Penetration and Toxicity of Nanomaterials in Higher Plants
Abstract
:1. Introduction
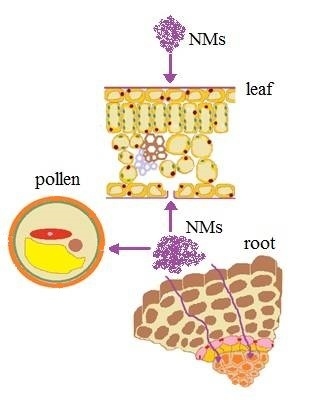
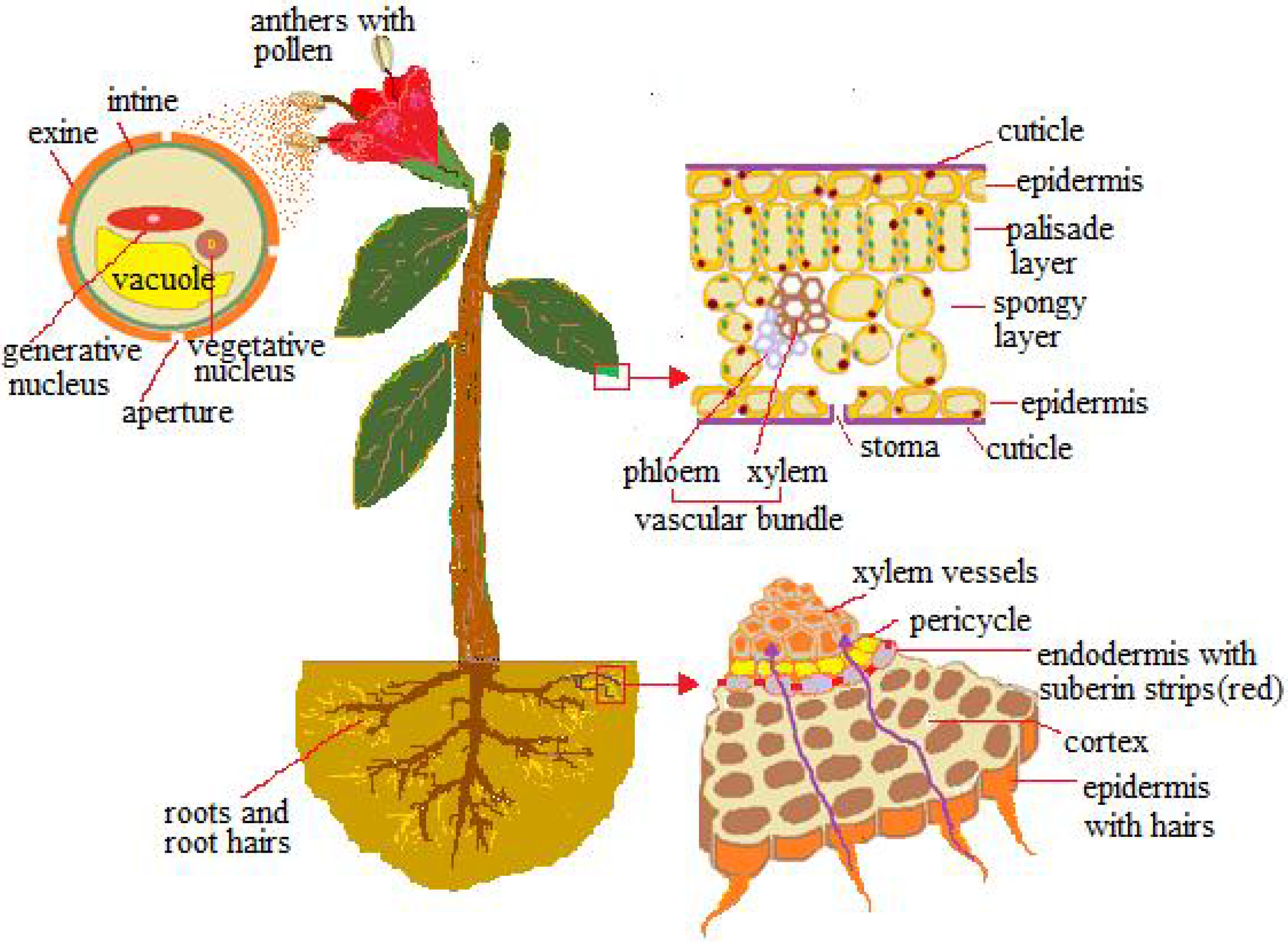
2. Vegetative System
3. Uptake and Toxicity of NMs
3.1. Leaf
3.1.1. Toxicity of Metal- and Metal Oxide-NPs
3.1.2. Toxicity of CNMs
3.2. Roots
3.2.1. Toxicity of Metal- and Metal Oxide-NPs
3.2.2. Genotoxicity of NPs
3.2.3. Toxicity of CNMs
3.2.4. Genotoxicity of CNTs
4. Reproductive Systems
5. Penetration and Toxicity of Metal- and Metal Oxide-NPs
6. Conclusions and Perspectives
Author Contributions
Conflicts of Interest
References
- Dietz, K.J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Veerapandian, M.; Yun, K.S. Nanoparticles: Functionalization and multifunctional applications in biomedical sciences. Curr. Med. Chem. 2010, 17, 4559–4577. [Google Scholar] [CrossRef] [PubMed]
- Treuel, L.; Eslahian, K.A.; Docter, D.; Lang, T.; Zellner, R.; Nienhaus, K.; Nienhaus, G.U.; Stauber, R.H.; Maskos, M. Physicochemical characterization of nanoparticles and their behavior in the biological environment. Phys. Chem. Chem. Phys. 2014, 16, 15053–15067. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, K.; Ma, J.; Gao, F. Cerium oxide nanoparticles in cancer. OncoTargets Ther. 2014, 7, 835–840. [Google Scholar] [CrossRef]
- Onelli, E.; Prescianotto-Baschong, C.; Caccianiga, M.; Moscatelli, A. Clathrin-dependent and independent endocytic pathways in tobacco protoplasts revealed by labelling with charged nanogold. J. Exp. Bot. 2008, 59, 3051–3068. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yang, B.; Xiong, H.; Zhou, Y.; Xue, S.; Xu, B.; Wang, S. Selective removal of heavy metal ions from aqueous solutions with surface functionalized silica nanoparticles by different functional groups. J. Cent. South Univ. 2014, 21, 3575–3579. [Google Scholar] [CrossRef]
- Poma, A.; Colafarina, S.; Fontecchio, G.; Chichiriccò, G. Transgenerational Effects of NMs. In Nanomaterials, Impacts on Cell Biology and Medicine; Capco, D., Chen, Y., Eds.; Springer Science and Business Media: Dordrecht, Germany, 2014; Volume 811, pp. 235–254. [Google Scholar]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef] [PubMed]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy and application. Nanoscale Res. Lett. 2014, 9, 229–252. [Google Scholar] [CrossRef] [PubMed]
- Eichert, T.; Goldbach, H.E. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surface—Further evidence for a stomatal pathway. Physiol. Plant. 2008, 132, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L. Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.; O’Neill, M.A.; Ehwald, R. The pore size of non-Graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 1999, 121, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.; Sabularse, D.; Montezinos, D.; Delmer, D.P. Determination of the pore size of cell walls of living plants. Science 1979, 205, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Tepfer, M.; Taylor, I.E.P. The permeability of plant cell walls as measured by gel filtration chromatography. Science 1981, 213, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Holbrook, N.M. Bordered pit structure and vessel wall surface properties. Implications for embolism repair. Plant Physiol. 2000, 123, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Hacke, U.G. Analysis of circular bordered pit function I. Angiosperm vessels with homogeneous pit membranes. Am. J. Bot. 2004, 91, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Pittermann, J.; Choat, B.; Jansen, S.; Stuart, S.A.; Lynn, L.; Dawson, T.E. The Relationships between xylem safety and hydraulic efficiency in the Cupressaceae: The evolution of pit membraneorm and function. Plant Physiol. 2010, 15, 1919–1931. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Birbaum, K.; Brogioli, R.; Schellenberg, M.; Martinoia, E.; Stark, W.J.; Günther, D.; Limbach, L.K. No evidence for cerium dioxide nanoparticle translocation in maize plants. Environ. Sci. Technol. 2010, 44, 8718–8723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultra-small anatase TiO2 alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-N.; Tarafdar, J.C.; Biswas, P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Taran, N.; Batsmanova, L.; Konotop, Y.; Okanenko, A. Redistribution of elements of metals in plant tissues under treatment by non-ionic colloidal solution of biogenic metal nanoparticles. Nanoscale Res. Lett. 2014, 9, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Peralta-Videa, J.R.; Rico, C.; Sahi, S.; Viveros, M.N.; Bartonio, J.; Zhao, L.; Gardea-Torresdey, J.L. Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on Cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2014, 48, 4376–4385. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Bureau, S.; Barthès, V.; Ouerdane, L.; Carrière, M.; Sarret, G. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation. J. Hazard. Mater. 2014, 261, 98–106. [Google Scholar] [CrossRef]
- Larue, C.; Veronesi, G.; Flank, A.M.; Surble, S.; Herlin-Boime, N.; Carriere, M. Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health A 2012, 75, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; White, J.C.; Xing, B. Interaction between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. Sci. A 2014, 15, 552–572. [Google Scholar] [CrossRef]
- Shen, C.-X.; Zhang, Q.F.; Li, J.; Bi, F.-C.; Yao, N. Induction of programmed cell death in Arabidopsis and rice by single wall carbon nanotubes. Am. J. Bot. 2010, 97, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; Mc Nicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplast of spinach. Biol. Trace Elem. Res. 2005, 105, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kurepa, J.; Smalle, J.A. Ultra-small TiO2 nanoparticles disrupt microtubular networks in Arabidopsis thaliana. Plant Cell Environ. 2011, 34, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Rãcuciu, M.; Creangã, D.E. TMA-OH coated magnetic nanoparticles internalized in vegetal tissue. Rom. J. Phys. 2007, 52, 395–402. [Google Scholar]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2011, 5, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.G.; Lòpez, M.L.; Gonzales, C.M.; Peralta-Videa, J.R.; Garrea-Torresdey, J.L. Toxicity and biotransformation of uncoated and coated nickel hydroxide nanoparticles on mesquite plants. Environ. Toxicol. Chem. 2010, 29, 1146–1154. [Google Scholar] [PubMed]
- Lin, C.; Fugetsu, B.; Su, Y.; Watari, F. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J. Hazard. Mater. 2009, 170, 578–583. [Google Scholar] [CrossRef]
- Tan, X.M.; Lin, C.; Fugetsu, B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 2009, 47, 3479–3487. [Google Scholar] [CrossRef]
- Santos, S.M.A.; Dinis, A.M.; Rodrigues, D.M.F.; Peixoto, F.; Videira, R.A.; Jurado, A.S. Studies on the toxicity of an aqueous suspension of C60 nanoparticles using a bacterium (gen. Bacillus) and an aquatic plant (Lemna gibba) as in vitro model systems. Aquat. Toxicol. 2013, 142–143, 347–354. [Google Scholar]
- Bianco, S. Graphene Phytotoxicity in the Seedling Stage of Cabbage, Tomato, Red Spinach, and Lettuce. In Carbon Nanotubes—From Research to Applications; ISBN 978-953-307-500-6. Bianco, S. InTech: Rijeka; Available online: http://www.intechopen.com/books/carbon-nanotubes-fromresearch-to-applications/graphene-phytotoxicity-in-the-seedling-stage-of cabbage-tomato-red-spinach-and-lettuce (accessed on 23 May 2015).
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Rãcuciu, M.; Creangã, D.E. Cytogenetical changes induced by β-cyclodextrin coated nanoparticles in plant seeds. Rom. J. Phys. 2009, 54, 125–131. [Google Scholar]
- Stampoulis, D.; Sinha, S.K.; White, J.C. White assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, G.; Lopez-Moreno, M.L.; Hernandez-Viescaz, J.; Montes, M.O.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity and biotransformation of ZnO nanoparticles in the desert plants Prosopis juliflora-velutina, Salsola tragus and Parkinsonia florida. Int. J. Nanotechnol. 2011, 8, 492–506. [Google Scholar] [CrossRef]
- Pokhrel, L.R.; Dubey, B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013, 452–453, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): Investigating a wide range of concentrations. Toxicol. Environ. Chem. 2014, 96, 861–868. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation, and accumulation of manufactured iron oxide by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.H.; Zhang, Z.Y.; He, X.; Zhang, J.; Guo, Z.; Tai, R.Z.; Zhao, Y.L.; Chai, Z.F. Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano 2012, 6, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peralta-Videa, J.R.; Varela-Ramirez, A.; Castillo-Michel, H.; Li, C.; Zhang, J.; Aguilera, R.J.; Keller, A.A.; Gardea-Torresdey, J.L. Effect of surface coating and organic matter on the uptake of CeO2–NPs by corn plants grown in soil: Insight into the uptake mechanism. J. Hazard. Mater. 2012, 225–226, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peralta-Videa, J.R.; Peng, B.; Bandyopadhyay, S.; Corral-Diaz, B.; Osuna-Avila, P.; Montes, M.O.; Keller, A.A.; Gardea-Torresdey, J.L. Alginate modifies the physiological impact of CeO2 nanoparticles in corn seedlings cultivated in soil. J. Environ. Sci. 2014, 26, 382–389. [Google Scholar] [CrossRef]
- Canas, J.E.; Long, M.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.; Ambikapathi, R.; Lee, E.H.; Oslzyk, D. Effects of functionalized and non-functionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; de Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Wild, E.; Jones, K.C. Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environ. Sci. Technol. 2009, 43, 290–294. [Google Scholar] [CrossRef]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.A.; Gusev, A.A.; Zaitseva, O.N.; Lazareva, E.M.; Onishchenko, G.E.; Kuznetsova, E.V.; Tkachev, A.G.; Feofanov, A.V.; Kirpichnikov, M.P. Multi-walled carbon nanotubes penetrate into plant cells and affect the growth of Onobrychis arenaria seedlings. Acta Nat. 2011, 3, 99–106. [Google Scholar]
- Cifuentes, Z.; Custardoy, L.; de la Fuente, J.M.; Marquina, C.; Ibarra, M.R.; Rubiales, D.; Alejandro Pérez-de-Luque, A. Absorption and translocation to the aerial part of magnetic carbon-coated nanoparticles through the root of different crop plants. J. Nanobiotechnol. 2010, 8. [Google Scholar] [CrossRef]
- Kole, C.; Kole, P.; Randunu, K.M.; Choudhary, P.; Podila, R.; Ke, P.; Rao, A.M.; Marcus, R.K. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol. 2013, 13, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Serag, M.F.; Kaji, N.; Venturelli, E.; Okamoto, Y.; Terasaka, K.; Tokeshi, M.; Mizukami, H.; Ugent, K.B.; Bianco, A.; Baba, Y.; et al. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano 2011, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Serag, M.F.; Kaji, N.; Gaillard, C.; Okamoto, Y.; Terasaka, K.; Jabasini, M.; Tokeshi, M.; Misukami, H.; Bianco, A.; Baba, Y.; et al. A functional platform for controlled subcellular distribution of carbon nanotubes. ACS Nano 2011, 5, 9264–9270. [Google Scholar] [CrossRef] [PubMed]
- Serag, M.F.; Kaji, N.; Tokeshi, M.; Baba, Y. Introducing carbon nanotubes into living walled plant cells through cellulase-induced nanoholes. RSC Adv. 2012, 2, 398–400. [Google Scholar] [CrossRef]
- Serag, M.F.; Braeckmans, K.; Habuchi, S.; Kaji, N.; Bianco, A.; Baba, Y. Spatiotemporal visualization of subcellular dynamics of carbon nanotubes. Nano Lett. 2012, 12, 6145–6151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Wang, Q.; Zhao, Y.; Rui, Q.; Wang, D. Toxicity and translocation of graphene oxide in Arabidopsis thaliana. Environ. Toxicol. Pharmacol. 2015, 39, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Bagheri, S.; Julkapli, N.M.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangon, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125–1140. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.; Luo, L.; Zhang, J.; Yang, K.; Christie, P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano 2015, 2, 68–77. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.; Parsons, J.G.; Troiani, H.E.; Yacaman, M.J. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Harris, A.T.; Bali, R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanopart. Res. 2008, 10, 691–695. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.S.; Shaheen, M.S.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi Chem. Soc. 2014, 18, 356–363. [Google Scholar] [CrossRef]
- Yin, S.L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the ions: The effects of silver nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, E.A.; Elsayed, B.A. Phytotoxicity of silver nanoparticles on Vicia faba seedlings. N. Y. Sci. J. 2013, 6, 148–156. [Google Scholar]
- Gardea-Torresdey, J.L.; Parsons, J.G.; Gomez, E.; Peralta-Videa, J.; Troiani, H.E.; Santiago, P.; Yacaman, M.J. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Zhai, G.; Walters, K.S.; Peate, D.W.; Alvarez, P.J.J.; Schnoor, J.L. Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ. Sci. Technol. Lett. 2014, 1, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bali, R.; Siegele, R.; Harris, A.T. Biogenic Pt uptake and nanoparticle formation in Medicago sativa and Brassica juncea. J. Nanopart. Res. 2010, 12, 3087–3095. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Zhang, W.; Pei, H.; Chen, Y. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics 2012, 4, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, F.; Schulin, R.; Limbach, L.K.; Stark, W.; Bürge, D.; Nowack, B. Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere 2013, 91, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Morales, M.I.; Barrios, A.C.; McCreary, R.; Hong, J.; Lee, W.-Y.; Nunez, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains. J. Agric. Food Chem. 2013, 61, 11278–11285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.H.; Zhang, Z.Y.; He, X.; Li, Y.; Zhang, J.; Zheng, L.; Zhao, Y.L. Species-Specific toxicity of ceria nanoparticles to Lactuga plants. Nanotoxicology 2013, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.H.; Zhang, Z.Y.; He, X.; Guo, Z.; Tai, R.Z.; Ding, Y.Y.; Zhao, Y.L.; Chai, Z.F. Comparative toxicity of nanoparticulate/bulk Yb2O3 and YbCl3 to Cucumber (Cucumis sativus). Environ. Sci. Technol. 2012, 46, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Bandyopadhyay, M.; Mukherjee, A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: Plant and human lymphocytes. Chemosphere 2010, 81, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Poulose, A.C.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Uptake of FITC labelled silica nanoparticls and quantum dots by rice seedlings: Effects on seed germination and their potential as biolabels for plants. J. Fluoresc. 2011, 21, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Navarro, D.A.; Bisson, M.A.; Aga, D.S. Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J. Hazard. Mater. 2012, 211, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef] [PubMed]
- Lòpez-Moreno, M.L.; de la Rosa, G.; Hernàndez-Viezcas, J.A.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the differential biotrasformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, C.M.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbodensis L. and Zea mays L. J. Nanopart. Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Atha, D.H.; Wang, H.; Ptersen, E.J.; Cleveland, D.; Holbrook, D.R.; Jaruga, P.; Dizdaroglu, M.; Xing, B.; Nelson, C.B. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 2012, 46, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, H.; Liu, X.; Gu, X.; Chen, K.; Lu, D. Multiwalled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanopart. Res. 2012, 14, 841–848. [Google Scholar] [CrossRef]
- Mondal, A.; Basu, R.; Das, S.; Nandy, P. Beneficial role of carbon nanotubes on mustard plant growth: An agricultural prospect. J. Nanopart. Res. 2011, 13, 4519–4528. [Google Scholar] [CrossRef]
- Villagarcia, H.; Dervishi, E.; de Silva, K.; Biris, A.S.; Khodakovskaya, M.V. Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants. Small 2012, 8, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 12, 115–123. [Google Scholar] [CrossRef]
- Flores, D.; Chacón, R.; Alvarado, L.; Schmidt, A.; Alvarado, C.; Chaves, J. Effect of using two different types of carbon nanotubes for blackberry (Rubus adenotrichos) in vitro plant rooting, growth and histology. Am. J. Plant Sci. 2014, 5, 3510–3518. [Google Scholar] [CrossRef]
- Anjum, N.A.; Singh, N.; Singh, M.K.; Sayeed, I.; Duarte, A.C.; Pereira, E.; Ahmad, I. Single-bilayer graphene oxide sheet impacts and underlying potential mechanism assessment in germinating faba bean (Vicia faba L.). Sci. Total Environ. 2014, 472, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Begum, P.; Fugetsu, B. Induction of cell death by graphene in Arabidopsis thaliana (Columbia ecotype) T87 cell suspensions. J. Hazard. Mater. 2013, 260, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Begum, P.; Fugetsu, B. Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L) and the role of ascorbic acid as an antioxidant. J. Hazard. Mater. 2012, 243, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kang, J.; Lu, K.; Zhou, R.; Mu, L.; Zhou, Q. Graphene oxide amplifies the phytotoxicity of arsenic in wheat. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; de Silva, K.; Dervishi, E.; Villagarcía, H. Carbon nanotubes induce growth enhancement of tobacco cell. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Chakraborty, A.; Bandyopadhyay, M.; Mukherjee, A. Multi-walled carbon nanotubes (MWCNT): Induction of DNA damage in plant and mammalian cells. J. Hazard. Mater. 2011, 197, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhao, L.; Li, H.; Zhang, Q.; Tan, J.; Huang, M.; He, S.; Li, L. Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J. Hazard. Mater. 2013, 246, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Punt, W.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of Pollen and Spore Terminology; LPP Contributions Series No.1; LPP Foundation: Utrecht, The Netherlands, 1994. [Google Scholar]
- Speranza, A.; Leopold, K.; Maier, M.; Taddei, A.R.; Scoccianti, V. Pd-Nanoparticles cause increased toxicity to kiwifruit pollen compared to soluble Pd (II). Environ. Pollut. 2010, 158, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Taddei, A.R.; Iacobucci, M.; Bhattacharya, P.; Chun Ke, P. In vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ. Pollut. 2013, 179, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ebbs, S.; Chen, Y.; Ma, X. Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics 2013, 5, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Irani, N.G.; Friml, J. Clathrin-mediated endocytosis: The gateway into plant cells. Curr. Opin. Plant Biol. 2011, 14, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Popp, C.; Burghardt, M.; Friedman, A.; Riederer, M. Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticolar membrane: Permeation of water and uncharged organic compounds. J. Exp. Bot. 2005, 56, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Kerstiens, G. Water transport in plant cuticles: An update. J. Exp. Bot. 2006, 57, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.W. Plant viral movement proteins: Agents for cell-to-cell trafficking of viral genomes. Virology 2006, 344, 169–184. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Roche, R.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; Wang, Q.; Ma, X.; Hamdi, H.; White, J.C.; et al. Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ. Sci. Technol. 2013, 47, 12539–12547. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Roche, R.; Hawthorne, J.; Musante, C.; Xing, B.; Newman, L.A.; Ma, X.; White, J.C. Impact of Ag nanoparticle exposure on p,p′-DDE bioaccumulation by Cucurbita pepo (zucchini) and Glycine max (soybean). Environ. Sci. Technol. 2013, 47, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals 2013, 26, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Husen, A.; Siddiqi, K.S. Carbon and fullerene nanomaterials in plant system. J. Nanotechnol. 2014, 12, 16–25. [Google Scholar]
- Maurer-Jones, M.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of Engineered Nanoparticles in the Environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2015. [Google Scholar] [CrossRef]
- Auffan, M.; Tella, M.; Santaella, C.; Brousset, L.; Paillès, C.; Barakat, M.; Espinasse, B.; Artells, E.; Issartel, J.; Masion, A.; et al. An adaptable mesocosm platform for performing integrated assessments of nanomaterial risk in complex environmental systems. Sci. Rep. 2014, 4, 5608–5615. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, D.; Long, S.E.; Pennington, P.L.; Cooper, E.; Fulton, M.H.; Scott, G.I.; Brewer, T.; Davis, J.; Petersen, E.J.; Wood, L. Pilot estuarine mesocosm study on the environmental fate of silver nanomaterials leached from consumer products. Sci. Total Environ. 2012, 421–422, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Herlekar, M.; Barve, S.; Kumar, R. Plant-mediated green synthesis of iron nanoparticles. J. Nanopart. 2014, 2014. [Google Scholar] [CrossRef]
- Petersen, E.J.; Henry, T.B.; Zhao, J.; MacCuspie, R.I.; Kirschling, T.L.; Dobrovolskaia, M.A.; Hackley, V.; Xing, B.; White, J.C. Identification and avoidance of potential artifacts and misinterpretations in nanomaterial ecotoxicity measurements. Environ. Sci. Technol. 2014, 48, 4226–4246. [Google Scholar] [CrossRef] [PubMed]
- Chichiriccò, G.; Picozzi, P. Reversible inhibition of the pollen germination and the stigma penetration in Crocus vernus ssp. vernus (Iridaceae) following fumigations with NO2, CO2, and O3 gases. Plant Biol. 2007, 9, 730–735. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chichiriccò, G.; Poma, A. Penetration and Toxicity of Nanomaterials in Higher Plants. Nanomaterials 2015, 5, 851-873. https://doi.org/10.3390/nano5020851
Chichiriccò G, Poma A. Penetration and Toxicity of Nanomaterials in Higher Plants. Nanomaterials. 2015; 5(2):851-873. https://doi.org/10.3390/nano5020851
Chicago/Turabian StyleChichiriccò, Giuseppe, and Anna Poma. 2015. "Penetration and Toxicity of Nanomaterials in Higher Plants" Nanomaterials 5, no. 2: 851-873. https://doi.org/10.3390/nano5020851