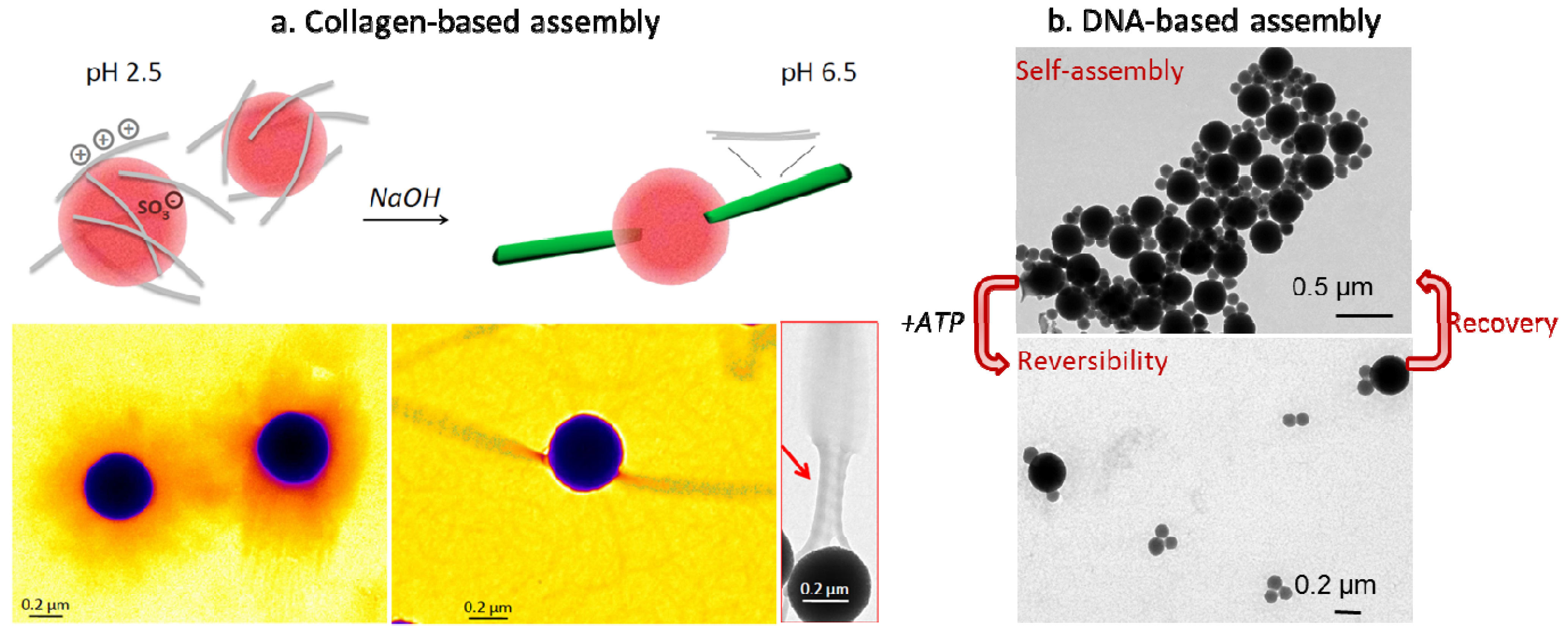
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar]
- Addadi, L.; Weiner, S. Control and design principles in biological mineralization. Angew. Chemie. Int. Ed. Engl. 1992, 31, 153–169. [Google Scholar] [CrossRef]
- Bäuerlein, E. Biomineralization of unicellular organisms: An unusual membrane biochemistry for the production of inorganic nano- and microstructures. Angew. Chem. Int. Ed. Engl. 2003, 42, 614–641. [Google Scholar]
- Mann, S. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature 1993, 365, 499–505. [Google Scholar] [CrossRef]
- Dujardin, E.; Mann, S. Bio-inspired materials chemistry. Adv. Mater. 2002, 14, 775–788. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Biomimetics—Using nature to inspire human innovation. Bioinspir. Biomim. 2006, 1, 1–12. [Google Scholar]
- Crookes-Goodson, W.J.; Slocik, J.M.; Naik, R.R. Bio-directed synthesis and assembly of nanomaterials. Chem. Soc. Rev. 2008, 37, 2403–2412. [Google Scholar]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological, biochemical and chemical studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Coradin, T.; Lopez, P.J. Biogenic silica patterning: Simple chemistry or subtle biology? ChemBioChem 2003, 4, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.J.; Gautier, C.; Livage, J.; Coradin, T. Mimicking biogenic silica nanostructures formation. Curr. Nanosci. 2005, 1, 73–83. [Google Scholar] [CrossRef]
- Sumper, M.; Brunner, E. Learning from diatoms: Nature’s tools for the production of nanostructured silica. Adv. Funct. Mater. 2006, 16, 17–26. [Google Scholar] [CrossRef]
- Patwardhan, S.V. Biomimetic and bioinspired silica: Recent developments and applications. Chem. Commun. 2011, 47, 7567–7582. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, M. Diatoms, biomineralization processes, and genomics. Chem. Rev. 2008, 108, 4855–4874. [Google Scholar] [PubMed]
- Tesson, B.; Masse, S.; Laurent, G.; Maquet, J.; Livage, J.; Martin-Jezequel, V.; Coradin, T. Contribution of multi-nuclear solid state NMR to the characterization of the Thalassiosira pseudonana diatom cell wall. Anal. Bioanal. Chem. 2008, 390, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Mitchell, J.G.; Lal, R.; Voelcker, N.H. Rapid fabrication of micro- and nanoscale patterns by replica molding from diatom biosilica. Adv. Funct. Mater. 2007, 17, 2439–2446. [Google Scholar] [CrossRef]
- Scheffel, A.; Poulsen, N.; Shian, S.; Kröger, N. Nanopatterned protein microrings from a diatom that direct silica morphogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Rosengarten, G.; Mitchell, J.G.; Voelcker, N.H. Pore architecture of diatom frustules: Potential nanostructured membranes for molecular and particle separations. J. Nanosci. Nanotechnol. 2006, 6, 982–989. [Google Scholar] [PubMed]
- Kröger, N.; Deutzmann, R.; Sumper, M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science 1999, 286, 1129–1132. [Google Scholar]
- Kröger, N.; Deutzmann, R.; Sumper, M. Silica-precipitating peptides from diatoms. The chemical structure of silaffin-A from Cylindrotheca fusiformis. J. Biol. Chem. 2001, 276, 26066–26070. [Google Scholar]
- Kröger, N.; Lorenz, S.; Brunner, E.; Sumper, M. Self-assembly of highly phosphorylated silaffins and their function in biosilica morphogenesis. Science 2002, 298, 584–586. [Google Scholar]
- Poulsen, N.; Sumper, M.; Kröger, N. Biosilica formation in diatoms: Characterization of native silaffin-2 and its role in silica morphogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 12075–12080. [Google Scholar] [CrossRef] [PubMed]
- Sumper, M.; Lorenz, S.; Brunner, E. Biomimetic control of size in the polyamine-directed formation of silica nanospheres. Angew. Chem. Int. Ed. Engl. 2003, 42, 5192–5195. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-specific polyamines from diatoms control silica morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar]
- Tesson, B.; Hildebrand, M. Extensive and intimate association of the cytoskeleton with forming silica in diatoms: control over patterning on the meso- and micro-scale. PLoS One 2010, 5, e14300. [Google Scholar] [PubMed]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. Engl. 2009, 48, 9724–9727. [Google Scholar] [CrossRef] [PubMed]
- Aluma, Y.; Ilan, M.; Sherman, D. Comments on a skeleton design paradigm for a demosponge. J. Struct. Biol. 2011, 175, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Pietrasanta, L.I.; Hedin, N.; Chmelka, B.F.; Hansma, P.K.; Morse, D.E. Nanostructural features of demosponge biosilica. J. Struct. Biol. 2003, 144, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.C.; Wang, X.; Tremel, W.; Ushijima, H.; Müller, W.E.G. Biofabrication of biosilica-glass by living organisms. Nat. Prod. Rep. 2008, 25, 455–474. [Google Scholar]
- Wang, X.; Schröder, H.C.; Wang, K.; Kaandorp, J.A.; Müller, W.E.G. Genetic, biological and structural hierarchies during sponge spicule formation: From soft sol–gels to solid 3D silica composite structures. Soft Matter 2012, 8, 9501–9518. [Google Scholar] [CrossRef]
- Shimizu, K.; Cha, J.; Stucky, G.D.; Morse, D.E. Silicatein alpha: Cathepsin L-like protein in sponge biosilica. Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238. [Google Scholar] [CrossRef]
- Schlossmacher, U.; Wiens, M.; Schröder, H.C.; Wang, X.; Jochum, K.P.; Müller, W.E.G. Silintaphin-1—Interaction with silicatein during structure-guiding bio-silica formation. FEBS J. 2011, 278, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Croce, G.; Frache, A.; Milanesio, M.; Viterbo, D.; Bavestrello, G.; Benatti, U.; Giovine, M.; Amenitsch, H. Fiber diffraction study of spicules from marine sponges. Microsc. Res. Tech. 2003, 62, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, X.; Wiens, M.; Schlossmacher, U.; Jochum, K.P.; Schröder, H.C. Hardening of bio-silica in sponge spicules involves an aging process after its enzymatic polycondensation: Evidence for an aquaporin-mediated water absorption. Biochim. Biophys. Acta 2011, 1810, 713–726. [Google Scholar]
- Pender, M.J.; Sowards, L.A.; Hartgerink, J.D.; Stone, M.O.; Naik, R.R. Peptide-mediated formation of single-wall carbon nanotube composites. Nano Lett. 2006, 6, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Pouget, E.; Dujardin, E.; Cavalier, A.; Moreac, A.; Valéry, C.; Marchi-Artzner, V.; Weiss, T.; Renault, A.; Paternostre, M.; Artzner, F. Hierarchical architectures by synergy between dynamical template self-assembly and biomineralization. Nat. Mater. 2007, 6, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V.; Mukherjee, N.; Steintz-Kannan, M.; Clarson, S.J. Bioinspired synthesis of new silica structures. Chem. Commun. 2003, 11, 1122–1123. [Google Scholar] [CrossRef]
- Coradin, T.; Durupthy, O.; Livage, J. Interactions of amino-containing peptides with sodium silicate and colloidal silica: A biomimetic approach of silicification. Langmuir 2002, 18, 2331–2336. [Google Scholar] [CrossRef]
- Bellomo, E.G.; Deming, T.J. Monoliths of aligned silica-polypeptide hexagonal platelets. J. Am. Chem. Soc. 2006, 128, 2276–2279. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.N.; Stucky, G.D.; Morse, D.E.; Deming, T.J. Biomimetic synthesis of ordered silica structures mediated by block copolypeptides. Nature 2000, 403, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.; Lee, S.; Carr, C.S.; Shantz, D.F. Biomimetic synthesis of inorganic nanospheres. Chem. Mater. 2005, 17, 4310–4317. [Google Scholar] [CrossRef]
- Niu, L.; Jiao, K.; Qi, Y.; Yiu, C.K.Y.; Ryou, H.; Arola, D.D.; Chen, J.; Breschi, L.; Pashley, D.H.; Tay, F.R. Infiltration of silica inside fibrillar collagen. Angew. Chemie Int. Ed. 2011, 50, 11688–11691. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Ge, X.; Han, S.; Wang, S.; Zhou, P.; Shan, H.; Zhao, X.; Lu, J.R. Twisted nanotubes formed from ultrashort amphiphilic peptide I3K and their templating for the fabrication of silica nanotubes. Chem. Mater. 2010, 22, 5165–5173. [Google Scholar] [CrossRef]
- Wang, S.; Xue, J.; Ge, X.; Fan, H.; Xu, H.; Lu, J.R. Biomimetic synthesis of silica nanostructures with controllable morphologies and sizes through tuning interfacial interactions. Chem. Commun. 2012, 48, 9415–9417. [Google Scholar] [CrossRef]
- Coradin, T.; Coupe, A.; Livage, J. Interactions of bovine serum albumin and lysozyme with sodium silicate solutions. Colloids Surf. B Biointerfaces 2003, 29, 189–196. [Google Scholar] [CrossRef]
- Heinemann, S.; Coradin, T.; Desimone, M.F. Bio-inspired silica–collagen materials: Applications and perspectives in the medical field. Biomater. Sci. 2013, 1, 688–702. [Google Scholar] [CrossRef]
- Giraud Guille, M.M.; Mosser, G.; Helary, C.; Eglin, D. Bone matrix like assemblies of collagen: From liquid crystals to gels and biomimetic materials. Micron 2005, 36, 602–608. [Google Scholar]
- Coradin, T.; Giraud-Guille, M.M.; Helary, C.; Livage, J.; Sanchez, C. A Novel Route to Collagen-Silica Biohybrids. San Francisco, CA, USA, 9–13 April 2002; Brinker, C.J., Laine, R.M., Sanchez, C., Yang, S., Eds.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Eglin, D.; Mosser, G.; Giraud-Guille, M.M.; Livage, J.; Coradin, T. Type I collagen, a versatile liquid crystal biological template for silica structuration from nano- to microscopic scales. Soft Matter 2005, 1, 129–131. [Google Scholar] [CrossRef]
- Desimone, M.F.; Helary, C.; Mosser, G.; Giraud-Guille, M.M.; Livage, J.; Coradin, T. Fibroblast encapsulation in hybrid silica-collagen hydrogels. J. Mater. Chem. 2009, 20, 666–668. [Google Scholar] [CrossRef]
- Coradin, T.; Bah, S.; Livage, J. Gelatine/silicate interactions: From nanoparticles to composite gels. Colloids Surf. B 2004, 35, 53–58. [Google Scholar] [CrossRef]
- Coradin, T.; Marchal, A.; Abdoul-Aribi, N.; Livage, J. Gelatine thin films as biomimetic surfaces for silica particles formation. Colloids Surf. B 2005, 44, 191–196. [Google Scholar]
- Shopsowitz, K.E.; Qi, H.; Hamad, W.Y.; Maclachlan, M.J. Free-standing mesoporous silica films with tunable chiral nematic structures. Nature 2010, 468, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Molvinger, K.; Quignard, F.; Brunel, D.; Boissière, M.; Devoisselle, J.M. Porous chitosan-silica hybrid microspheres as a potential catalyst. Chem. Mater. 2004, 16, 3367–3372. [Google Scholar] [CrossRef]
- Chang, J.S.; Kong, Z.L.; Hwang, D.F.; Chang, K.L.B. Chitosan-catalyzed aggregation during the biomimetic synthesis of silica nanoparticles. Chem. Mater. 2006, 18, 702–707. [Google Scholar] [CrossRef]
- Gautier, C.; Abdoul-Aribi, N.; Roux, C.; Lopez, P.J.; Livage, J.; Coradin, T. Biomimetic dual templating of silica by polysaccharide/protein assemblies. Colloids Surf. B 2008, 65, 140–145. [Google Scholar] [CrossRef]
- Erni, P.; Dardelle, G.; Sillick, M.; Wong, K.; Beaussoubre, P.; Fieber, W. Turning coacervates into biohybrid glass: Core/shell capsules formed by silica precipitation in protein/polysaccharide scaffolds. Angew. Chem. Int. Ed. Engl. 2013, 52, 10334–10338. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, A.; Illa, O.; Ortuño, R.M. Amphiphiles in aqueous solution: Well beyond a soap bubble. Chem. Soc. Rev. 2013, 42, 8200–8219. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, M.; Fang, X.; Wu, L. Fabrication and application of inorganic hollow spheres. Chem. Soc. Rev. 2011, 40, 5472–5491. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Nakashima, K.; Sano, M.; Kanekiyo, Y.; Inoue, K.; Shinkai, S.; Hojo, J. Organic gels are useful as a template for the preparation of hollow fiber silica. Chem. Commun. 1998, 14, 1477–1478. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Hanabusa, K. Creation of both right-handed and left-Handed silica structures by sol-gel transcription of organogel fibers comprised of chiral diaminocyclohexane derivatives. J. Am. Chem. Soc. 2000, 122, 5008–5009. [Google Scholar]
- Yang, X.; Tang, H.; Cao, K.; Song, H.; Sheng, W.; Wu, Q. Templated-assisted one-dimensional silica nanotubes: Synthesis and applications. J. Mater. Chem. 2011, 21, 6122–6135. [Google Scholar] [CrossRef]
- Harada, M.; Adachi, M. Surfactant-mediated fabrication of silica nanotubes. Adv. Mater. 2000, 12, 839–841. [Google Scholar] [CrossRef]
- Brizard, A.; Aimé, C.; Labrot, T.; Huc, I.; Berthier, D.; Artzner, F.; Desbat, B.; Oda, R. Counterion, temperature, and time modulation of nanometric chiral ribbons from gemini-tartrate amphiphiles. J. Am. Chem. Soc. 2007, 129, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Delclos, T.; Aimé, C.; Pouget, E.; Brizard, A.; Huc, I.; Delville, M.H.; Oda, R. Individualized silica nanohelices and nanotubes: Tuning inorganic nanostructures using lipidic self-assemblies. Nano Lett. 2008, 8, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Klug, A. The tobacco mosaic virus particle: Structure and assembly. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Chen, Z.; Usha, R.; Stauffacher, C.V.; Dai, J.B.; Schmidt, T.; Johnson, J.E. The refined crystal structure of cowpea mosaic virus at 2.8 A resolution. Virology 1999, 265, 20–34. [Google Scholar]
- Douglas, T.; Young, M. Host-guest encapsulation of materials by assembled virus protein cages. Nature 1998, 393, 152–155. [Google Scholar]
- Shenton, W.; Douglas, T.; Young, M.; Stubbs, G.; Mann, S. Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virus. Adv. Mater. 1999, 11, 253–256. [Google Scholar] [CrossRef]
- Fowler, C.E.; Shenton, W.; Stubbs, G.; Mann, S. Tobacco mosaic virus liquid crystals as templates for the interior design of silica. Adv. Mater. 2001, 13, 1266–1269. [Google Scholar] [CrossRef]
- Mao, C.; Wang, F.; Cao, B. Controlling nanostructures of mesoporous silica fibers by supramolecular assembly of genetically modifiable bacteriophages. Angew. Chemie 2012, 51, 6411–6415. [Google Scholar] [CrossRef]
- Niu, Z.; Kabisatpathy, S.; He, J.; Lee, L.A.; Rong, J.; Yang, L.; Sikha, G.; Popov, B.N.; Emrick, T.S.; Russell, T.P.; et al. Synthesis and characterization of bionanoparticle—Silica composites and mesoporous silica with large pores. Nano Res. 2009, 2, 474–483. [Google Scholar] [CrossRef]
- Li, K.; Nguyen, H.G.; Lu, X.; Wang, Q. Viruses and their potential in bioimaging and biosensing applications. Analyst 2010, 135, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hsu, F.C.; Battigelli, D.; Chang, H.C. Capture and release of viruses using amino-functionalized silica particles. Anal. Chim. Acta 2006, 569, 76–82. [Google Scholar] [CrossRef]
- Kangasniemi, L.; Koskinen, M.; Jokinen, M.; Toriseva, M.; Ala-Aho, R.; Kähäri, V.M.; Jalonen, H.; Ylä-Herttuala, S.; Moilanen, H.; Stenman, U.H.; et al. Extended release of adenovirus from silica implants in vitro and in vivo. Gene Ther. 2009, 16, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Laidler, J.R.; Shugart, J.A.; Cady, S.L.; Bahjat, K.S.; Stedman, K.M. Reversible inactivation and desiccation tolerance of silicified viruses. J. Virol. 2013, 87, 13927–13929. [Google Scholar] [CrossRef] [PubMed]
- Laidler, J.R.; Stedman, K.M. Virus silicification under simulated hot spring conditions. Astrobiology 2010, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, H.; Jones, B.; Chen, S.; Zhou, H. Silicified virus-like nanoparticles in an extreme thermal environment: Implications for the preservation of viruses in the geological record. Geobiology 2013, 11, 511–526. [Google Scholar] [PubMed]
- Puddu, V.; Perry, C.C. Peptide adsorption on silica nanoparticles: Evidence of hydrophobic interactions. ACS Nano 2012, 6, 6356–6363. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V.; Emami, F.S.; Berry, R.J.; Jones, S.E.; Naik, R.R.; Deschaume, O.; Heinz, H.; Perry, C.C. Chemistry of aqueous silica nanoparticle surfaces and the mechanism of selective peptide adsorption. J. Am. Chem. Soc. 2012, 134, 6244–6256. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.B.; Gu, W.; Kleitz, F.; Liu, J.; He, L.; Middelberg, A.P.J.; Yu, C.; Lu, G.Q.M.; Qiao, S.Z. Poly-l-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012, 6, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Feifel, S.C.; Lisdat, F. Silica nanoparticles for the layer-by-layer assembly of fully electro-active cytochrome c multilayers. J. Nanobiotechnol. 2011, 9. [Google Scholar] [CrossRef]
- Sharma, M.K.; Gilchrist, M.L. Templated assembly of biomembranes on silica microspheres using bacteriorhodopsin conjugates as structural anchors. Langmuir 2007, 23, 7101–7112. [Google Scholar] [CrossRef] [PubMed]
- Aimé, C.; Mosser, G.; Pembouong, G.; Bouteiller, L.; Coradin, T. Controlling the nano-bio interface to build collagen-silica self-assembled networks. Nanoscale 2012, 4, 7127–7134. [Google Scholar]
- Bancelin, S.; Decencière, E.; Machairas, V.; Albert, C.; Coradin, T.; Schanne-Klein, M.C.; Aimé, C. Fibrillogenesis from nanosurfaces: Multiphoton imaging and stereological analysis of collagen 3D self-assembly dynamics. Soft Matter 2014, 10, 6651–6657. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Coradin, T.; Aimé, C. Reversible bioresponsive aptamer-based nanocomposites: ATP binding and removal from DNA-grafted silica nanoparticles. J. Mater. Chem. B 2013, 1, 5353–5359. [Google Scholar] [CrossRef]
- Macfarlane, R.J.; Lee, B.; Jones, M.R.; Harris, N.; Schatz, G.C.; Mirkin, C. Nanoparticle superlattice engineering with DNA. Science 2011, 334, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Do, J.; Roller, E.M.; Zhang, T.; Schüller, V.J.; Nickels, P.C.; Feldmann, J.; Liedl, T. Hierarchical assembly of metal nanoparticles, quantum dots and organic dyes using DNA origami scaffolds. Nat. Nanotechnol. 2014, 9, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, L.R.; Zhao, X.; Tan, W. Immobilization of oligonucleotides onto silica nanoparticles for DNA hybridization studies. Anal. Chim. Acta 2002, 470, 51–56. [Google Scholar] [CrossRef]
- Wu, J.; Silvent, J.; Coradin, T.; Aimé, C. Biochemical investigation of the formation of three-dimensional networks from DNA-grafted large silica particles. Langmuir 2012, 28, 2156–2165. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernandes, F.M.; Coradin, T.; Aimé, C. Self-Assembly in Biosilicification and Biotemplated Silica Materials. Nanomaterials 2014, 4, 792-812. https://doi.org/10.3390/nano4030792
Fernandes FM, Coradin T, Aimé C. Self-Assembly in Biosilicification and Biotemplated Silica Materials. Nanomaterials. 2014; 4(3):792-812. https://doi.org/10.3390/nano4030792
Chicago/Turabian StyleFernandes, Francisco M., Thibaud Coradin, and Carole Aimé. 2014. "Self-Assembly in Biosilicification and Biotemplated Silica Materials" Nanomaterials 4, no. 3: 792-812. https://doi.org/10.3390/nano4030792