Rare Earth Ion-Doped Upconversion Nanocrystals: Synthesis and Surface Modification
Abstract
:1. Introduction
2. Synthetic Approaches
2.1. Thermal Decomposition
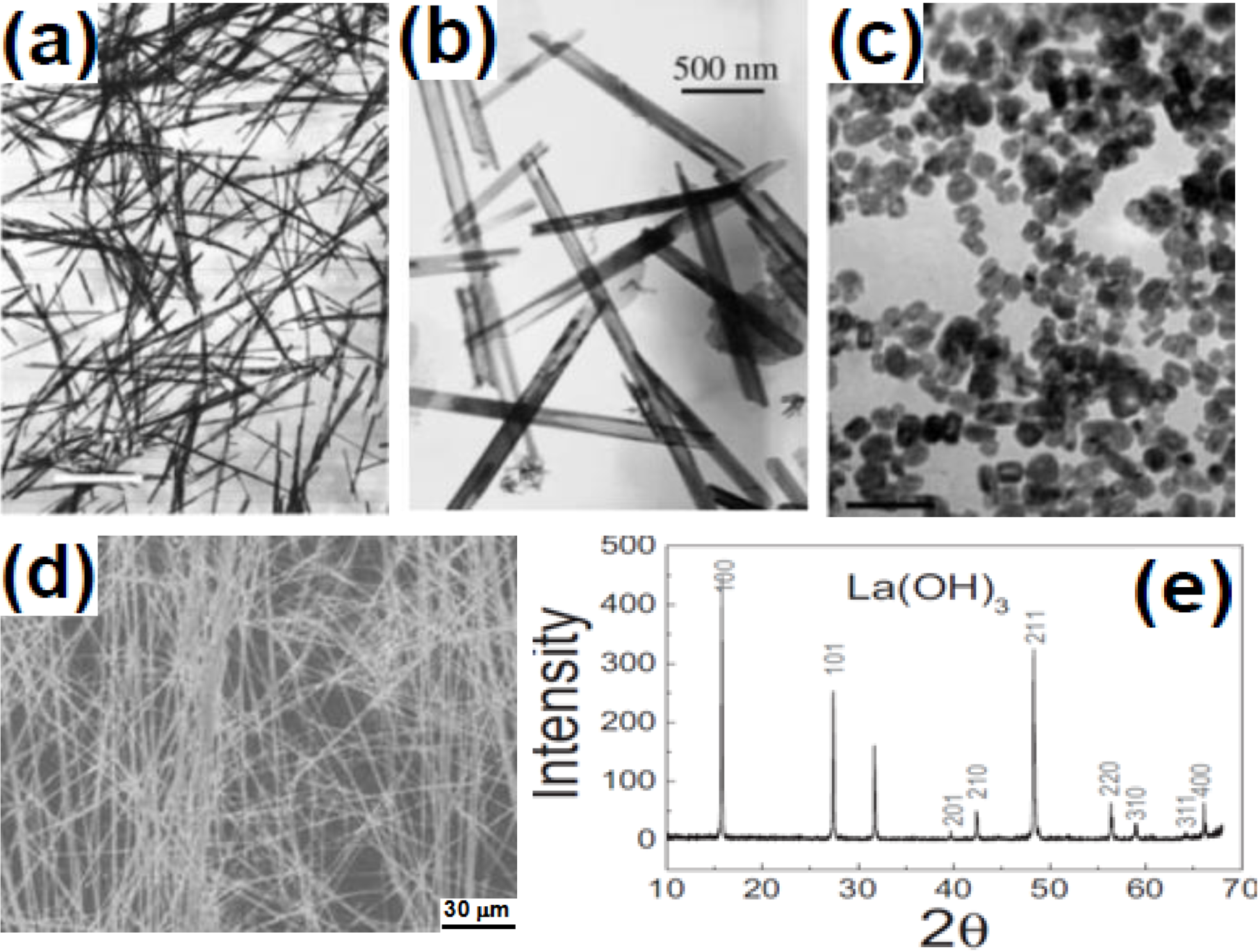
2.2. Thermal Coprecipitation
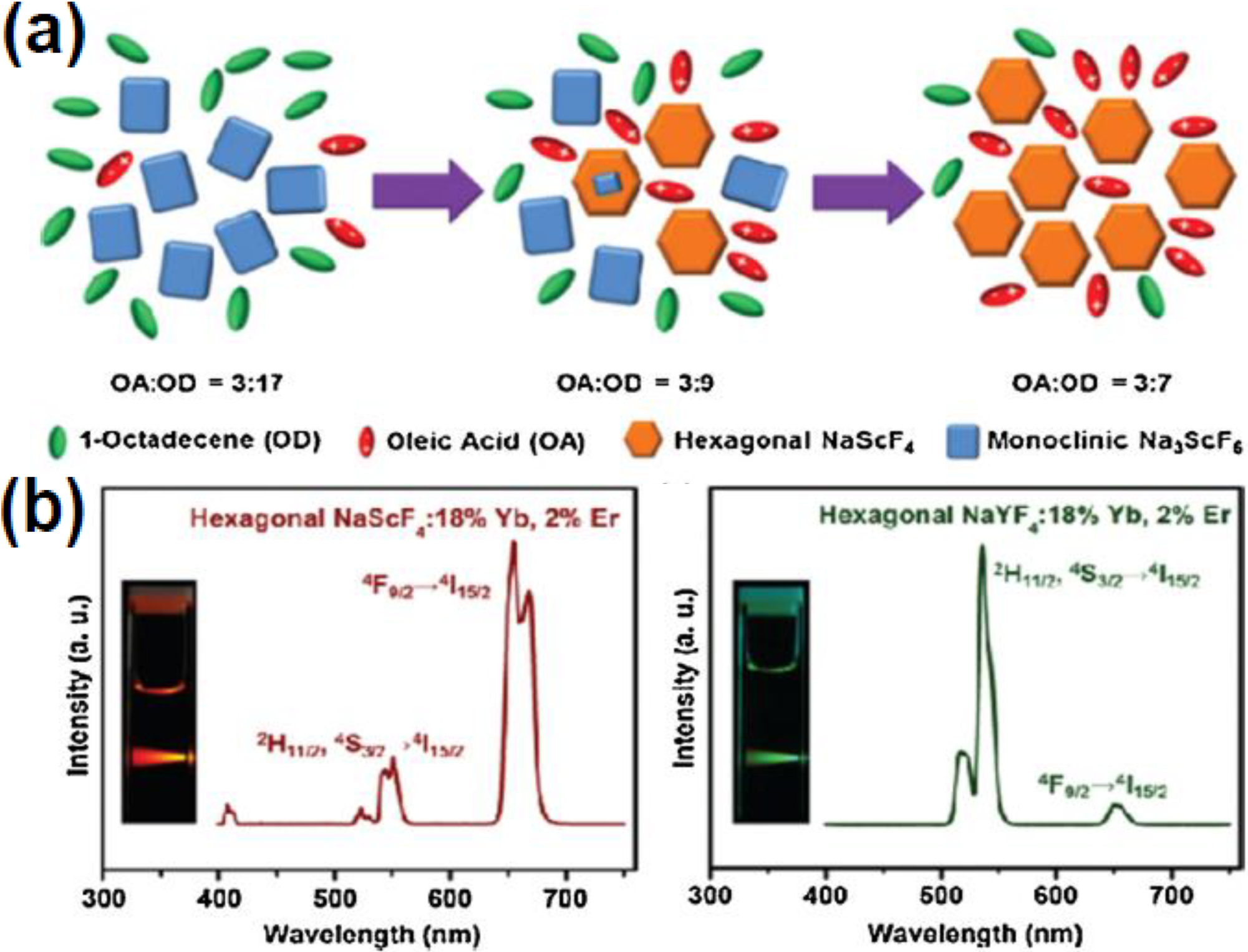
2.3. Hydro/Solvothermal Synthesis
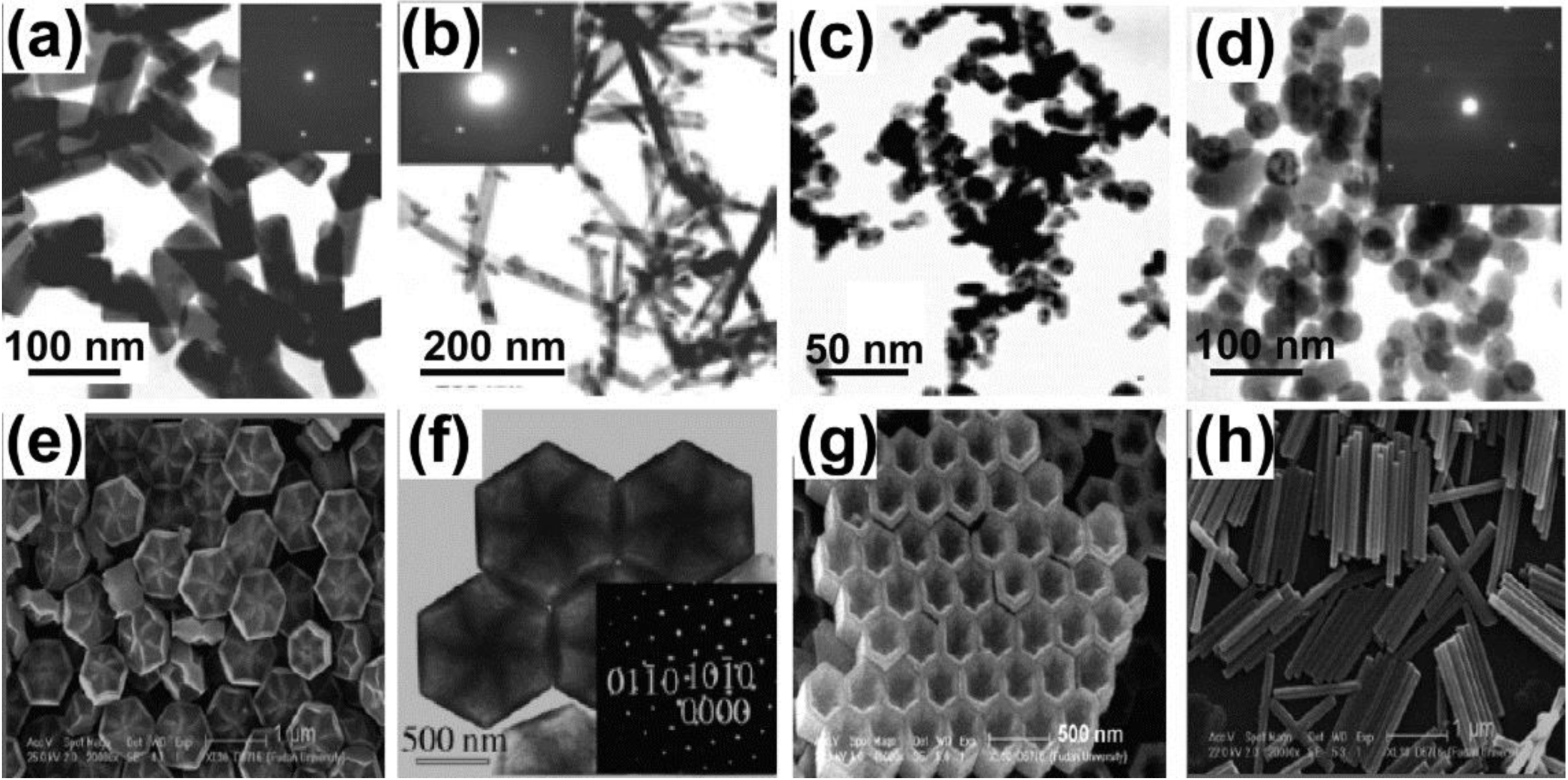
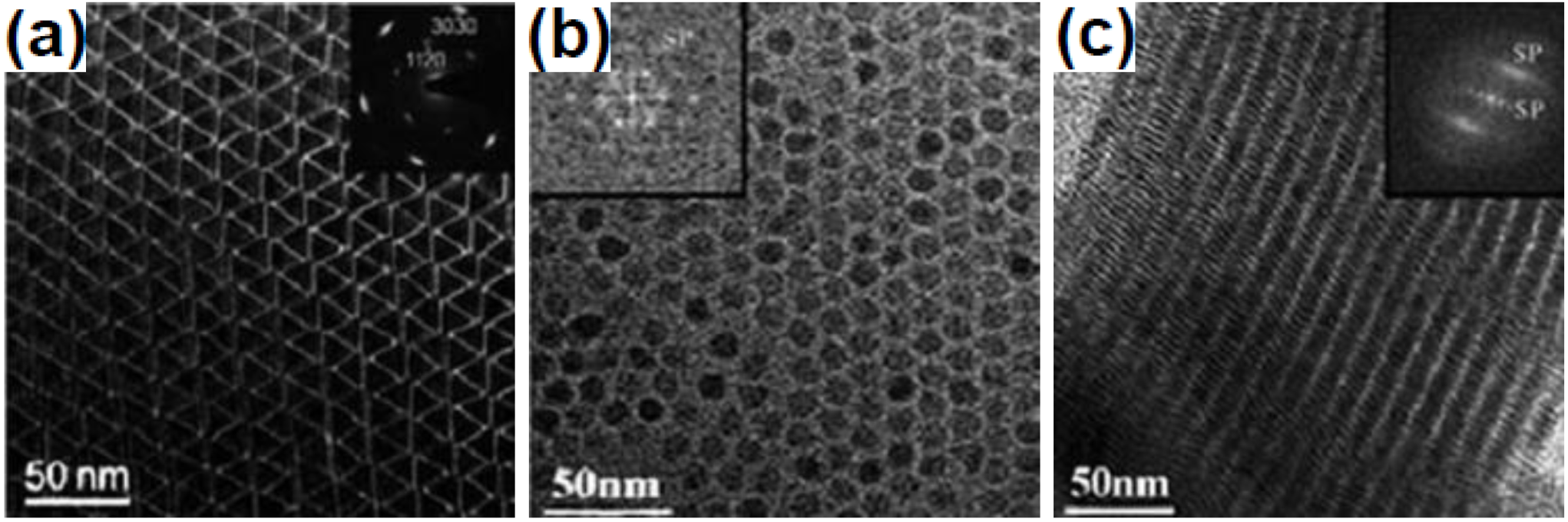
2.4. Sol-Gel Method
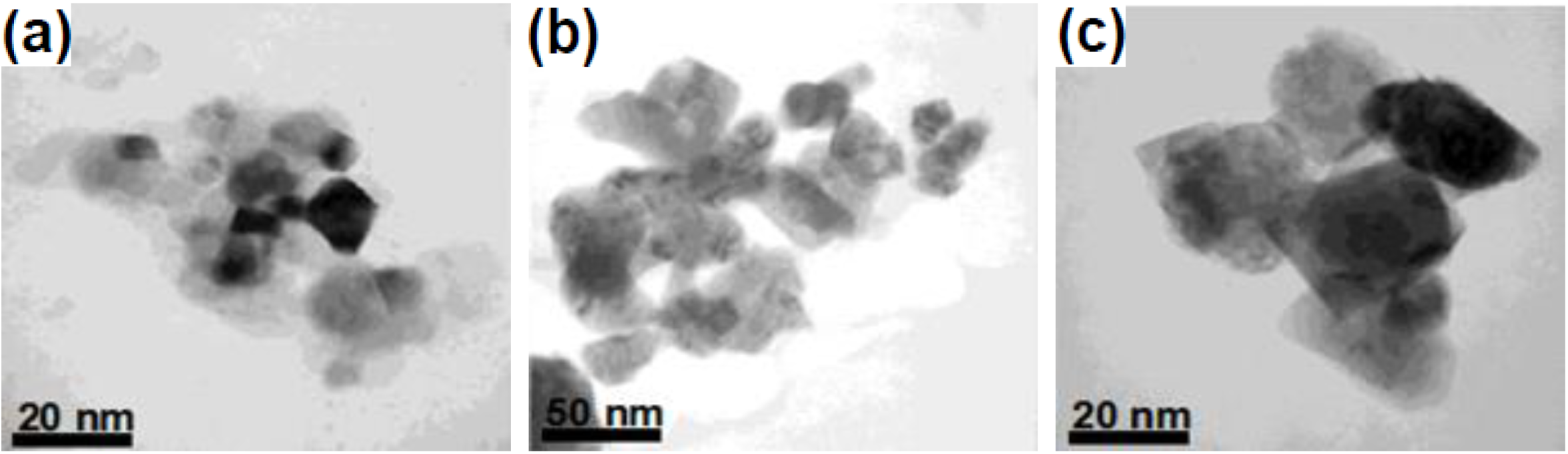
2.5. Microemulsion
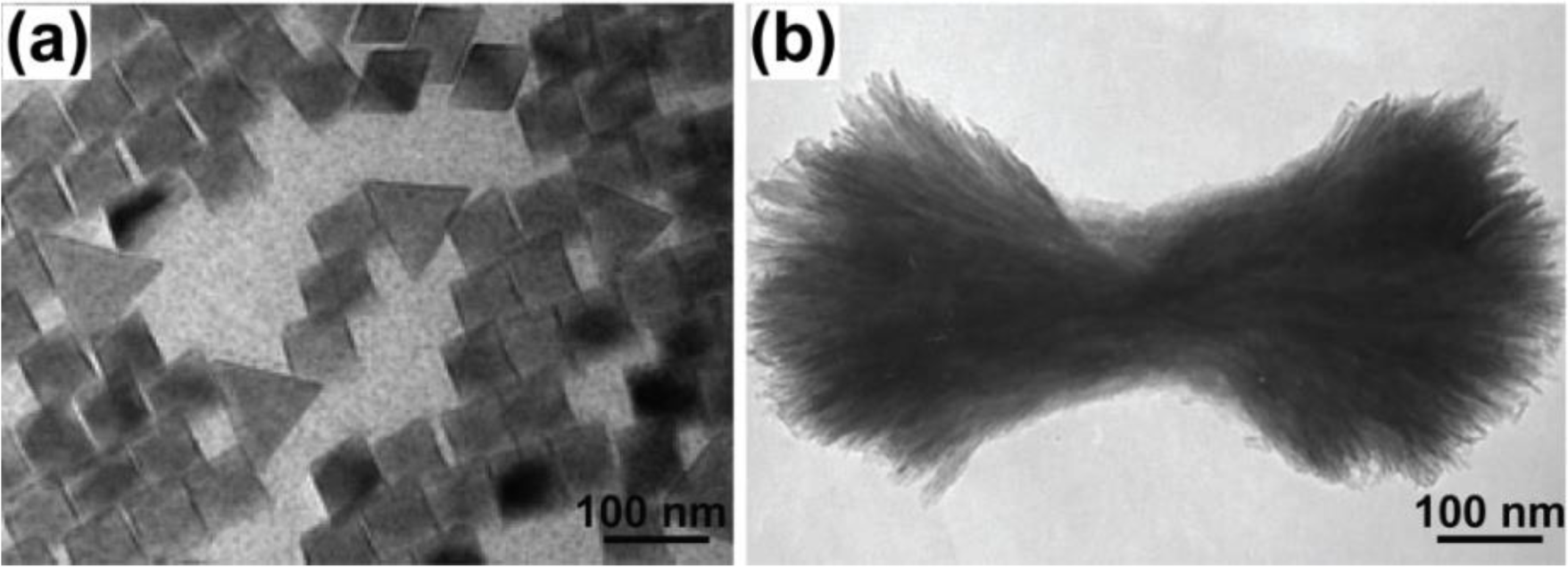
2.6. Combustion Synthesis
2.7. Flaming Synthesis
2.8. Electrospinning
2.9. Microwave Synthesis
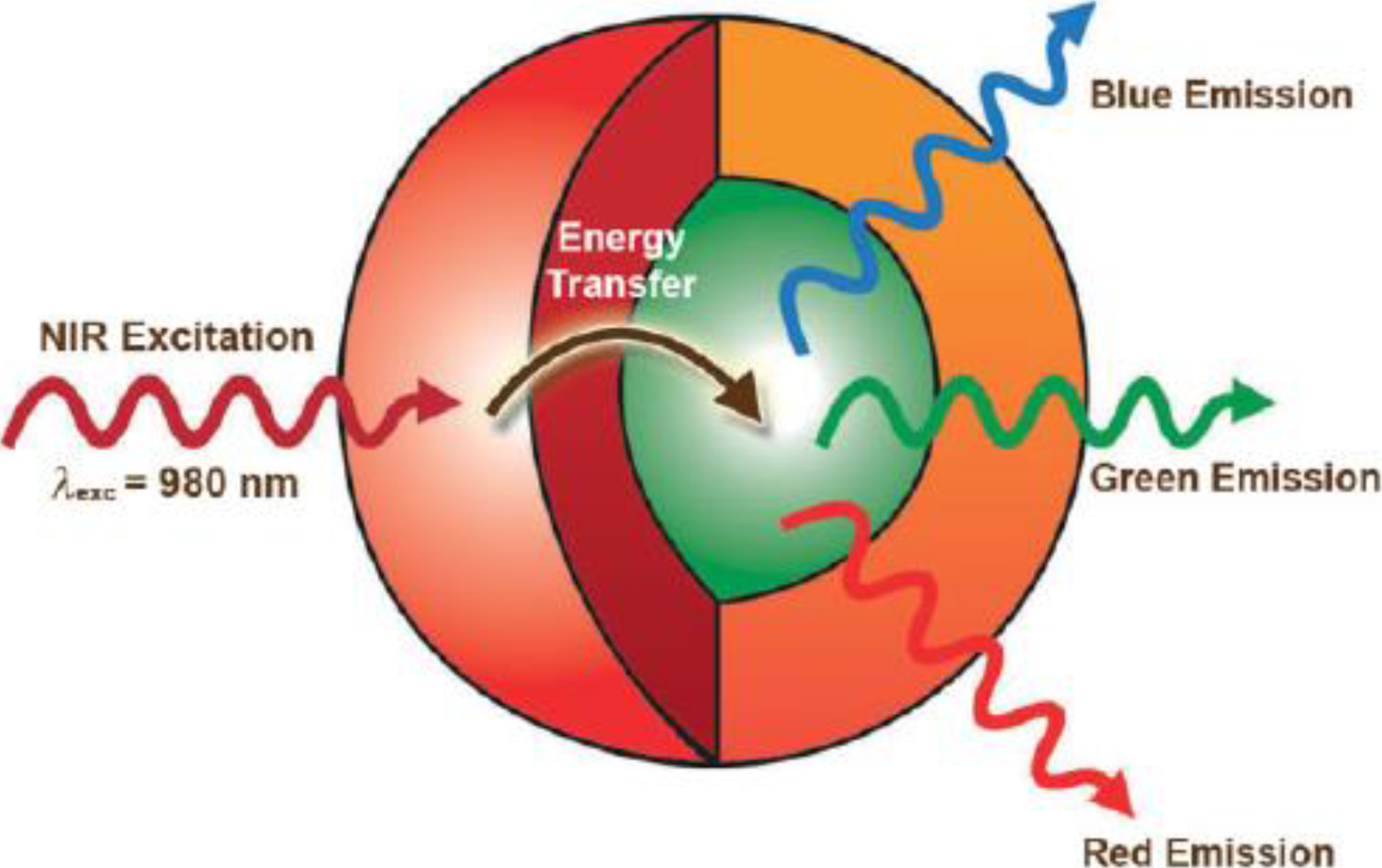
3. Surface Modification of UCNPs
3.1. SiO2 Encapsulation
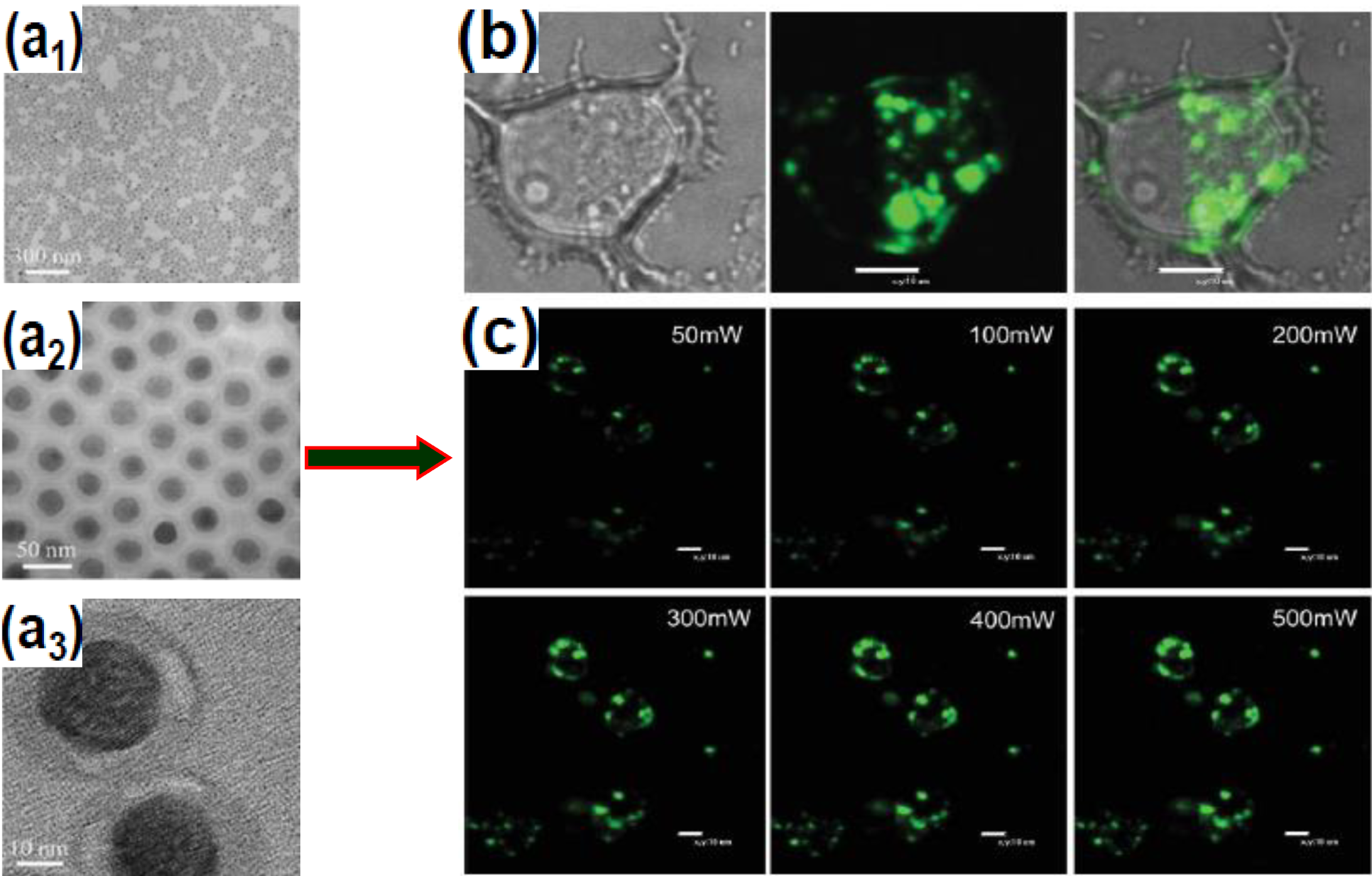
3.2. Polymer Encapsulation
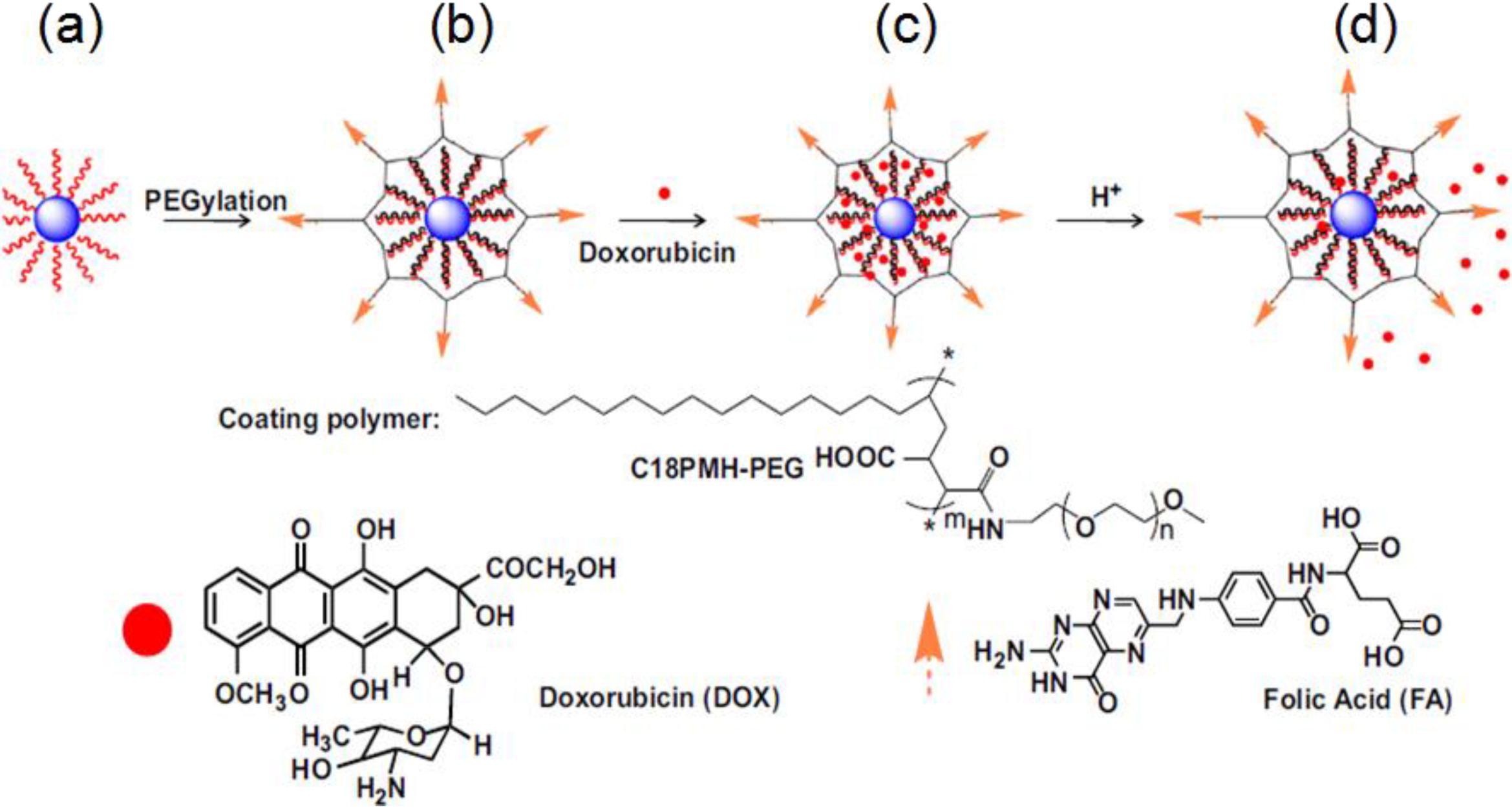
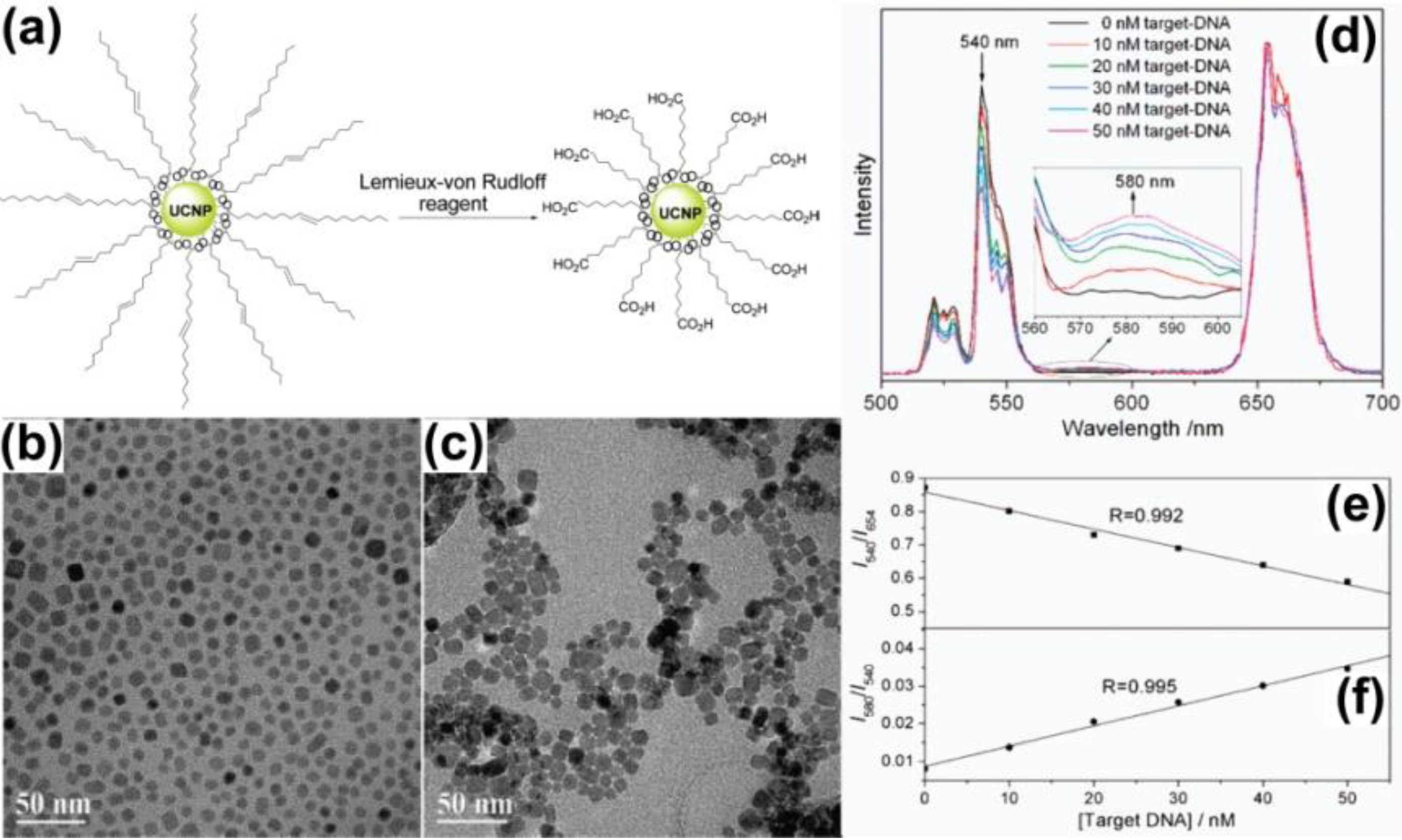
3.3. Ligand Oxidation
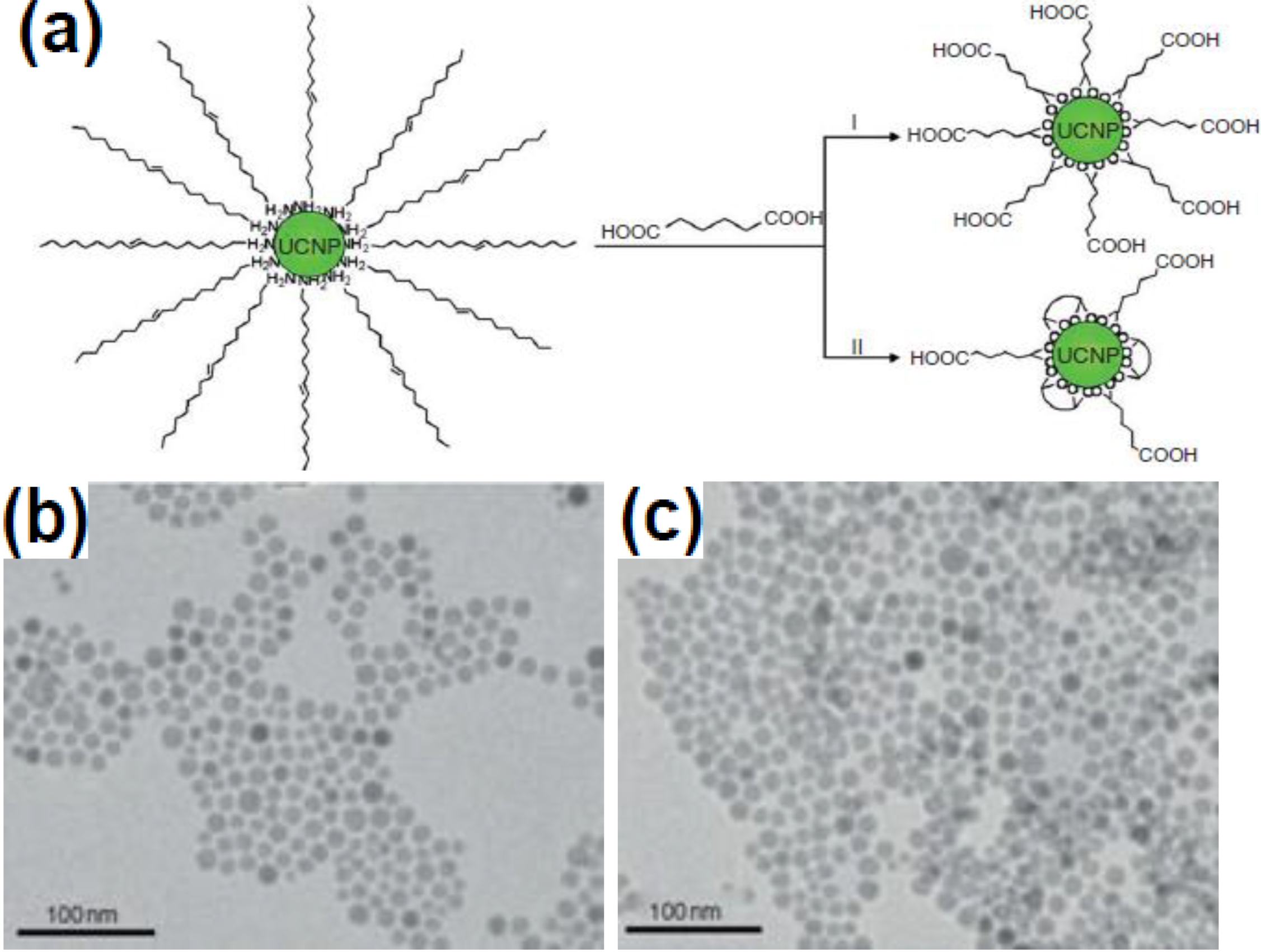
3.4. Ligand Exchange
4. Conclusions
Acknowledges
Author Contributions
Conflicts of Interest
References
- Law, M.; Goldberger, J.; Yang, P.D. Semiconductor nanowires and nanotubes. Annu. Rev. Mater. Sci. 2004, 34, 83–122. [Google Scholar] [CrossRef]
- Yoffe, A.D. Low-dimensional systems: Quantum size effects and electronic properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-two-dimensional systems. Adv. Phys. 2002, 42, 173–266. [Google Scholar] [CrossRef]
- Su, V.C.; Chen, P.H.; Lin, R.M.; Lee, M.L.; You, Y.H.; Ho, C.I.; Chen, Y.C.; Chen, W.F.; Kuan, C.H. Suppressed quantum-confined stark effect in InGaN-based LEDs with nano-sized patterned sapphire substrates. Opt. Exp. 2013, 21, 30066–30071. [Google Scholar]
- Wang, F.; Liu, X.G. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.S.; Lu, H.C.; Zhao, S.Y.; Yue, G.; Yang, W.J.; Chen, D.P. Synthesis, characterization, and biological application of size-controlled nanocrystalline NaYF4:Yb,Er infrared-to-visible up-conversion phosphors. Nano Lett. 2004, 4, 2191–2196. [Google Scholar] [CrossRef]
- Carlos, L.D.; Ferreira, R.A.; Ribeiro, S.J. Lanthanide-containing light-emitting organic-inorganic hybrids: A bet on the future. Adv. Mater. 2009, 21, 509–534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Z.; Li, F.Y. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, C.M.; Li, F.Y. Upconversion-nanophosphor-based functional nanocomposites. Adv. Mater. 2013, 25, 5287–5303. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, C.R.; Wang, M.; Wang, L.P.; Mao, C.B.; Xu, S.K. Controllable synthesis of NaYF4:Yb,Er upconversion nanophosphors and their application to in vivo imaging of Caenorhabditis elegans. J. Mater. Chem. 2011, 21, 2632–2638. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Liu, X.G. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Hampl, J.; Hall, M.; Mufti, N.A.; Yao, Y.M.; Mac Queen, D.B.; Wright, W.H.; Cooper, D.E. Upconverting phosphor reporters in immunochromatographic assays. Anal. Biochem. 2001, 288, 176–187. [Google Scholar] [CrossRef] [PubMed]
- VandeRijke, F.; Zijlmans, H.; Li, S.; Vail, T.; Raap, A.K.; Niedala, R.S.; Tanke, H.J. Up-converting phosphor reporters for nucleic acid microarrays. Nat. Biotechnol. 2001, 19, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.F.; Riehn, R.; Ryu, W.S.; Khanarian, N.; Tung, C.-K.; Tank, D.; Austin, R.H. In vivo and scanning electron microscopy imaging of upconverting nanophosphors in Caenorhabditis elegans. Nano Lett. 2006, 6, 169–174. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Rufaihah, A.J.; Zhang, Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials 2008, 29, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Rogelj, S.; Nguyen, K.; Wheeler, D. Design of a highly sensitive and specific nucleotide sensor based on photon upconverting particles. J. Am. Chem. Soc. 2006, 128, 12410–12411. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.L.; Yang, P.P.; Li, C.X.; Wang, W.X.; Dai, Y.L.; Niu, N.; Lin, J. Nanomorphology and charge generation in bulk heterojunctions based on low-bandgap dithiophene polymers with different bridging atoms. Adv. Funct. Mater. 2010, 20, 1166–1172. [Google Scholar] [CrossRef]
- Hinklin, T.R.; Rand, S.C.; Laine, R.M. Transparent, polycrystalline upconverting nanoceramics: Towards 3-D displays. Adv. Mater. 2008, 20, 1270–1273. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Sun, X.; Si, R.; You, L.P.; Yan, C.H. Single-crystalline and monodisperse LaF3 triangular nanoplates from a single-source precursor. J. Am. Chem. Soc. 2005, 127, 3260–3261. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.W.; Du, Y.P.; Yan, Z.G.; Si, R.; You, L.P.; Yan, C.H. From trifluoroacetate complex precursors to monodisperse rare-earth fluoride and oxyfluoride nanocrystals with diverse shapes through controlled fluorination in solution phase. Chem. Eur. J. 2007, 13, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.C.; Cuccia, L.A.; Capobianco, J.A. Synthesis of colloidal upconverting NaYF4:Er3+/Yb3+ and Tm3+/Yb3+ monodisperse nanocrystals. Nano Lett. 2007, 7, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Thomas, N. Monodisperse upconverting nanocrystals by microwave-assisted synthesis. ACS Nano 2009, 3, 3804–3808. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.S.; Lee, W.B.; Chow, G.M. Synthesis of LiYF4, BaYF5, and NaLaF4 optical nanocrystals. J. Nanosci. Nanotechnol. 2007, 7, 2790–2794. [Google Scholar] [CrossRef]
- Naccache, R.; Vetrone, F.; Mahalingam, V.; Capobianco, J.A. Controlled synthesis and water dispersibility of hexagonal phase NaGdF4:Ho/Yb nanoparticles. Chem. Mater. 2009, 21, 717–723. [Google Scholar] [CrossRef]
- Mahalingam, V.; Vetrone, F.; Naccache, R.; Speghini, A.; Capobianco, J.A. Colloidal Tm3+/Yb3+-doped LiYF4 nanocrystals: Multiple luminescence spanning the UV to NIR regions via low-energy excitation. Adv. Mater. 2009, 21, 4025–4028. [Google Scholar] [CrossRef]
- Mahalingam, V.; Vetrone, F.; Naccache, R.; Speghini, A.; Capobianco, J.A. Structural and optical investigation of colloidal Ln3+/Yb3+ co-doped KY3F10 nanocrystals. J. Mater. Chem. 2009, 19, 3149–3152. [Google Scholar] [CrossRef]
- Vetrone, F.; Mahalingam, V.; Capobianco, J.A. Near-infrared-to-blue upconversion in colloidal BaYF5:Tm3+,Yb3+ nanocrystals. Chem. Mater. 2009, 21, 1847–1851. [Google Scholar] [CrossRef]
- Zhang, P.D.; Zhang, Y.W.; Zheng, Z.G.; Sun, L.D.; Yan, C.H. Highly luminescent self-organized sub-2-nm EuOF nanowires. J. Am. Chem. Soc. 2009, 131, 16364–16365. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.P.; Zhang, Y.W.; Sun, L.D.; Yan, C.H. Luminescent monodisperse nanocrystals of lanthanide oxyfluorides synthesized from trifluoroacetate precursors in high-boiling solvents. J. Phys. Chem. C 2008, 112, 405–415. [Google Scholar] [CrossRef]
- Du, Y.P.; Zhang, Y.W.; Sun, L.D.; Yan, C.H. Atomically efficient synthesis of self-assembled monodisperse and ultrathin lanthanide oxychloride nanoplates. J. Am. Chem. Soc. 2009, 131, 3162–3163. [Google Scholar] [CrossRef] [PubMed]
- Stouwdam, J.W.; van Veggel, F.C.J.M. Near-infrared emission of redispersible Er3+, Nd3+, and Ho3+ doped LaF3 nanoparticles. Nano Lett. 2002, 2, 733–737. [Google Scholar] [CrossRef]
- Yi, G.S.; Chow, G.M. Colloidal LaF3:Yb,Er, LaF3:Yb,Ho and LaF3:Yb,Tm nanocrystals with multicolor upconversion fluorescence. J. Mater. Chem. 2005, 15, 4460–4464. [Google Scholar] [CrossRef]
- Heer, S.; Kompe, K.; Gudel, H.U.; Haase, M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Heer, S.; Lehmann, O.; Haase, M.; Gudel, H.U. Blue, green, and red upconversion emission from lanthanide-doped LuPO4 and YbPO4 nanocrystals in a transparent colloidal solution. Angew. Chem. Int. Ed. 2003, 42, 3179–3182. [Google Scholar] [CrossRef]
- Zeng, J.H.; Su, J.; Li, Z.H.; Yan, R.X.; Li, Y.D. Synthesis and upconversion luminescence of hexagonal-phase NaYF4:Yb3+,Er3+ phosphors of controlled size and morphology. Adv. Mater. 2005, 17, 2119–2123. [Google Scholar] [CrossRef]
- Teng, X.; Zhu, Y.H.; Wei, W.; Wang, S.C.; Huang, J.F.; Naccache, R.; Hu, W.B.; Han, Y.; Zhang, Q.C.; Fan, Q.L.; et al. Lanthanide-doped NaxScF3+x nanocrystals: Crystal structure evolution and multicolor tuning. J. Am. Chem. Soc. 2012, 134, 8340–8343. [Google Scholar] [CrossRef]
- Liang, L.F.; Xu, H.F.; Su, Q.; Konishi, H.; Jiang, Y.B.; Wu, M.M.; Wang, Y.F.; Xia, D.Y. Hydrothermal synthesis of prismatic NaHoF4 microtubes and NaSmF4 nanotubes. Inorg. Chem. 2004, 43, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.F.; Fan, H.; Xi, B.J.; Wang, X.Y.; Dong, C.; Qian, Y.T. Synthesis, characterization, and luminescence properties of uniform Ln3+-doped YF3 nanospindles. J. Phys. Chem. C 2007, 111, 6652–6657. [Google Scholar] [CrossRef]
- Liang, X.; Wang, X.; Zhuang, J.; Peng, Q.; Li, Y.D. Branched NaYF4 nanocrystals with luminescent properties. Inorg. Chem. 2007, 46, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.W.; Suan, Y.J.; Kong, X.G.; Tian, L.J.; Wang, Y.; Tu, L.P.; Zhao, J.L.; Zhang, H. Controlled synthesis, formation mechanism, and great enhancement of red upconversion luminescence of NaYF4:Yb3+,Er3+ nanocrystals/submicroplates at low doping level. J. Phys. Chem. B 2008, 112, 15666–15672. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y.D. Hydrothermal synthesis of rare-earth fluoride nanocrystals. Inorg. Chem. 2006, 45, 6661–6665. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.D. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.Y.; Chen, C.; Li, Y.D. Systematic synthesis of lanthanide phosphate nanocrystals. Chem. Eur. J. 2007, 13, 7708–7714. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Li, Y.D. Systematic synthesis of lanthanide phosphate nanocrystals. Na(Y1.5Na0.5)F6 single-crystal nanorods as multicolor luminescent materials. Nano Lett. 2006, 6, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Li, Y.D. Controlled synthesis and luminescence of lanthanide doped NaYF4 nanocrystals. Chem. Mater. 2007, 19, 727–734. [Google Scholar] [CrossRef]
- Wang, G.F.; Peng, Q.; Li, Y.D. Luminescence tuning of upconversion nanocrystals. Chem. Eur. J. 2010, 16, 4923–4931. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Li, P.; Li, Y.D. Down and upconversion luminescent nanorods. Adv. Mater. 2007, 19, 3304–3307. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.D. Synthesis and characterization of lanthanide hydroxide single-crystal nanowires. Angew. Chem. Int. Ed. 2002, 41, 4790–4793. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.M.; Yu, D.P.; Zou, B.S.; Li, Y.D. Rare earth compound nanotubes. Adv. Mater. 2003, 15, 1442–1445. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.D. Fullerene-like rare-earth nanoparticles. Angew. Chem. Int. Ed. 2003, 42, 3497–3500. [Google Scholar] [CrossRef]
- Hu, C.G.; Liu, H.; Wang, Z.L. La(OH)3 and La2O3 nanobelts-synthesis and physical properties. Adv. Mater. 2007, 19, 470–474. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Yu, T.; Zhang, F.; Shi, Y.; Xie, S.; Li, Y.; Xu, L.; Tu, B.; Zhao, D. Uniform nanostructured arrays of sodium rare-earth fluorides for highly efficient multicolor upconversion luminescence. Angew. Chem. Int. Ed. 2007, 46, 7976–7979. [Google Scholar] [CrossRef]
- Liu, C.; Chen, D. Controlled synthesis of hexagon shaped lanthanide-doped LaF3 nanoplates with multicolor upconversion fluorescence. J. Mater. Chem. 2007, 17, 3875–3880. [Google Scholar] [CrossRef]
- Pan, S.H.; Deng, R.P.; Feng, J.; Wang, S.Y.; Wang, S.; Zhu, M.; Zhang, H.J. Microwave-assisted synthesis and down and upconversion luminescent properties of BaYF5:Ln (Ln = Yb/Er, Ce/Tb) nanocrystals. CrystEngComm 2013, 15, 7640–7643. [Google Scholar] [CrossRef]
- Yang, M.; You, H.P.; Zheng, Y.H.; Liu, K.; Jia, G.; Song, Y.H.; Huang, Y.J.; Zhang, L.H.; Zhang, H. Hydrothermal synthesis and luminescent properties of novel ordered sphere CePO4 hierarchical architectures. J. Inorg. Chem. 2009, 48, 11559–11565. [Google Scholar] [CrossRef]
- Yang, M.; You, H.P.; Huang, Y.H.; Zhang, H.J. Synthesis and luminescent properties of orderly YPO4:Eu3+ olivary architectures self-assembled by nanoflakes. CrystEngComm 2010, 12, 4141–4145. [Google Scholar] [CrossRef]
- Zheng, Y.H.; You, H.P.; Jia, K.; Liu, K.; Song, Y.H.; Yang, M.; Zhang, H.J. Highly uniform Gd2O3 hollow microspheres: Template-directed synthesis and luminescence properties. Langmuir 2010, 26, 5122–5128. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; You, H.P.; Liu, K.; Zheng, Y.H.; Guo, N.; Zhang, H.J. Facile hydrothermal synthesis and luminescent properties of large-scale GdVO4:Eu3+ nanowires. Cryst. Growth Des. 2009, 9, 5101–5107. [Google Scholar] [CrossRef]
- Li, C.X.; Hou, Z.Y.; Zhang, C.M.; Yang, P.P.; Li, G.G.; Xu, Z.H.; Fan, Y.; Lin, J. Controlled synthesis of Ln3+ (Ln = Tb, Eu, Dy) and V5+ ion-doped YPO4 nano-/microstructures with tunable luminescent colors. Chem. Mater. 2009, 21, 4598–4607. [Google Scholar] [CrossRef]
- Li, C.X.; Yang, J.; Yang, P.P.; Lian, H.Z.; Lin, J. Hydrothermal synthesis of lanthanide fluorides LnF3 (Ln = La to Lu) nano/microcrystals with multiform structures and morphologies. Chem. Mater. 2008, 20, 4317–4326. [Google Scholar] [CrossRef]
- Li, C.X.; Yang, J.; Quan, Z.W.; Yang, P.P.; Kong, D.Y.; Lin, J. Different microstructures of ss-NaYF4 fabricated by hydrothermal process: Effects of pH values and fluoride sources. Chem. Mater. 2007, 19, 4933–4942. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Wang, C.; Liu, Z.; Liu, X.G. Single-band upconversion emission in lanthanide-doped KMnF3 nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 10369–10372. [Google Scholar] [CrossRef]
- Tian, G.; Gu, Z.J.; Zhou, L.J.; Yin, W.Y.; Liu, X.X.; Yan, L.; Jin, S.; Ren, W.L.; G, M.; Li, S.G.; et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.J.; Yi, Z.G.; Lu, W.; Qian, C.; Wang, H.B.; Rao, L.; Zeng, T.M.; Liu, H.R.; Liu, H.G.; Fei, B.; et al. Simultaneous realization of phase/size manipulation, upconversion luminescence enhancement, and blood vessel imaging in multifunctional nanoprobes through transition metal Mn2+ doping. Adv. Funct. Mater. 2014, 24, 4051–4059. [Google Scholar] [CrossRef]
- Lin, J.; Yu, M.; Lin, C.K.; Liu, X.M. Multiform oxide optical materials via the versatile Pechini-type sol-gel process: Synthesis and characteristics. J. Phys. Chem. C 2007, 111, 5835–5845. [Google Scholar] [CrossRef]
- Patra, A.; Friend, C.S.; Kapoor, R.; Prasad, P.N. Upconversion in Er3+:ZrO2 nanocrystals. J. Phys. Chem. B 2002, 106, 1909–1912. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Xing, R.B.; Hou, Z.Y.; Huang, S.S.; Lin, J. Patterning of light-emitting YVO4:Eu3+ thin films via inkjet printing. J. Phys. Chem. C 2010, 114, 9883–9888. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Xu, W.; Zhang, H.Z.; Wang, W.; Xu, S.; Song, H.W. Inhibited long-scale energy transfer in dysprosium doped yttrium vanadate inverse opal. J. Phys. Chem. C 2012, 116, 2297–2302. [Google Scholar] [CrossRef]
- Wang, W.; Song, H.W.; Liu, Q.; Bai, X.; Wang, Y.; Dong, B. Modified optical properties in a samarium doped titania inverse opal. Opt. Lett. 2010, 35, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, H.W.; Wang, W.; Bai, X.; Wang, Y.; Dong, B.; Xu, L.; Han, W. Observation of lamb shift and modified spontaneous emission dynamics in the YBO3:Eu3+ inverse opal. Opt. Lett. 2010, 35, 2898–2900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Sun, Z.P.; Yin, Z.; Song, H.W.; Xu, W.; Wang, Y.F.; Zhang, L.G.; Zhang, H.Z. Self-assembly, highly modified spontaneous emission and energy transfer properties of LaPO4:Ce3+,Tb3+ inverse opals. Dalton Trans. 2013, 42, 8049–8057. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Friend, C.S.; Kapoor, R.; Prasad, P.N. Fluorescence upconversion properties of Er3+-doped TiO2 and BaTiO3 nanocrystallites. Chem. Mater. 2003, 15, 3650–3655. [Google Scholar] [CrossRef]
- Venkatramu, V.; Falcomer, D.; Speghini, A.; Bettinelli, M.; Jayasankar, C.K. Synthesis and luminescence properties of Er3+-doped Lu3Ga5O12 nanocrystals. J. Lumin. 2008, 128, 811–813. [Google Scholar] [CrossRef]
- Yang, K.S.; Zheng, F.; Wu, R.; Li, H.; Zhang, X. Upconversion luminescent properties of YVO4:Yb3+,Er3+ nano-powder by sol-gel method. J. Rare Earth 2006, 24, 162–166. [Google Scholar] [CrossRef]
- Lemyre, J.L.; Ritcey, A.M. Synthesis of lanthanide fluoride nanoparticles of varying shape and size. Chem. Mater. 2005, 17, 3040–3043. [Google Scholar] [CrossRef]
- Wang, G.F.; Qin, W.P.; Zhang, J.S. Synthesis, growth mechanism, and tunable upconversion luminescence of Yb3+/Tm3+-codoped YF3 nanobundles. J. Phys. Chem C 2008, 112, 12161–12167. [Google Scholar] [CrossRef]
- Wang, G.F.; Qin, W.P.; Wei, G.D. Synthesis and upconversion luminescence properties of Yb3+/Tm3+-codoped BaSiF6 nanorods. J. Fluorine Chem. 2009, 130, 158–161. [Google Scholar] [CrossRef]
- Vetrone, F.; Boyer, J.C.; Capobianco, J.A.; Speghini, A.; Bettinelli, M. Significance of Yb3+ concentration on the upconversion mechanisms in codoped Y2O3:Er3+,Yb3+ nanocrystals. J. Appl. Phys. 2004, 96, 661–667. [Google Scholar] [CrossRef]
- Pandozzi, F.; Vetrone, F.; Boyer, J.C.; Naccache, R.; Capobianco, J.A.; Speghini, A.; Bettinellin, M. A spectroscopic analysis of blue and ultraviolet upconverted emissions from Gd3Ga5O12:Tm3+,Yb3+ nanocrystals. J. Phys. Chem. B 2005, 109, 17400–17405. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.X.; Cao, W.H. Ethanol-assistant solution combustion method to prepare La2O3:Yb,Pr nanometer phosphor. J. Alloys. Compds. 2008, 46, 529–534. [Google Scholar] [CrossRef]
- Xu, L.L.; Yu, Y.N.; Li, X.G. Synthesis and upconversion properties of monoclinic Gd2O3:Er3+ nanocrystals. Opt. Mater. 2008, 30, 1284–1288. [Google Scholar] [CrossRef]
- Qin, X.; Yokomori, T.; Ju, Y.G. Flame synthesis and characterization of rare-earth (Er3+, Ho3+, and Tm3+) doped upconversion nanocryphosphors. Appl. Phys. Lett. 2007, 90, 073104. [Google Scholar] [CrossRef]
- Dong, B.; Song, H.W.; Yu, H.Q.; Zhang, H.; Qin, R.F.; Bai, X.; Pan, G.H.; Lu, S.Z.; Wang, F.; Fan, L.B.; et al. Upconversion properties of Ln3+ doped NaYF4/polymer composite fibers prepared by electrospinning. J. Phys. Chem. C 2008, 112, 1435–1440. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.X.; Zheng, Y.Z.; Zhao, J.; Xu, Z.D. Microwave-assisted hydrothermal synthesis and characterizations of PrF3 hollow nanoparticles. Mater. Lett. 2007, 61, 2765–2768. [Google Scholar] [CrossRef]
- Chan, E.M. Combinatorial approaches for developing upconverting nanomaterials: High-throughput screening, modeling, and applications. Chem. Soc. Rev. 2014. [CrossRef]
- Liu, H.; Jakobsson, O.; Xu, C.T.; Xie, H.; Laurell, T.; Andersson-Engels, S. Synthesis of NaYF4:Yb3+/Er3+ Upconverting Nanoparticles in a Capillary-Based Continuous Microfluidic Reaction System. In Colloidal Quantum Dots/Nanocrystals for Biomedical Applications; SPIE: Bellingham, WA, USA, 2011; Volume 7909, p. 790917. [Google Scholar]
- Xu, C.T.; Zhan, Q.; Liu, H.; Somesfalean, G.; Qian, J.; He, S.; Andersson-Engels, S. Upconverting nanoparticles for pre-clinical diffuse optical imaging, microscopy and sensing: Current trends and future challenges. Laser Photon. Rev. 2013, 7, 663–697. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Q.; Li, Y.; Wang, H. Redispersible and water-soluble LaF3:Ce,Tb nanocrystalsvia a microfluidic reactor with temperature steps. J. Mater. Chem. 2008, 18, 5060–5062. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Q.; Li, Y.; Wang, H.J. Facile crystallization control of LaF3/LaPO4:Ce,Tb nanocrystals in a microfluidic reactor using microwave irradiation. J. Mater. Chem. 2010, 20, 1766–1771. [Google Scholar] [CrossRef]
- Yi, G.S.; Chow, G.M. Water-soluble NaYF4:Yb,Er(Tm)/NaYF4/polymer core/shell/shell nanoparticles with significant enhancement of upconversion fluorescence. Chem. Mater. 2007, 19, 341–343. [Google Scholar] [CrossRef]
- Liu, Y.S.; Tu, D.T.; Zhu, H.M.; Li, R.F.; Luo, W.Q.; Chen, X.Y. A strategy to achieve efficient dual-mode luminescence of Eu3+ in lanthanides doped multifunctional NaGdF4 nanocrystals. Adv. Mater. 2010, 22, 3266–3271. [Google Scholar] [CrossRef] [PubMed]
- Vetrone, F.; Naccache, R.; Mahalingam, V.; Morgan, C.G.; Capobianco, J.A. The active-core/active-shell approach: A strategy to enhance the upconversion luminescence in lanthanide-doped nanoparticles. Adv. Funct. Mater. 2009, 19, 2924–2929. [Google Scholar] [CrossRef]
- Liu, X.M.; Kong, X.G.; Zhang, Y.L.; Tu, L.P.; Wang, Y.; Zeng, Q.H.; Li, C.G.; Shi, Z.; Zhang, H. Breakthrough in concentration quenching threshold of upconversion luminescence via spatial separation of the emitter doping area for bio-applications. Chem. Commun. 2011, 47, 11957–11959. [Google Scholar] [CrossRef]
- Wang, Z.L.; Quan, Z.W.; Jia, P.Y.; Lin, C.K.; Luo, Y.; Chen, Y.; Fang, J.; Zhou, W.; O’Connor, C.J.; Lin, J. A facile synthesis and photoluminescent properties of redispersible CeF3,CeF3:Tb3+, and CeF3:Tb3+/LaF3 (core/shell) nanoparticles. Chem. Mater. 2006, 18, 2030–2037. [Google Scholar] [CrossRef]
- Mai, H.X.; Zhang, Y.W.; Yan, C.H. Highly efficient multicolor up-conversion emissions and their mechanisms of monodisperse NaYF4:Yb,Er core and core/shell-structured nanocrystals. J. Phys. Chem. C 2007, 111, 13721–13729. [Google Scholar] [CrossRef]
- Boyer, J.C.; Gagnon, J.; Capobianco, J.A. Synthesis, characterization, and spectroscopy of NaGdF4:Ce3+,Tb3+/NaYF4 core/shell nanoparticles. Chem. Mater. 2007, 19, 3358–3360. [Google Scholar] [CrossRef]
- Helmut, S.; Pavel, P.; Otmanef, Z.; Markus, H. Synthesis and optical properties of KYF4/Yb,Er nanocrystals, and their surface modification with undoped KYF4. Adv. Funct. Mater. 2008, 18, 2913–2918. [Google Scholar] [CrossRef]
- Xie, X.J.; Gao, N.Y.; Deng, R.R.; Sun, Q.; Xu, Q.H.; Liu, X.G. Mechanistic investigation of photon upconversion in Nd3+-sensitized core-shell nanoparticles. J. Am. Chem. Soc. 2013, 135, 12608–12611. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zheng, W.; Zhou, S.Y.; Tu, D.T.; Chen, Z.; Zhu, H.M.; Li, R.F.; Ma, E.; Huang, M.D.; Chen, X.Y. Lanthanide-doped LiLuF4 upconversion nanoprobes for the detection of disease biomarkers. Angew. Chem. Int. Ed. 2014, 53, 1252–1257. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.R.; Liu, X.G. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 2014, 9, 1634–1644. [Google Scholar] [CrossRef]
- Wang, Y.F.; Sun, L.D.; Xia, J.W.; Feng, W.; Zhou, J.C.; Shen, J.; Yan, C.H. Rare-earth nanoparticles with enhanced upconversion emission and suppressed rare-earth-ion leakage. Chem. Eur. J. 2012, 18, 5558–5564. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, M.; Nann, T. One-pot synthesis of YF3@silica core/shell nanoparticles. Chem. Commun. 2006, 7, 776–778. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, Y. Monodisperse silica-coated polyvinylpyrrolidone/NaYF4 nanocrystals with multicolor upconversion fluorescence emission. Angew. Chem. Int. Ed. 2006, 45, 7732–7735. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, Y.; Jiang, S. Multicolor core/shell-structured upconvers fluorescent nanoparticles. Adv. Mater. 2008, 20, 4765–4769. [Google Scholar] [CrossRef]
- Chen, B.T.; Dong, B.; Wang, J.; Zhang, S.; Xu, L.; Yu, W.; Song, H.W. Amphiphilic silane modified NaYF4:Yb,Er loaded with Eu(TTA)3(TPPO)2 nanoparticles and their multi-functions: Dual mode temperature sensing and cell imaging. Nanoscale 2013, 5, 8541–8549. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Diamenteand, P.R.; van Veggel, F.C.J.M. Silica-coated Ln3+-doped LaF3 nanoparticles as robust down and upconverting biolabels. Chem. Eur. J. 2006, 12, 5878–5884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Yi, G.S.; Zhang, H.T.; Ding, J.; Zhang, Y.W.; Xue, J.M. Monodisperse silica nanoparticles encapsulating upconversion fluorescent and superparamagnetic nanocrystals. Chem. Commun. 2008, 6, 694–696. [Google Scholar] [CrossRef]
- Wang, M.; Mi, C.C.; Zhang, Y.Z.; Liu, J.L.; Li, F.; Mao, C.B.; Xu, S.K. NIR-responsive silica-coated NaYbF4:Er/Tm/Ho upconversion fluorescent nanoparticles with tunable emission colors and their applications in immunolabeling and fluorescent imaging of cancer cells. J. Phys. Chem. C 2009, 113, 19021–19027. [Google Scholar] [CrossRef]
- Yu, X.F.; Chen, L.D.; Li, M.; Xie, M.Y.; Zhou, L.; Li, Y.; Wang, Q.Q. Highly efficient fluorescence of NdF3/SiO2 core/shell nanoparticles and the applications for in vivo NIR detection. Adv. Mater. 2008, 20, 4118–4123. [Google Scholar] [CrossRef]
- Li, X.H.; Wu, Y.Q.; Liu, Y.; Zou, X.M.; Yao, L.M.; Lia, F.Y.; Feng, W. Cyclometallated ruthenium complex-modified upconversion nanophosphors for selective detection of Hg2+ ions in water. Nanoscale 2014, 6, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.; Liu, Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Budijono, S.J.; Shan, J.N.; Yao, N.; Miura, Y.; Hoye, T.; Austin, R.H.; Ju, Y.G.; Prud’homme, R.K. Synthesis of stable block-copolymer-protected NaYF4:Yb3+,Er3+ up-converting phosphor nanoparticles. Chem. Mater. 2010, 22, 311–318. [Google Scholar] [CrossRef]
- Chen, Z.G.; Chen, H.L.; Hu, H.; Yu, M.X.; Li, F.Y.; Zhang, Q.; Zhou, Z.G.; Yi, T.; Huang, C.H. Versatile synthesis strategy for carboxylic acid-functionalized upconverting nanophosphors as biological labels. J. Am. Chem. Soc. 2008, 130, 3023–3029. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, N.; Vetrone, F.; Ozin, G.A.; Capobianco, J.A. Synthesis of ligand-free colloidally stable water dispersible brightly luminescent lanthanide-doped upconverting nanoparticles. Nano Lett. 2011, 11, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.S.; Chow, G.M. Synthesis of hexagonal-phase NaYF4:Yb,Er and NaYF4:Yb,Tm nanocrystals with efficient up-conversion fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Chen, G.Y.; Ohulchanskyy, T.Y.; Law, W.C.; Agren, H.; Prasad, P.N. Monodisperse NaYbF4: Tm3+/NaGdF4 core/shell nanocrystals with near-infrared to near-infrared upconversion photoluminescence and magnetic resonance properties. Nanoscale 2011, 3, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.C.; Manseau, M.P.; Murray, J.I.; van Veggel, F.C.J.M. Surface modification of upconverting NaYF4 nanoparticles with PEG-phosphate ligands for NIR (800 nm) biolabeling within the biological window. Langmuir 2010, 26, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Nyk, M.; Kumar, R.; Ohulchanskyy, T.Y.; Bergey, E.J.; Prasad, P.N. High contrast in vitro and in vivo photoluminescence bioimaging using near Infrared to near infrared up-conversion in Tm3+ and Yb3+ doped fluoride nanophosphors. Nano Lett. 2008, 8, 3834–3838. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.Y.; Yang, T.S.; Gao, Y.; Yang, Y.; Hu, H.; Li, F.Y. Water-soluble NaYF4:Yb/Er upconversion nanophosphors: Synthesis, characteristics and application in bioimaging. Inorg. Chem. Commun. 2010, 13, 392–394. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, M.X.; Sun, Y.; Zhang, X.Z.; Zhu, X.J.; Wu, Z.H.; Wu, D.M.; Li, F.Y. Fluorine-18-labeled Gd3+/Yb3+/Er3+ co-doped NaYF4 nanophosphors formultimodality PET/MR/UCL imaging. Biomaterials 2011, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Naczynski, D.J.; Andelman, T.; Pal, D.; Chen, S.; Richard, E.; Riman, R.E.; Roth, C.M.; Moghe, P.V. Albumin nanoshell encapsulation of near-infrared-excitable rare-earth nanoparticles enhances biocompatibility and enables targeted cell imaging. Small 2010, 6, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.; Xie, J.; Zhao, B.; Liu, B.; Xu, S.; Ren, N.; Xie, X.; Huang, L.; Huang, W. Rare Earth Ion-Doped Upconversion Nanocrystals: Synthesis and Surface Modification. Nanomaterials 2015, 5, 1-25. https://doi.org/10.3390/nano5010001
Chang H, Xie J, Zhao B, Liu B, Xu S, Ren N, Xie X, Huang L, Huang W. Rare Earth Ion-Doped Upconversion Nanocrystals: Synthesis and Surface Modification. Nanomaterials. 2015; 5(1):1-25. https://doi.org/10.3390/nano5010001
Chicago/Turabian StyleChang, Hongjin, Juan Xie, Baozhou Zhao, Botong Liu, Shuilin Xu, Na Ren, Xiaoji Xie, Ling Huang, and Wei Huang. 2015. "Rare Earth Ion-Doped Upconversion Nanocrystals: Synthesis and Surface Modification" Nanomaterials 5, no. 1: 1-25. https://doi.org/10.3390/nano5010001




