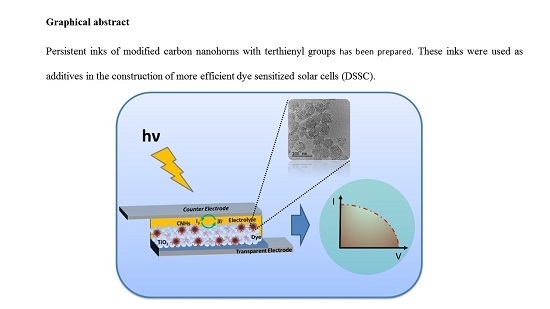
Carbon Nanohorns Modified with Conjugated Terthienyl/Terthiophene Structures: Additives to Enhance the Performance of Dye-Sensitized Solar Cells
Abstract
:1. Introduction
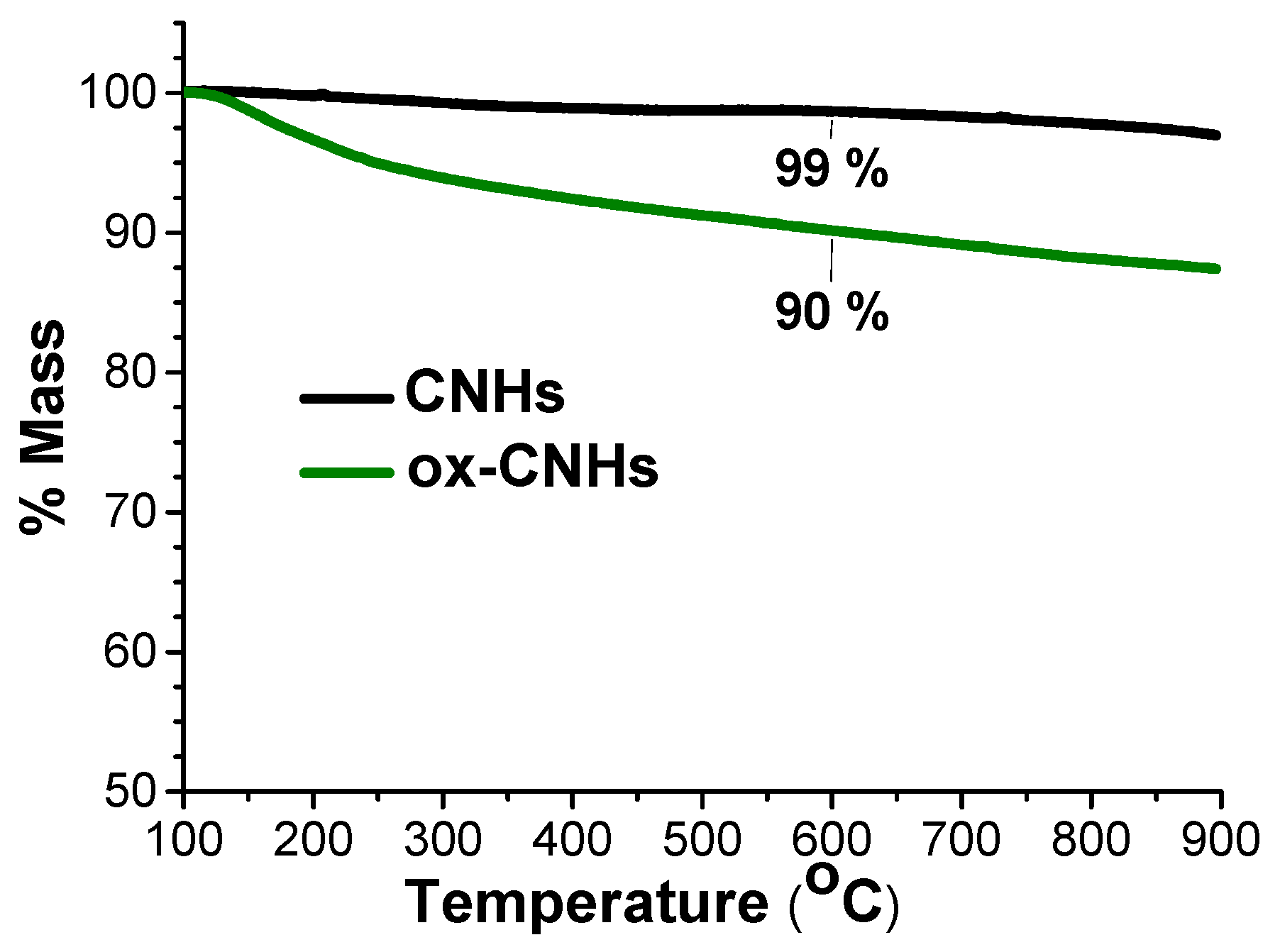
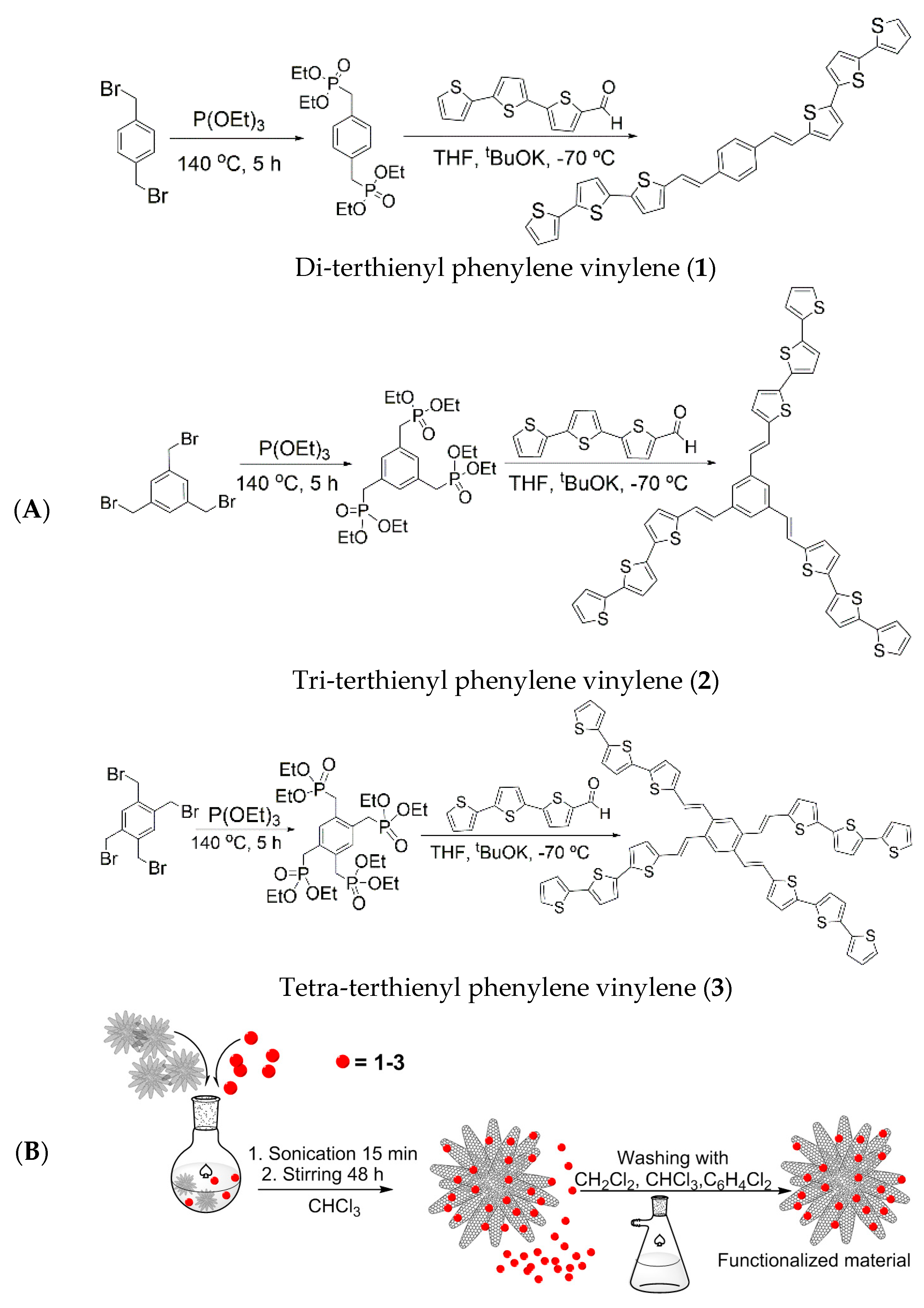
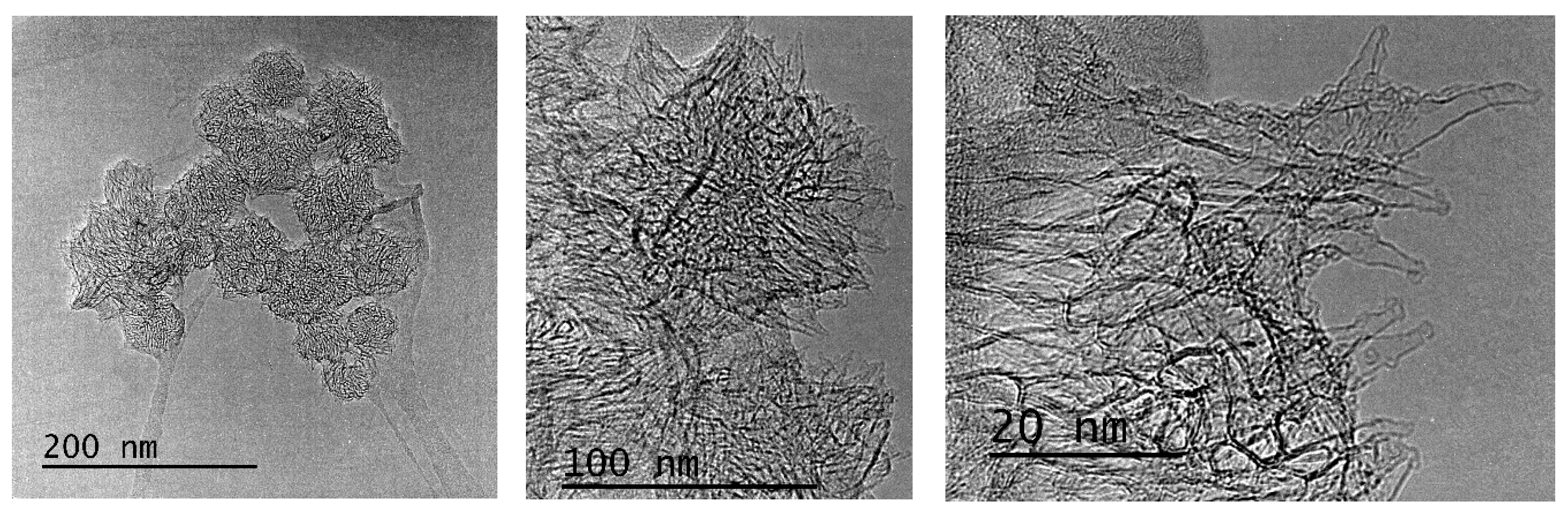
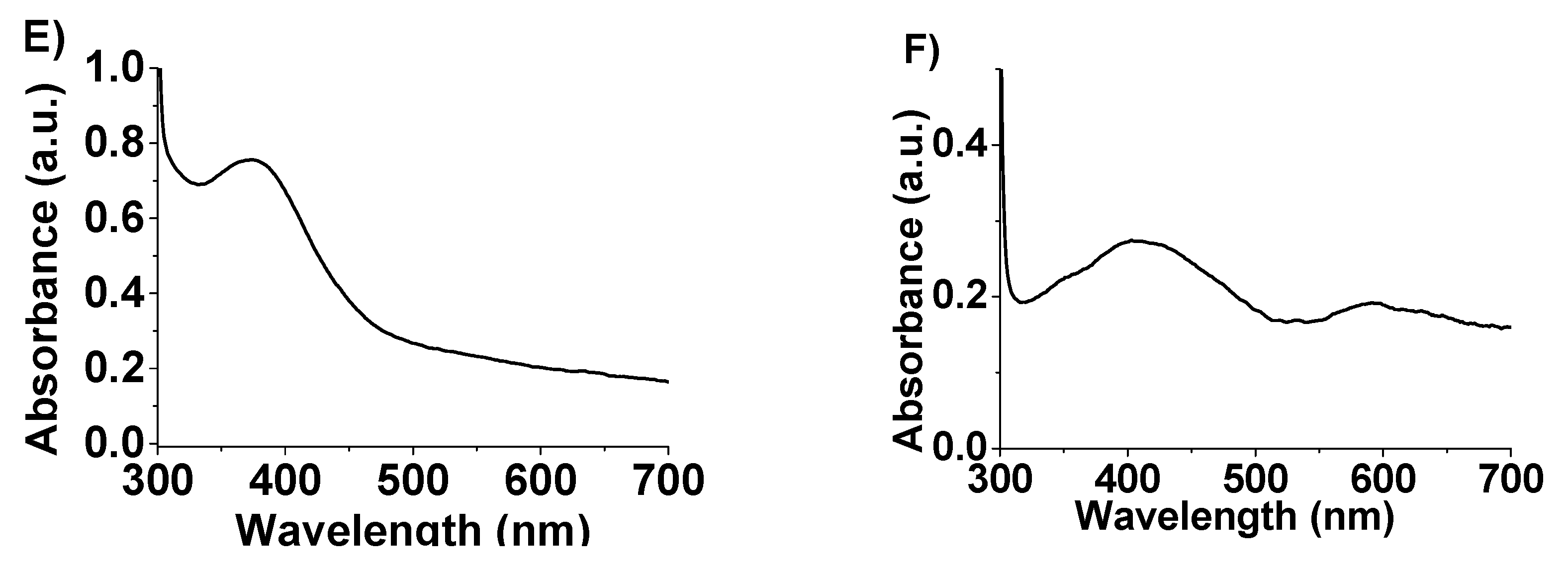
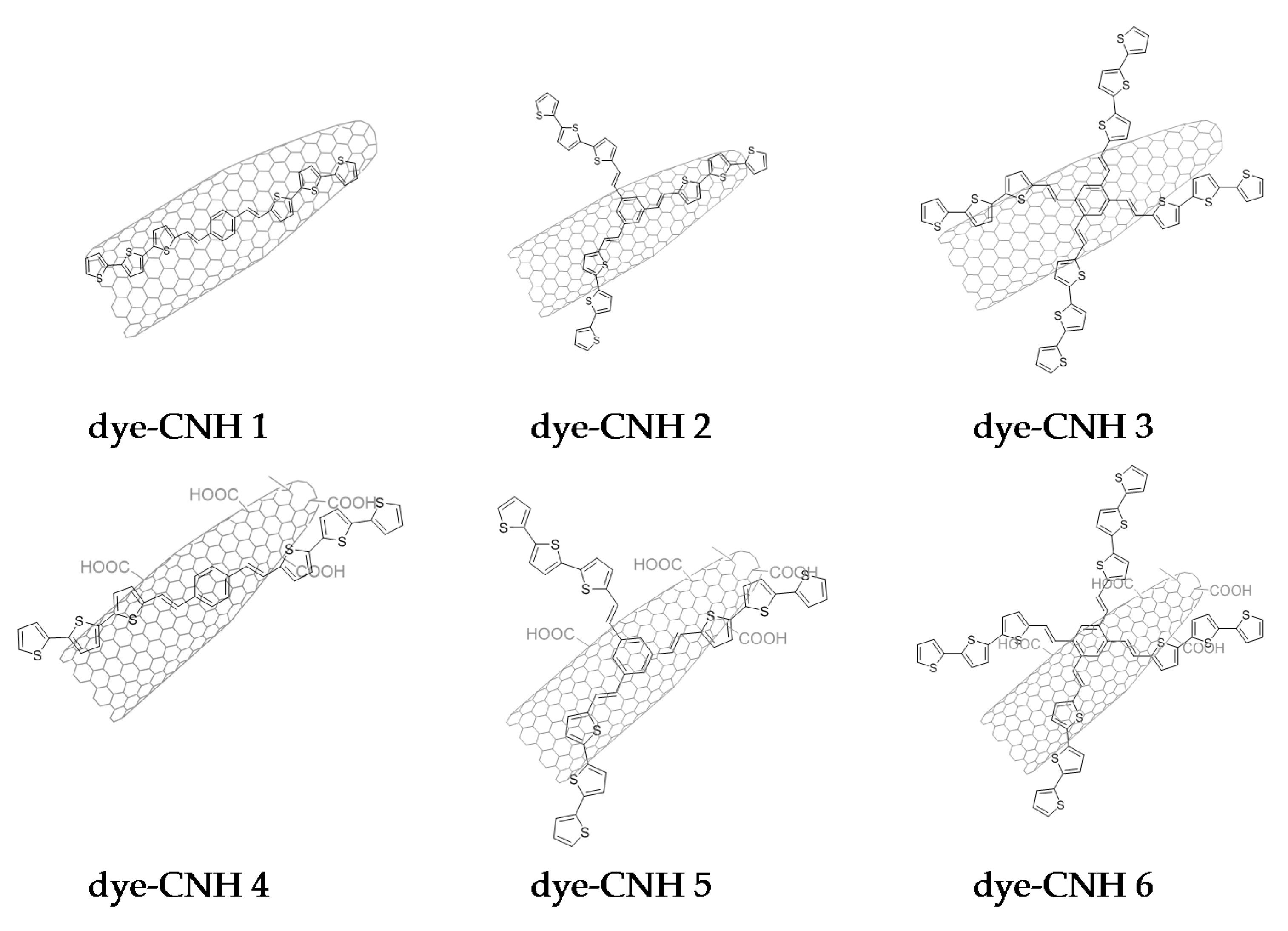
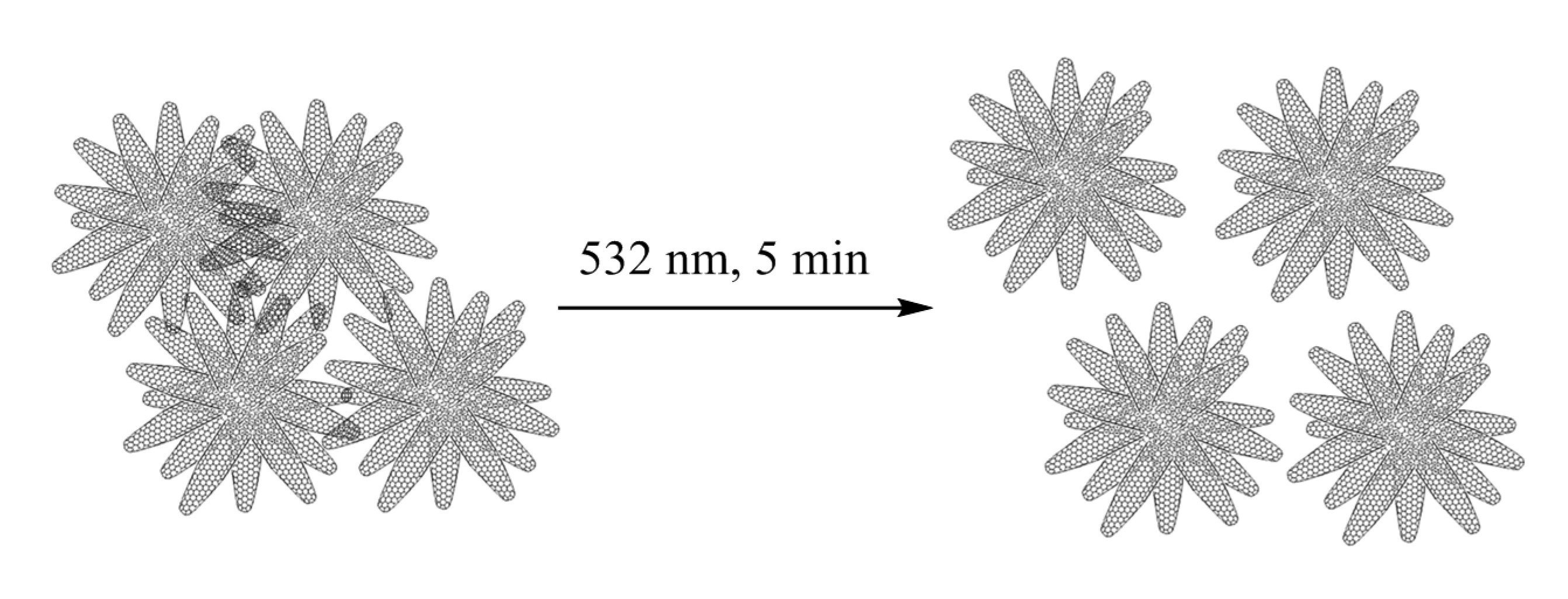
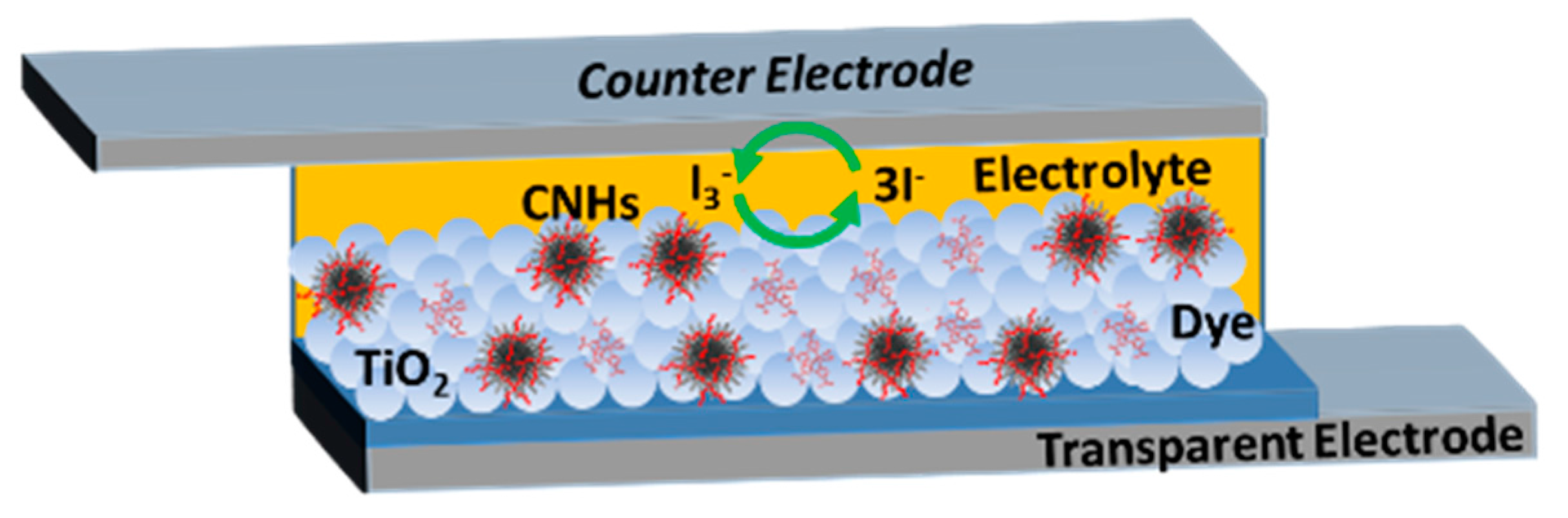
2. Results and Discussion
3. Materials and Methods
3.1. Electron Microscopy Characterization
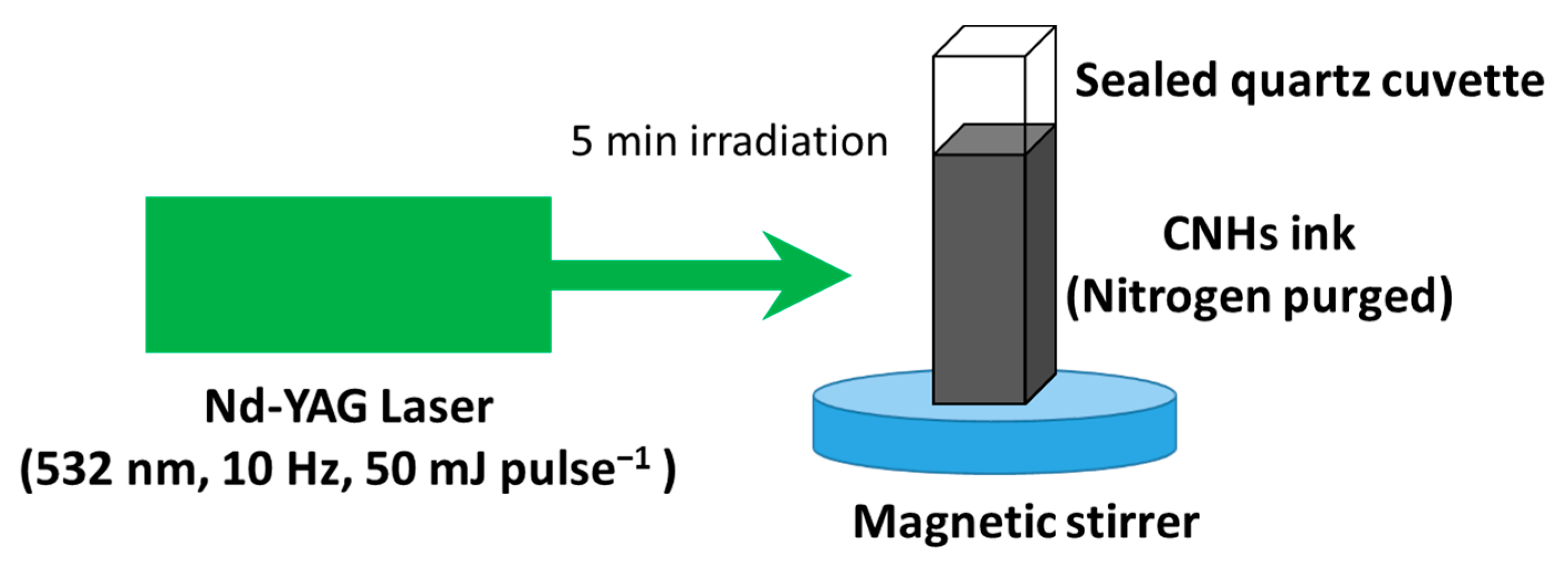
3.2. Preparation of the CNHs Samples
3.3. Preparation of the CNHs Inks
3.4. Preparation of the Photovoltaic Devices
3.5. Photovoltaic Response Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Albero, J.; Atienzar, P.; Corma, A.; Garcia, H. Efficiency records in mesoscopic dye-sensitized solar cells. Chem. Rec. 2015, 15, 803–828. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.S.; Chang, J.A.; Lee, Y.H.; Kim, H.J.; Sarkar, A.; NazeeruddinMd, K.; et al. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; CurchodBasile, F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, L.J.; Byrne, M.T.; Bari, M.; Gun’ko, Y.K. Carbon nanomaterials for dye-sensitized solar cell applications: A bright future. Adv. Energy Mater. 2011, 1, 472–485. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaguchi, M.; Kumagai, M.; Yanagida, S. Application of carbon nanotubes to counter electrodes of dye-sensitized solar cells. Chem. Lett. 2003, 32, 28–29. [Google Scholar] [CrossRef]
- Casillas, R.; Lodermeyer, F.; Costa, R.D.; Prato, M.; Guldi, D.M. Substituting TiCl4-Carbon nanohorn interfaces for dye-sensitized solar cells. Adv. Energy Mater. 2014, 4, 1301577. [Google Scholar] [CrossRef]
- Costa, R.D.; Feihl, S.; Kahnt, A.; Gambhir, S.; Officer, D.L.; Wallace, G.G.; Lucio, M.I.; Herrero, M.A.; Vázquez, E.; Syrgiannis, Z.; et al. Carbon nanohorns as integrative materials for efficient dye-sensitized solar cells. Adv. Mater. 2013, 25, 6513–6518. [Google Scholar] [CrossRef] [PubMed]
- Batmunkh, M.; Biggs, M.J.; Shapter, J.G. Carbon nanotubes for dye-sensitized solar cells. Small 2015, 11, 2963–2989. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Luo, Y.H.; Lv, W.; Yu, W.J.; Wu, S.D.; Hou, P.X.; Yang, Q.H.; Meng, Q.B.; Liu, C.; Cheng, H.M. Vertically aligned carbon nanotubes grown on graphene paper as electrodes in lithium-ion batteries and dye-sensitized solar cells. Adv. Energy Mater. 2011, 1, 486–490. [Google Scholar] [CrossRef]
- Nam, J.G.; Park, Y.J.; Kim, B.S.; Lee, J.S. Enhancement of the efficiency of dye-sensitized solar cell by utilizing carbon nanotube counter electrode. Scr. Mater. 2010, 62, 148–150. [Google Scholar] [CrossRef]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Wang, H.; Chhowalla, M.; Sano, N.; Jia, S.; Amaratunga, G.A.J. Large-scale synthesis of single-walled carbon nanohorns by submerged arc. Nanotechnology 2004, 15, 546. [Google Scholar] [CrossRef]
- Guldi, D.M.; Martín, N. Carbon Nanotubes and Related Structures: Synthesis, Characterization, Functionalization, and Applications; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Nelson, J. Polymer: Fullerene bulk heterojunction solar cells. Mater. Today 2011, 14, 462–470. [Google Scholar] [CrossRef]
- Peters, C.H.; Sachs-Quintana, I.T.; Kastrop, J.P.; Beaupré, S.; Leclerc, M.; McGehee, M.D. High efficiency polymer solar cells with long operating lifetimes. Adv. Energy Mater. 2011, 1, 491–494. [Google Scholar] [CrossRef]
- Garcia, H. Allotropic carbon nanoforms as advanced metal-free catalysts or as supports. Adv. Chem. 2014, 2014, 20. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, B.; Itkis, M.E.; Haddon, R.C. Nitric acid purification of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 13838–13842. [Google Scholar] [CrossRef]
- Iglesias, D.; Guerra, J.; Gómez, M.V.; Rodríguez, A.M.; Prieto, P.; Vázquez, E.; Herrero, M.A. Design of assembled systems based on conjugated polyphenylene derivatives and carbon nanohorns. Chem. A Eur. J. 2016, 22, 11643–11651. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Scaiano, J.C. Photochemical generation of radical cations from. alpha.-terthienyl and related thiophenes: Knetic behavior and magnetic field effects on radical-ion pairs in micellar solution. J. Am. Chem. Soc. 1990, 112, 2694–2701. [Google Scholar] [CrossRef]
- Reyftmann, J.P.; Kagan, J.; Santus, R.; Morliere, P. Excited state properties of ∝ al-terthienyl and related molecules. Photochem. Photobiol. 1985, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, F.; Janssen, R.A.J.; Meijer, E.W. Synthesis and characterization of new copolymers of thiophene and vinylene: Poly(thienylenevinylene)s and poly(terthienylenevinylene)s with thioether side chains. J. Polym. Sci. A Polym. Chem. 1999, 37, 4629–4639. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Brinkhaus, L.; Katsukis, G.; Malig, J.; Costa, R.D.; Garcia-Iglesias, M.; Vázquez, P.; Torres, T.; Guldi, D.M. Tuning the stability of graphene layers by phthalocyanine-based oPPV oligomers towards photo- and redoxactive materials. Small 2013, 9, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Englert, J.M.; Hauke, F. Wet chemical functionalization of graphene. Acc. Chem. Res. 2013, 46, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Feng, Y.Y.; Tang, S.D.; Feng, W. Preparation of a graphene oxide-phthalocyanine hybrid through strong π–π interactions. Carbon 2010, 48, 211–216. [Google Scholar] [CrossRef]
- Guldi, D.M.; Rahman, G.M.A.; Zerbetto, F.; Prato, M. Carbon nanotubes in electron donor-acceptor nanocomposites. Acc. Chem. Res. 2005, 38, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wohlgenannt, M.; Vardeny, Z.V.; Blau, W.J.; Dalton, A.B.; Baughman, R.; Zakhidov, A.A. Photoinduced charge transfer in poly(p-phenylene vinylene) derivatives and carbon nanotube/c60 composites. Phys. B Condens. Matter 2003, 338, 366–369. [Google Scholar] [CrossRef]
- Pagona, G.; Fan, J.; Maignè, A.; Yudasaka, M.; Iijima, S.; Tagmatarchis, N. Aqueous carbon nanohorn-pyrene-porphyrin nanoensembles: Controlling charge-transfer interactions. Diam. Relat. Mater. 2007, 16, 1150–1153. [Google Scholar] [CrossRef]
- Alvaro, M.; Aprile, C.; Ferrer, B.; Garcia, H. Functional molecules from single wall carbon nanotubes. Photoinduced solubility of short single wall carbon nanotube residues by covalent anchoring of 2,4,6-triarylpyrylium units. J. Am. Chem. Soc. 2007, 129, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.O.; Sobarzo, P.; Gatica, N. Electronic and structural properties of polymers based on phenylene vinylene and thiophene units. Control of the gap by gradual increases of thiophene moieties. New J. Chem. 2015, 39, 7979–7987. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Wang, L.; Li, Y.; Ren, Y.; Shum, K. Energy barrier at the N719-dye/CsSnI3 interface for photogenerated holes in dye-sensitized solar cells. Sci. Rep. 2014, 4, 6954. [Google Scholar] [CrossRef] [PubMed]
- Mauro, S. Device and Method for Production of Carbon Nanotubes, Fullerene and Their Derivatives. U.S. Patent 7,125,525, 24 October 2006. [Google Scholar]
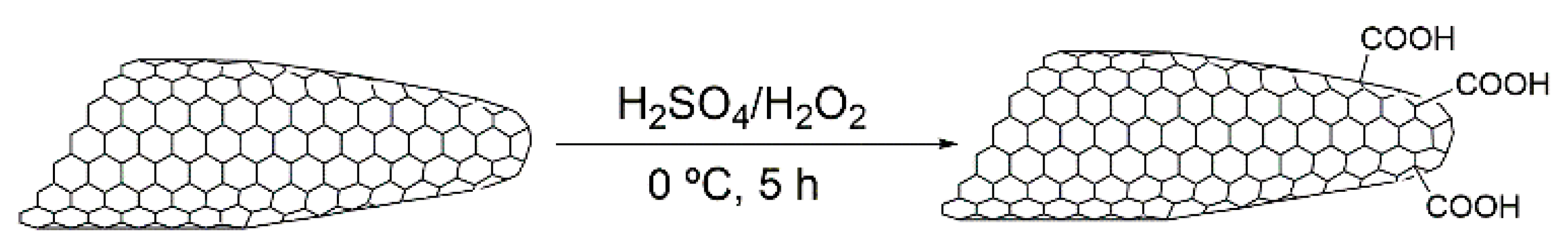


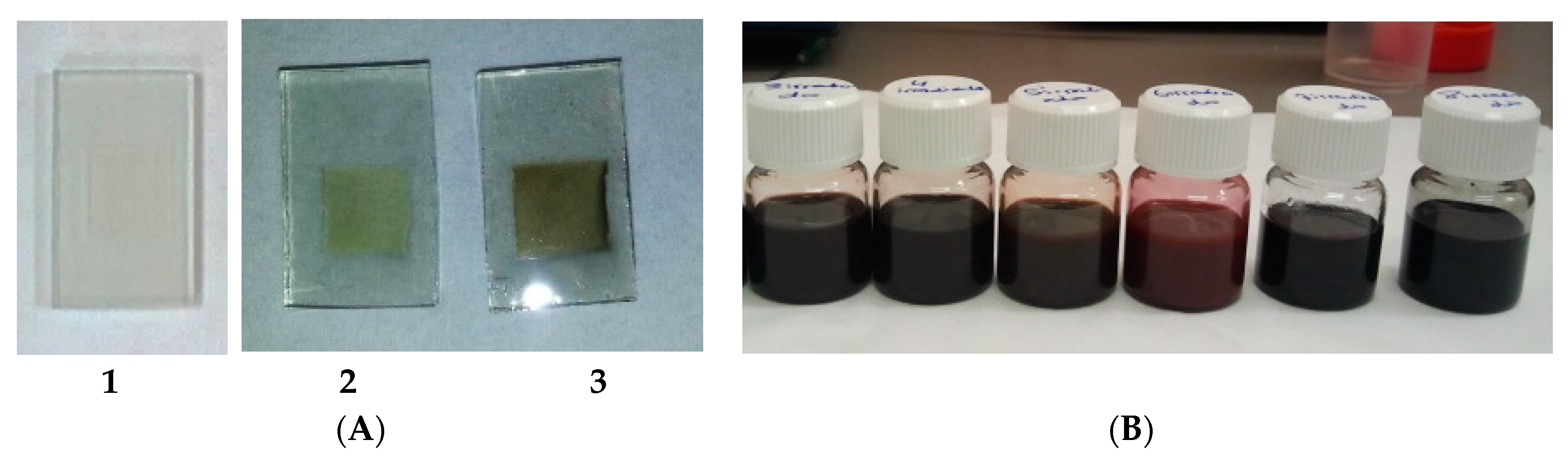
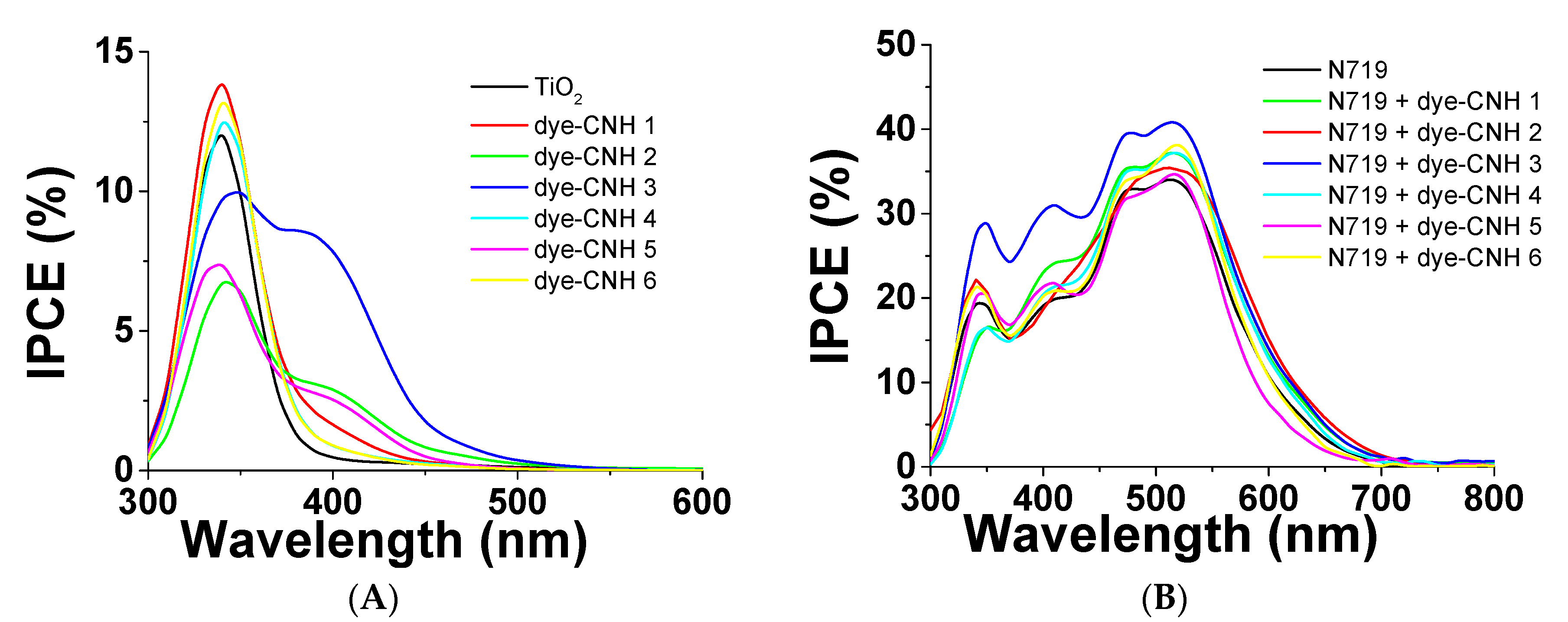

| Sample | Voc (Volt.) | Jsc (mA/cm2) | Ff | Ef (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Without | With | Without | With | Without | With | Without | With | |
| Reference | 0.314 | 0.761 | 0.208 | 7.20 | 0.444 | 0.74 | 0.03 | 4.07 |
| dye-CNH 1 | 0.286 | 0.795 | 0.274 | 7.74 | 0.384 | 0.67 | 0.03 | 4.14 |
| dye-CNH 2 | 0.220 | 0.635 | 0.157 | 8.28 | 0.323 | 0.67 | 0.02 | 4.50 |
| dye-CNH 3 | 0.486 | 0.808 | 0.533 | 10.37 | 0.476 | 0.75 | 0.12 | 6.24 |
| dye-CNH 4 | 0.431 | 0.785 | 0.205 | 8.20 | 0.527 | 0.72 | 0.05 | 4.63 |
| dye-CNH 5 | 0.472 | 0.797 | 0.226 | 7.48 | 0.624 | 0.72 | 0.07 | 4.29 |
| dye-CNH 6 | 0.431 | 0.796 | 0.204 | 7.77 | 0.532 | 0.72 | 0.05 | 4.47 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, D.; Atienzar, P.; Vázquez, E.; Herrero, M.A.; García, H. Carbon Nanohorns Modified with Conjugated Terthienyl/Terthiophene Structures: Additives to Enhance the Performance of Dye-Sensitized Solar Cells. Nanomaterials 2017, 7, 294. https://doi.org/10.3390/nano7100294
Iglesias D, Atienzar P, Vázquez E, Herrero MA, García H. Carbon Nanohorns Modified with Conjugated Terthienyl/Terthiophene Structures: Additives to Enhance the Performance of Dye-Sensitized Solar Cells. Nanomaterials. 2017; 7(10):294. https://doi.org/10.3390/nano7100294
Chicago/Turabian StyleIglesias, Daniel, Pedro Atienzar, Ester Vázquez, María Antonia Herrero, and Hermenegildo García. 2017. "Carbon Nanohorns Modified with Conjugated Terthienyl/Terthiophene Structures: Additives to Enhance the Performance of Dye-Sensitized Solar Cells" Nanomaterials 7, no. 10: 294. https://doi.org/10.3390/nano7100294