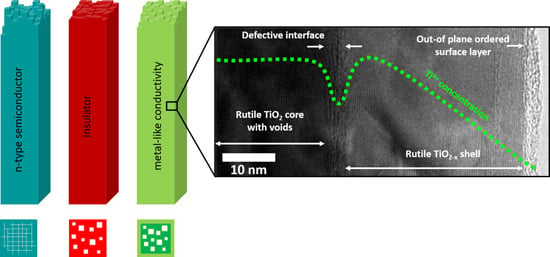
Tuning the Electronic Conductivity in Hydrothermally Grown Rutile TiO2 Nanowires: Effect of Heat Treatment in Different Environments
Abstract
:1. Introduction
2. Results
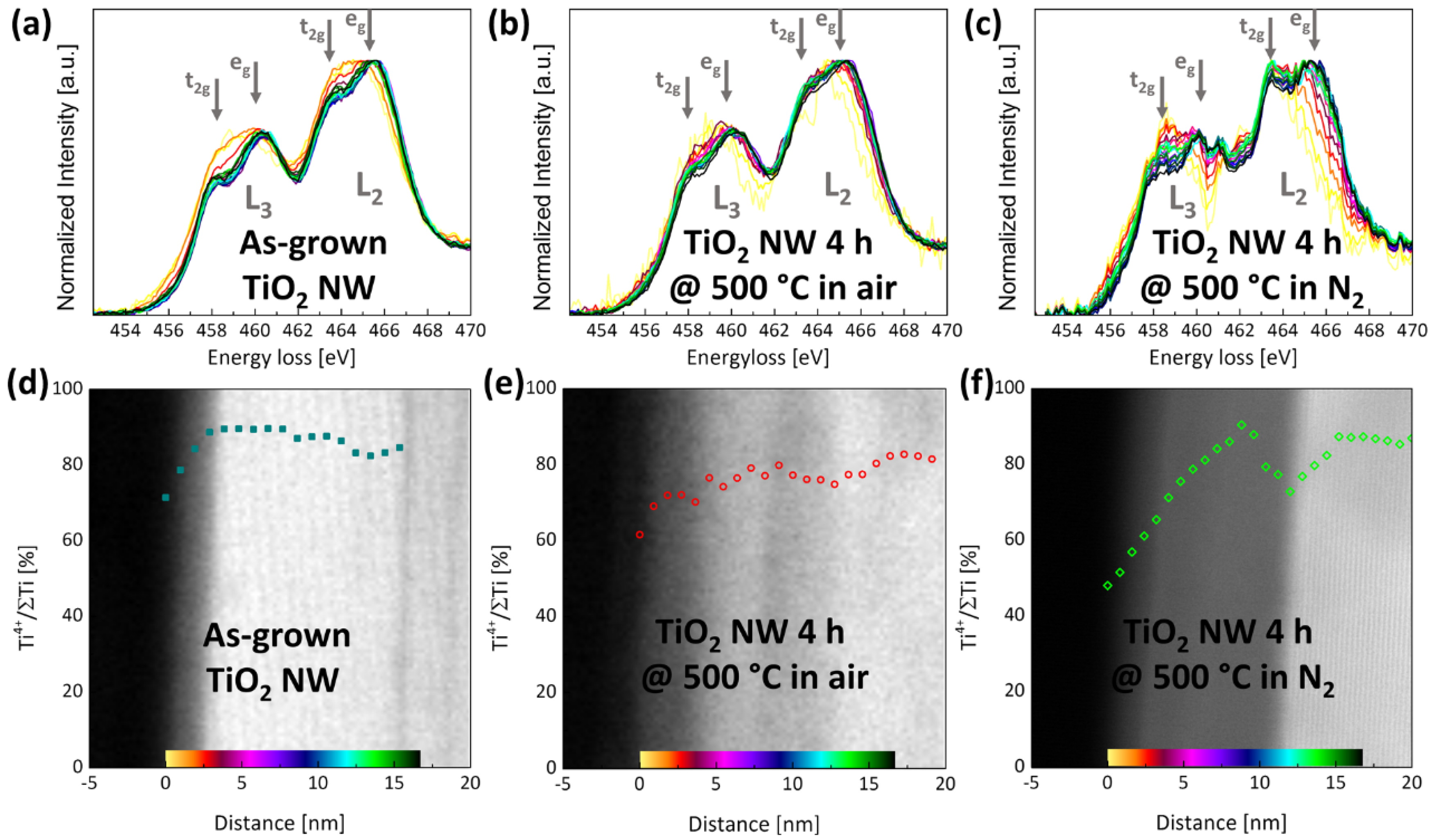
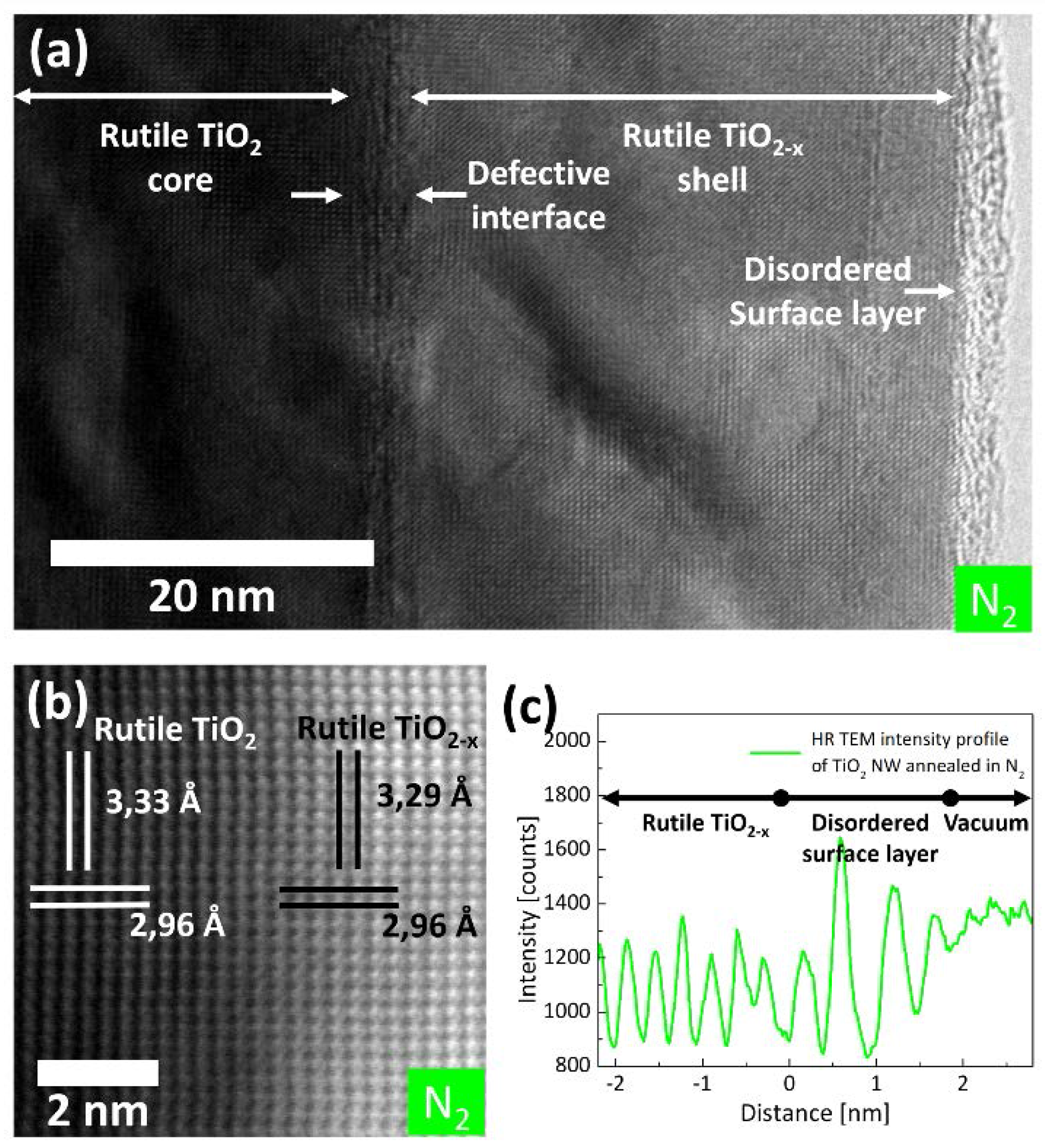
2.1. (Internal) Nanostructure and Local Chemical Environment
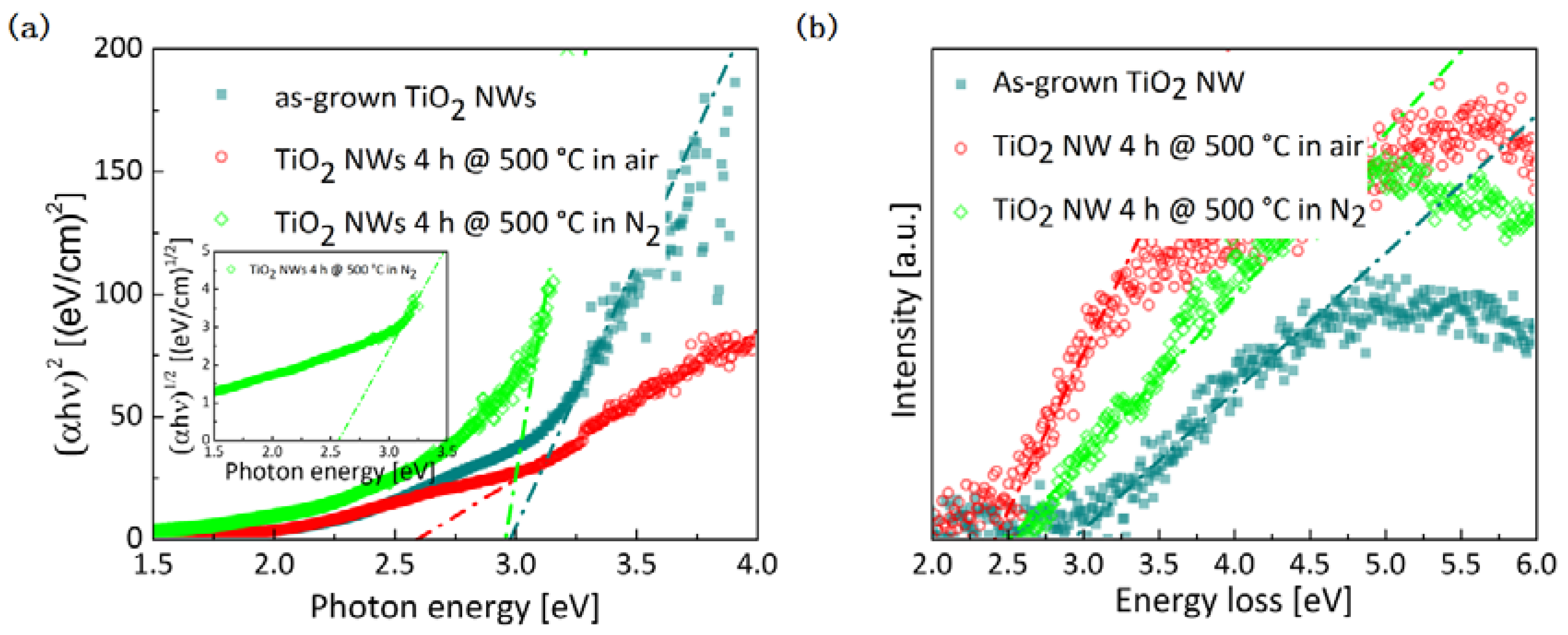
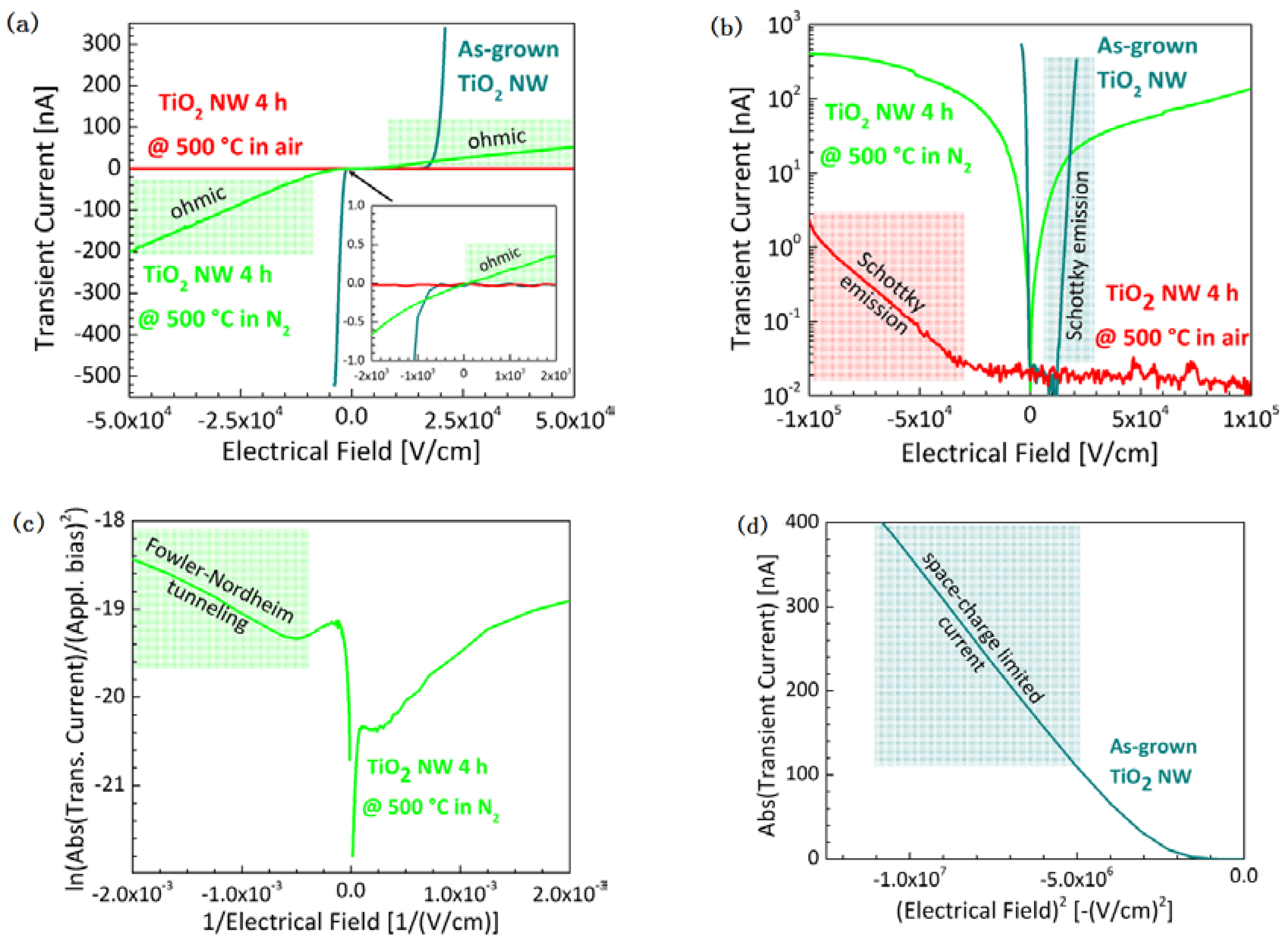
2.2. Optical and Electrical Properties
3. Discussion
4. Materials and Methods
4.1. Synthesis Procedure
4.2. Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weng, Z.; Guo, H.; Liu, X.; Wu, S.; Yeung, K.W.K.; Chu, P.K. Nanostructured TiO2 for energy conversion and storage. RSC Adv. 2013, 3, 24758. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Yukio, K.; Ryozo, A.; Kichinosuke, Y. Electrical properties of rutile (TiO2) thin film. Jpn. J. Appl. Phys. 1971, 10, 976. [Google Scholar]
- Tang, H.; Prasad, K.; Sanjinès, R.; Schmid, P.E.; Lévy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Liu, B.; Nakata, K.; Liu, S.; Sakai, M.; Ochiai, T.; Murakami, T.; Takagi, K.; Fujishima, A. Theoretical kinetic analysis of heterogeneous photocatalysis by TiO2 nanotube arrays: The effects of nanotube geometry on photocatalytic activity. J. Phys. Chem. C 2012, 116, 7471–7479. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.M.; Liu, C.; Andrews, S.C.; Hahn, C.; Yang, P. Large-scale synthesis of transition-metal-doped TiO2 nanowires with controllable overpotential. J. Am. Chem. Soc. 2013, 135, 9995–9998. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (n-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Lin, T.; Yang, C.; Wang, Z.; Yin, H.; Lü, X.; Huang, F.; Lin, J.; Xie, X.; Jiang, M. Effective nonmetal incorporation in black titania with enhanced solar energy utilization. Energy Environ. Sci. 2014, 7, 967. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Meng, X.; Wang, J.; Wang, S.; Lou, Z.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. Metallic zinc-assisted synthesis of Ti3+ self-doped TiO2 with tunable phase composition and visible-light photocatalytic activity. Chem. Commun. 2013, 49, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bi, Y. In situ preparation of oxygen-deficient TiO2 microspheres with modified {001} facets for enhanced photocatalytic activity. RSC Adv. 2017, 7, 9902–9907. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P. A ‘one pot’ gel combustion strategy towards Ti3+ self-doped ‘black’ anatase TiO2−x solar photocatalyst. J. Mater. Chem. A 2016, 4, 5854–5858. [Google Scholar] [CrossRef]
- Tian, M.; Mahjouri-Samani, M.; Eres, G.; Sachan, R.; Yoon, M.; Chisholm, M.F.; Wang, K.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; et al. Structure and formation mechanism of black TiO2 nanoparticles. ACS Nano 2015, 9, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Liu, Z.; Marcus, M.A.; Wang, W.C.; Oyler, N.A.; Grass, M.E.; Mao, B.; Glans, P.A.; Yu, P.Y.; et al. Properties of disorder-engineered black titanium dioxide nanoparticles through hydrogenation. Sci. Rep. 2013, 3, 1510. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Schneider, C.; Freitag, D.; Hartmann, M.; Venkatesan, U.; Muller, J.; Spiecker, E.; Schmuki, P. Black TiO2 nanotubes: Cocatalyst-free open-circuit hydrogen generation. Nano Lett. 2014, 14, 3309–3313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Lepcha, A.; Maccato, C.; Mettenbörger, A.; Andreu, T.; Mayrhofer, L.; Walter, M.; Olthof, S.; Ruoko, T.P.; Klein, A.; Moseler, M.; et al. Electrospun black titania nanofibers: Influence of hydrogen plasma-induced disorder on the electronic structure and photoelectrochemical performance. J. Phys. Chem. C 2015, 119, 18835–18842. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, H.; Zhao, H.; Lv, Y.; Zhou, L.J.; Song, Y.; Sun, Z. Reduced TiO2 rutile nanorods with well-defined facets and their visible-light photocatalytic activity. Chem. Commun. 2014, 50, 2755–2757. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.-P.; Yu, J.-S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Environ. Sci. 2015, 8, 3539–3544. [Google Scholar] [CrossRef]
- Das, T.K.; Ilaiyaraja, P.; Mocherla, P.S.V.; Bhalerao, G.M.; Sudakar, C. Influence of surface disorder, oxygen defects and bandgap in TiO2 nanostructures on the photovoltaic properties of dye sensitized solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 194–209. [Google Scholar] [CrossRef]
- Shah, M.W.; Zhu, Y.; Fan, X.; Zhao, J.; Li, Y.; Asim, S.; Wang, C. Facile synthesis of defective TiO2−x nanocrystals with high surface area and tailoring bandgap for visible-light photocatalysis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Sheppard, L.R.; Bak, T.; Nowotny, J. Defect chemistry of titanium dioxide. Application of defect engineering in processing of TiO2-based photocatalysts. J. Phys. Chem. C 2008, 112, 5275–5300. [Google Scholar] [CrossRef]
- Bak, T.; Bogdanoff, P.; Fiechter, S.; Nowotny, J. Defect engineering of titanium dioxide: Full defect disorder. Adv. Appl. Ceram. 2013, 111, 62–71. [Google Scholar] [CrossRef]
- Nowotny, J.; Bak, T.; Burg, T. Electrical properties of polycrystalline TiO2 at elevated temperatures. Electrical conductivity. Phys. Status Solidi B 2007, 244, 2037–2054. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Z.; Yan, K.; Bai, Y.; Yang, S. Unveiling two electron-transport modes in oxygen-deficient TiO2 nanowires and their influence on photoelectrochemical operation. J. Phys. Chem. Lett. 2014, 5, 2890–2896. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, A.; Luo, Y.; Lu, P.; Dai, Y.; Enriquez, E.; Dowden, P.; Xu, H.; Kotula, P.G.; Azad, A.K.; et al. Conducting interface in oxide homojunction: Understanding of superior properties in black TiO2. Nano Lett. 2016, 16, 5751–5755. [Google Scholar] [CrossRef] [PubMed]
- Wisnet, A.; Betzler, S.B.; Zucker, R.V.; Dorman, J.A.; Wagatha, P.; Matich, S.; Okunishi, E.; Schmidt-Mende, L.; Scheu, C. Model for hydrothermal growth of rutile wires and the associated development of defect structures. Cryst. Growth Des. 2014, 14, 4658–4663. [Google Scholar] [CrossRef]
- Wisnet, A.; Bader, K.; Betzler, S.B.; Handloser, M.; Ehrenreich, P.; Pfadler, T.; Weickert, J.; Hartschuh, A.; Schmidt-Mende, L.; Scheu, C.; et al. Defeating loss mechanisms in 1D TiO2-based hybrid solar cells. Adv. Funct. Mater. 2015, 25, 2601–2608. [Google Scholar] [CrossRef]
- Folger, A.; Ebbinghaus, P.; Erbe, A.; Scheu, C. Role of vacancy condensation in the formation of voids in rutile TiO2 nanowires. ACS Appl. Mater. Interfaces 2017, 9, 13471–13479. [Google Scholar] [CrossRef] [PubMed]
- Brydson, R.; Sauer, H.; Engel, W.; Thomass, J.M.; Zeitler, E.; Kosugi, N.; Kuroda, H. Electron energy loss and X-ray absorption spectroscopy of rutile and anatase: A test of structural sensitivity. J. Phys. Condens. Matter 1989, 1, 797–812. [Google Scholar] [CrossRef]
- Stoyanov, E.; Langenhorst, F.; Steinle-Neumann, G. The effect of valence state and site geometry on Ti L3,2 and O K electron energy-loss spectra of TixOy phases. Am. Mineral. 2007, 92, 577–586. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths (IMFPs). IV. Evaluation of calculated IMFPs and of the predictive IMFP formula TPP-2 for electron energies between 50 and 2000 eV. Surf. Interface Anal. 1993, 20, 77–89. [Google Scholar] [CrossRef]
- Egerton, R.F.; Cheng, S.C. Measurement of local thickness by electron energy-loss spectroscopy. Ultramicroscopy 1987, 21, 231–244. [Google Scholar] [CrossRef]
- Oh, S.H.; Kauffmann, Y.; Scheu, C.; Kaplan, W.D.; Ruhle, M. Ordered liquid aluminum at the interface with sapphire. Science 2005, 310, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.D.; Redfield, D. Toward a unified theory of urbach's rule and exponential absorption edges. Phys. Rev. B 1972, 5, 594–610. [Google Scholar] [CrossRef]
- Chiu, F.-C. A review on conduction mechanisms in dielectric films. Adv. Mater. Sci. Eng. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Choudhury, A. Oxygen defect dependent variation of band gap, urbach energy and luminescence property of anatase, anatase—Rutile mixed phase and of rutile phases of TiO2 nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2014, 56, 364–371. [Google Scholar] [CrossRef]
- Kofstad, P. Nonstoichiometry, diffusion, and electrical conductivity in binary metal oxides. Mater. Corros. Werkst. Korros. 1974, 25, 801–802. [Google Scholar]
- Soerensen, O.T. Nonstoichiometric Oxides; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Nowotny, M.K.; Bak, T.; Nowotny, J. Electrical properties and defect chemistry of TiO2 single crystal. I. Electrical conductivity. J. Phys. Chem. B 2006, 110, 16270–16282. [Google Scholar] [CrossRef] [PubMed]
- Austin, I.G.; Mott, N.F. Metallic and nonmetallic behavior in transition metal oxides. Science 1970, 168, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Klusek, Z.; Pierzgalski, S.; Datta, S. Insulator–metal transition on heavily reduced TiO2 (110) surface studied by high temperature-scanning tunnelling spectroscopy (HT-STS). Appl. Surf. Sci. 2004, 221, 120–128. [Google Scholar] [CrossRef]
- Lei, Y.; Leng, Y.; Yang, P.; Wan, G.; Huang, N. Theoretical calculation and experimental study of influence of oxygen vacancy on the electronic structure and hemocompatibility of rutile TiO2. Sci. China Ser. E Technol. Sci. 2009, 52, 2742–2748. [Google Scholar] [CrossRef]
- Bhatt, P.J.; Tomar, L.J.; Desai, R.K.; Chakrabarty, B.S. Pure single crystallographic form of TiO2 nanoparticles: Preparation and characterization. AIP Conf. Proc. 2015, 1667. [Google Scholar] [CrossRef]
- Axelevitch, A.; Gorenstein, B.; Golan, G. Investigation of optical transmission in thin metal films. Phys. Procedia 2012, 32, 1–13. [Google Scholar] [CrossRef]
- Koslowski, U.; Ellmer, K.; Bogdanoff, P.; Dittrich, T.; Guminskaya, T.; Tributsch, H. Structural, electrical, optical, and photoelectrochemical properties of thin titanium oxinitride films (TiO2–2xNx with 0 ≤ x ≤ 1). J. Vac. Sci. Technol. A Vac. Surf. Films 2006, 24, 2199–2205. [Google Scholar] [CrossRef]
- Kwon, D.H.; Kim, K.M.; Jang, J.H.; Jeon, J.M.; Lee, M.H.; Kim, G.H.; Li, X.S.; Park, G.S.; Lee, B.; Han, S.; et al. Atomic structure of conducting nanofilaments in TiO2 resistive switching memory. Nat. Nanotechnol. 2010, 5, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Jeong, D.S.; Hwang, C.S. Nanofilamentary resistive switching in binary oxide system: A review on the present status and outlook. Nanotechnology 2011, 22, 254002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gan, X.; Li, X.; Wu, L.; Gao, X.; Zheng, R.; He, Y.; Liu, X.; Yang, R. Realization of rectifying and resistive switching behaviors of TiO2 nanorod arrays for nonvolatile memory. Electrochem. Solid State Lett. 2011, 14, H422. [Google Scholar] [CrossRef]
- Park, J.; Heo, S.; Chung, J.; Kim, H.; Park, G.S. Bandgap measurement of dielectric thin films by using monochromated STEM-EELS. Microsc. Microanal. 2007, 13, 1306–1307. [Google Scholar] [CrossRef]
- Scott, J.; Thomas, P.J.; Mackenzie, M.; McFadzean, S.; Wilbrink, J.; Craven, A.J.; Nicholson, W.A. Near-simultaneous dual energy range EELS spectrum imaging. Ultramicroscopy 2008, 108, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Egerton, R.F. Electron energy-loss spectroscopy in the TEM. Rep. Prog. Phys. 2009, 72, 16502–16525. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folger, A.; Kalb, J.; Schmidt-Mende, L.; Scheu, C. Tuning the Electronic Conductivity in Hydrothermally Grown Rutile TiO2 Nanowires: Effect of Heat Treatment in Different Environments. Nanomaterials 2017, 7, 289. https://doi.org/10.3390/nano7100289
Folger A, Kalb J, Schmidt-Mende L, Scheu C. Tuning the Electronic Conductivity in Hydrothermally Grown Rutile TiO2 Nanowires: Effect of Heat Treatment in Different Environments. Nanomaterials. 2017; 7(10):289. https://doi.org/10.3390/nano7100289
Chicago/Turabian StyleFolger, Alena, Julian Kalb, Lukas Schmidt-Mende, and Christina Scheu. 2017. "Tuning the Electronic Conductivity in Hydrothermally Grown Rutile TiO2 Nanowires: Effect of Heat Treatment in Different Environments" Nanomaterials 7, no. 10: 289. https://doi.org/10.3390/nano7100289