Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties
Abstract
:1. Introduction
2. Preparation of TiO2 and TiO2-Based Composites with Different Morphologies
2.1. Micro- and Nano-Spheres
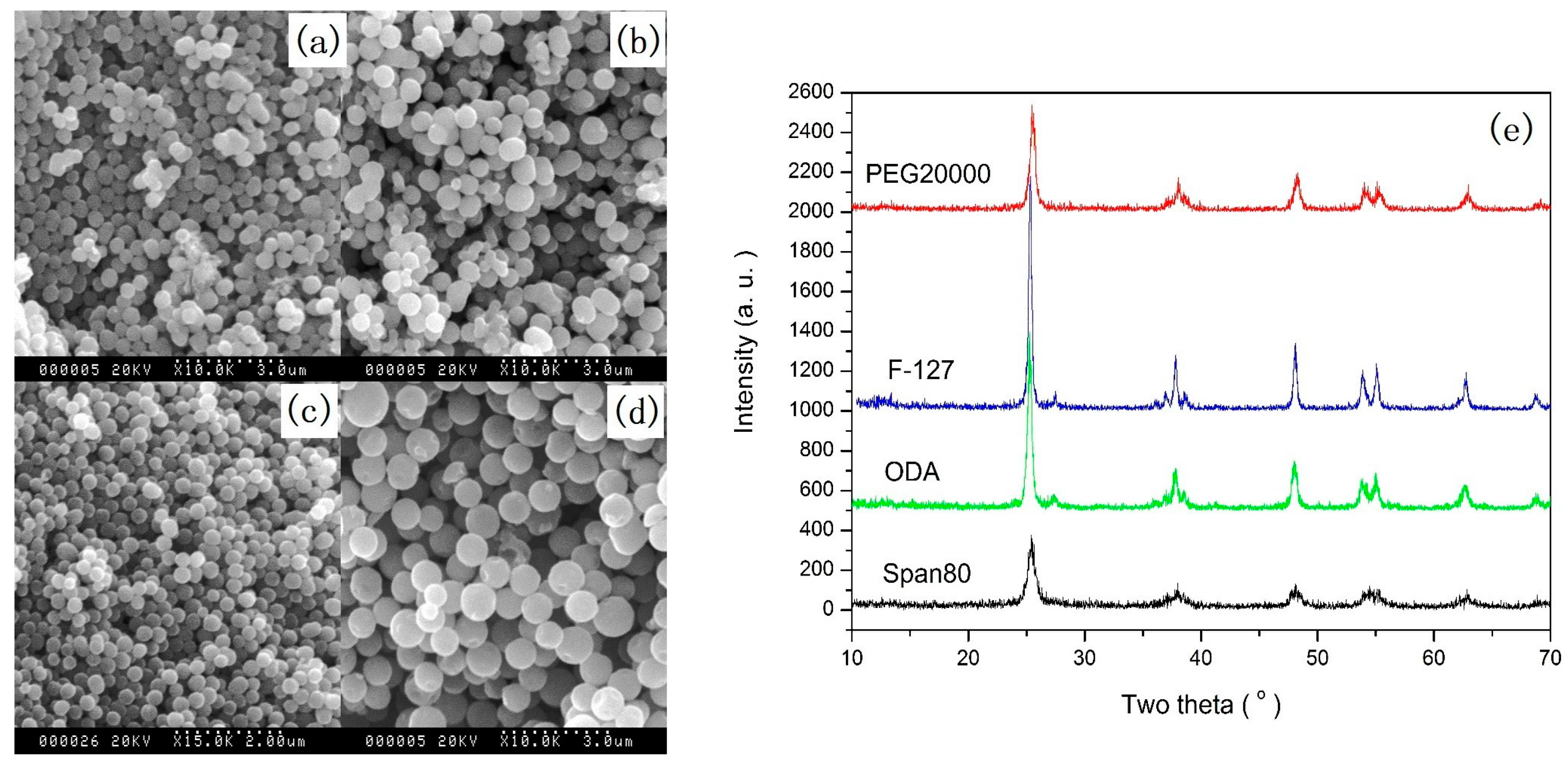
2.1.1. Solid Spheres
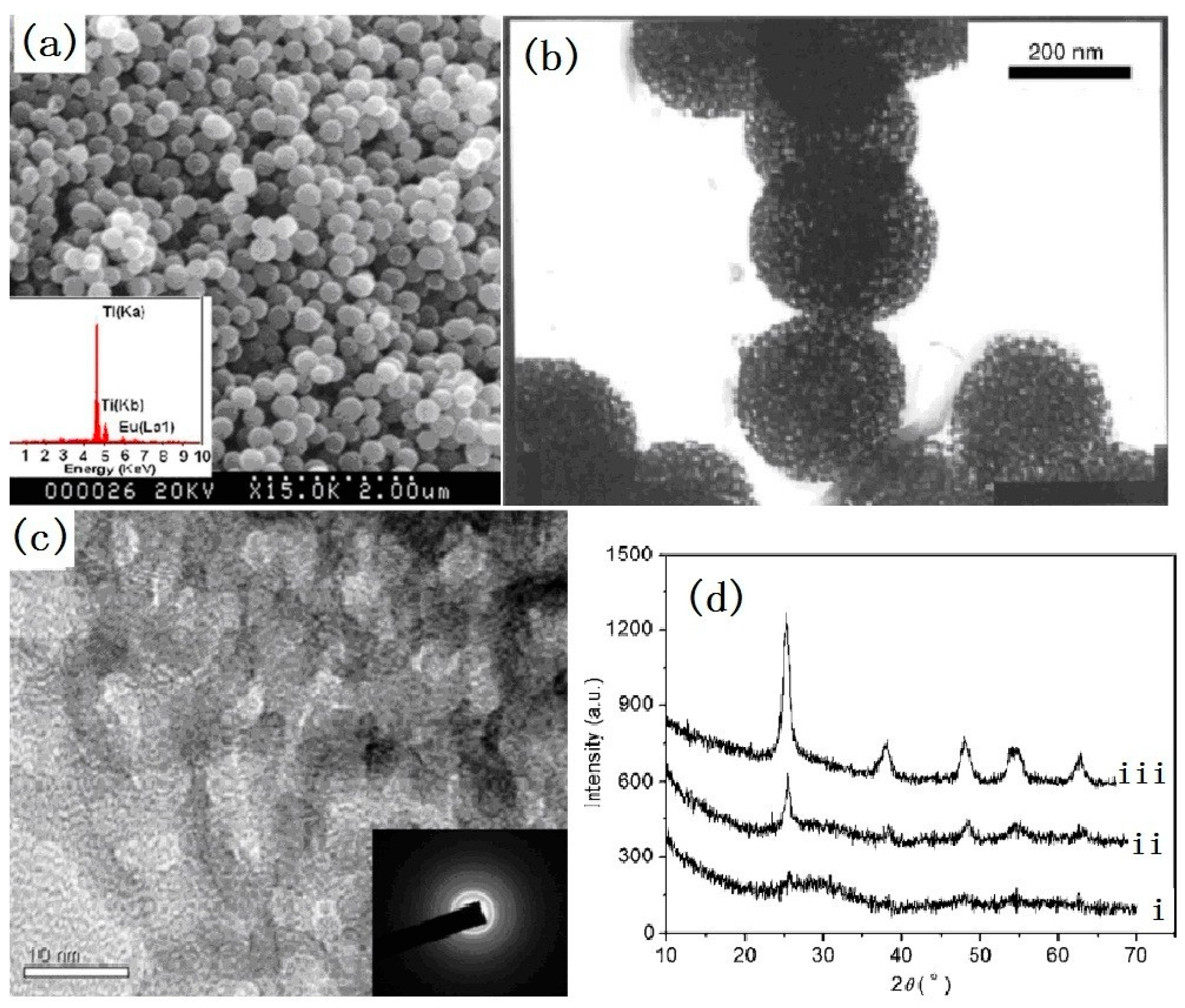
2.1.2. Hollow Spheres
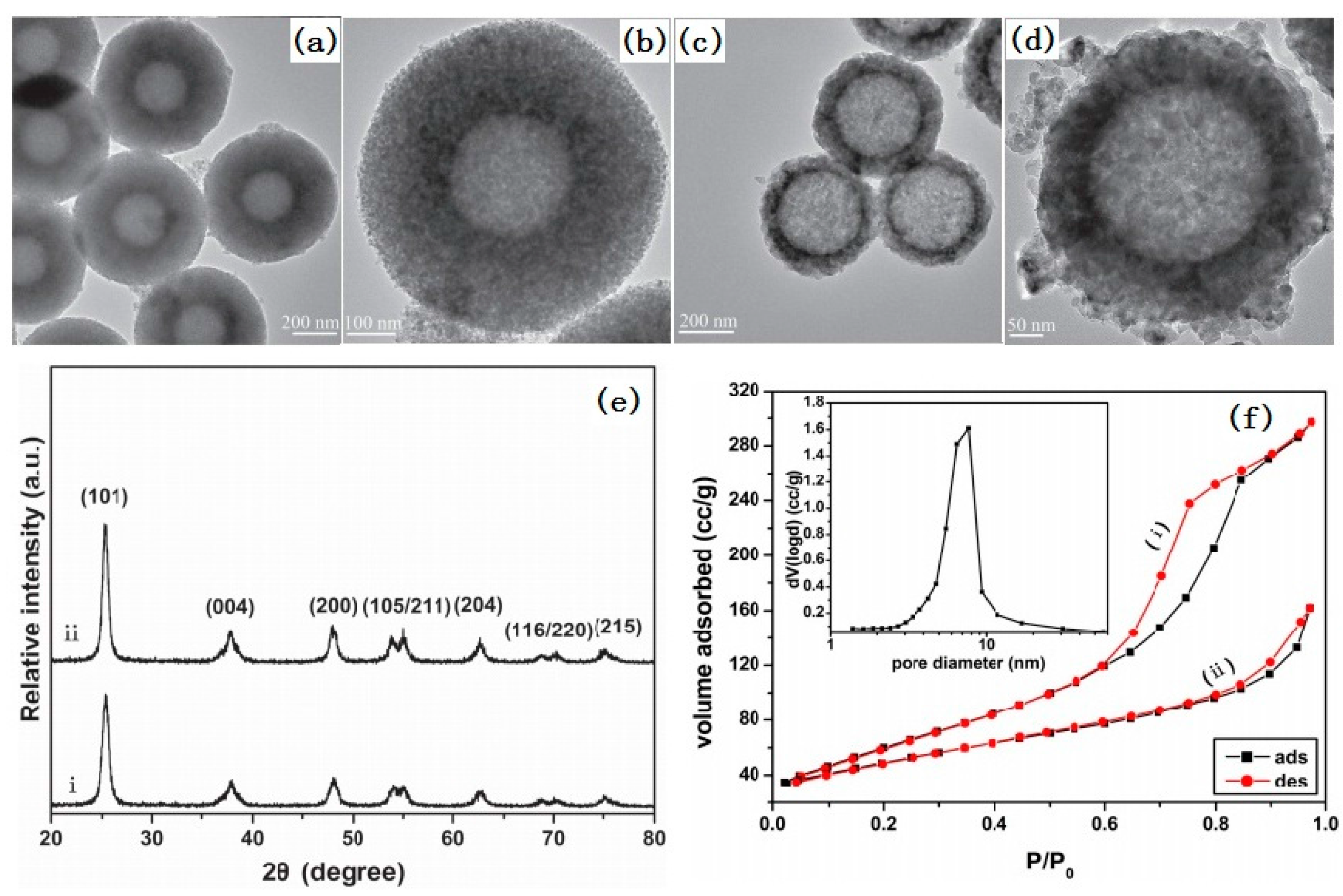
2.1.3. Porous Spheres
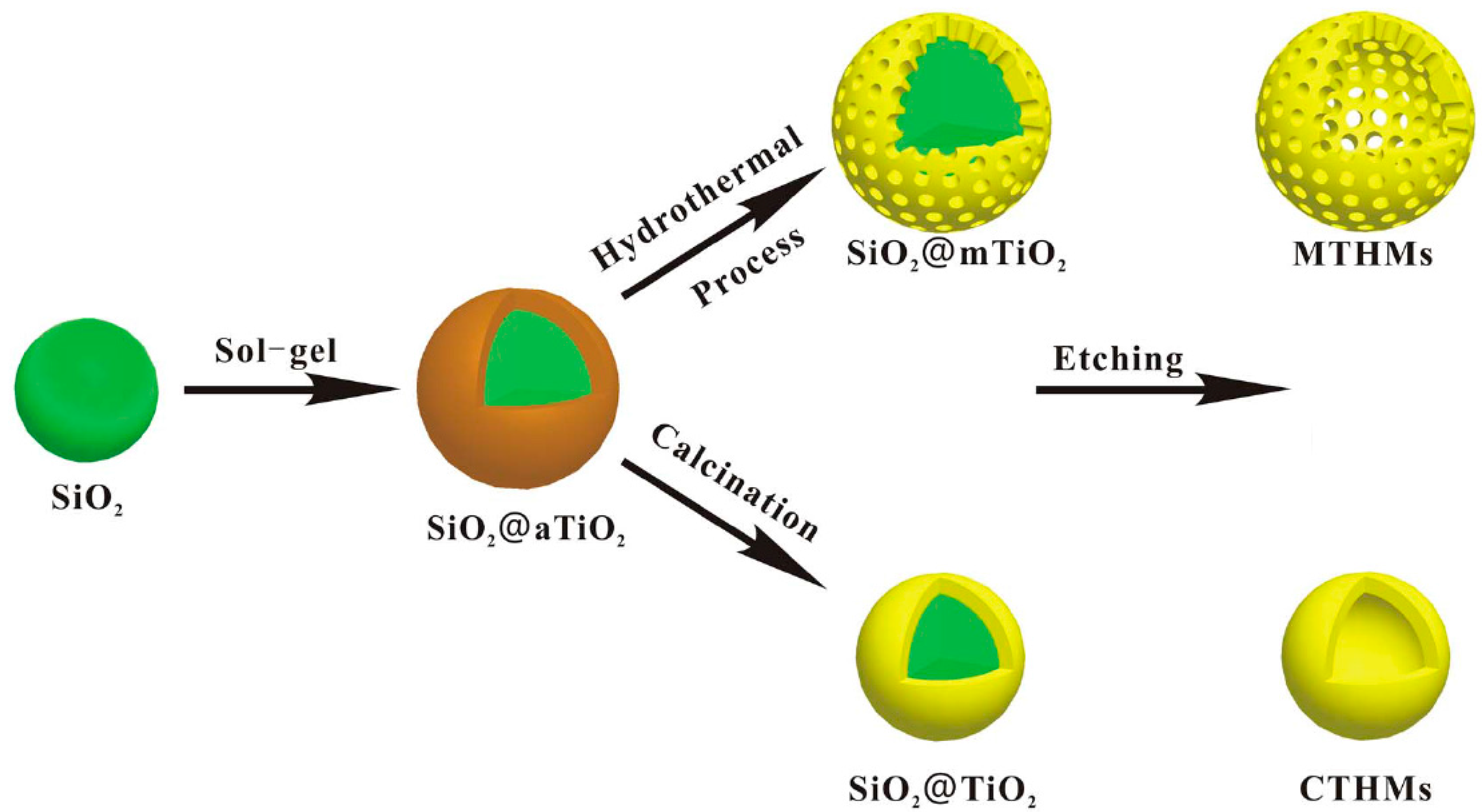
2.1.4. Hollow and Porous Spheres
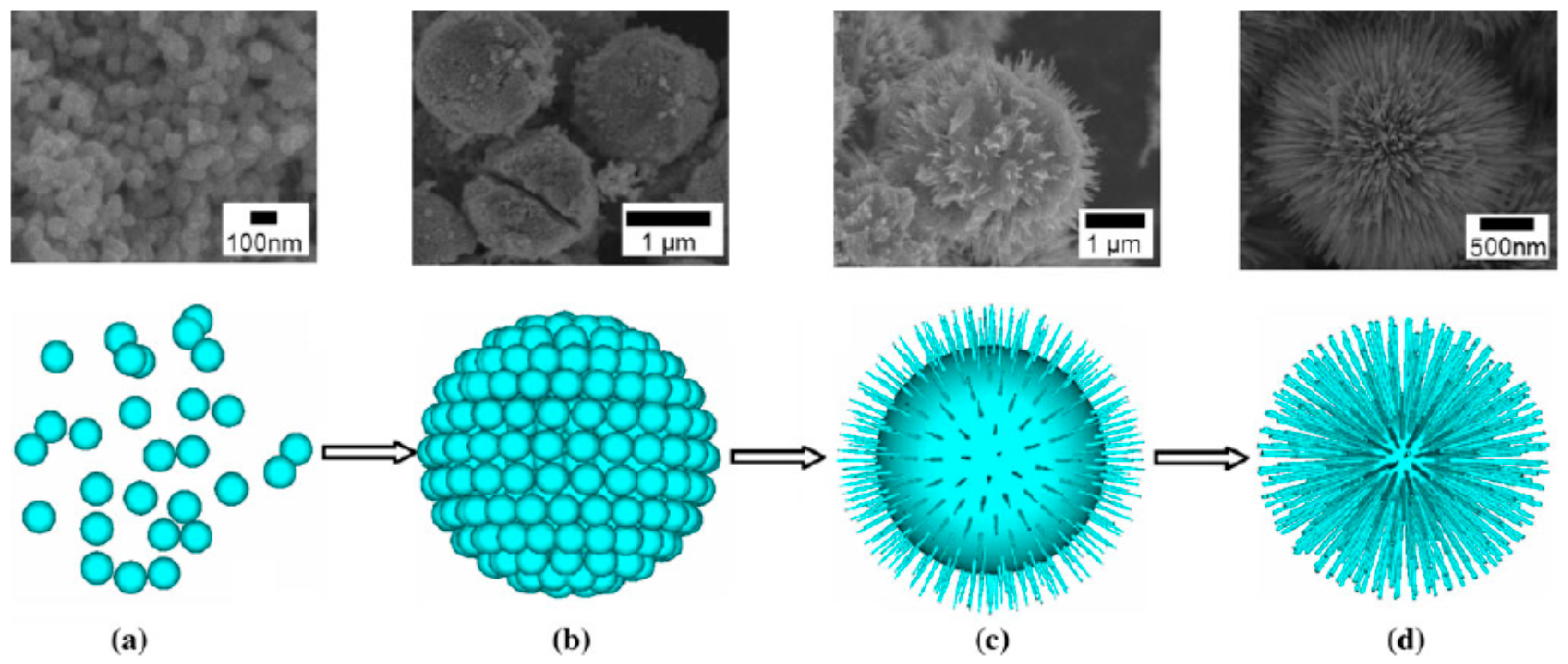
2.2. 3D Urchin-Like Hierarchical Particles
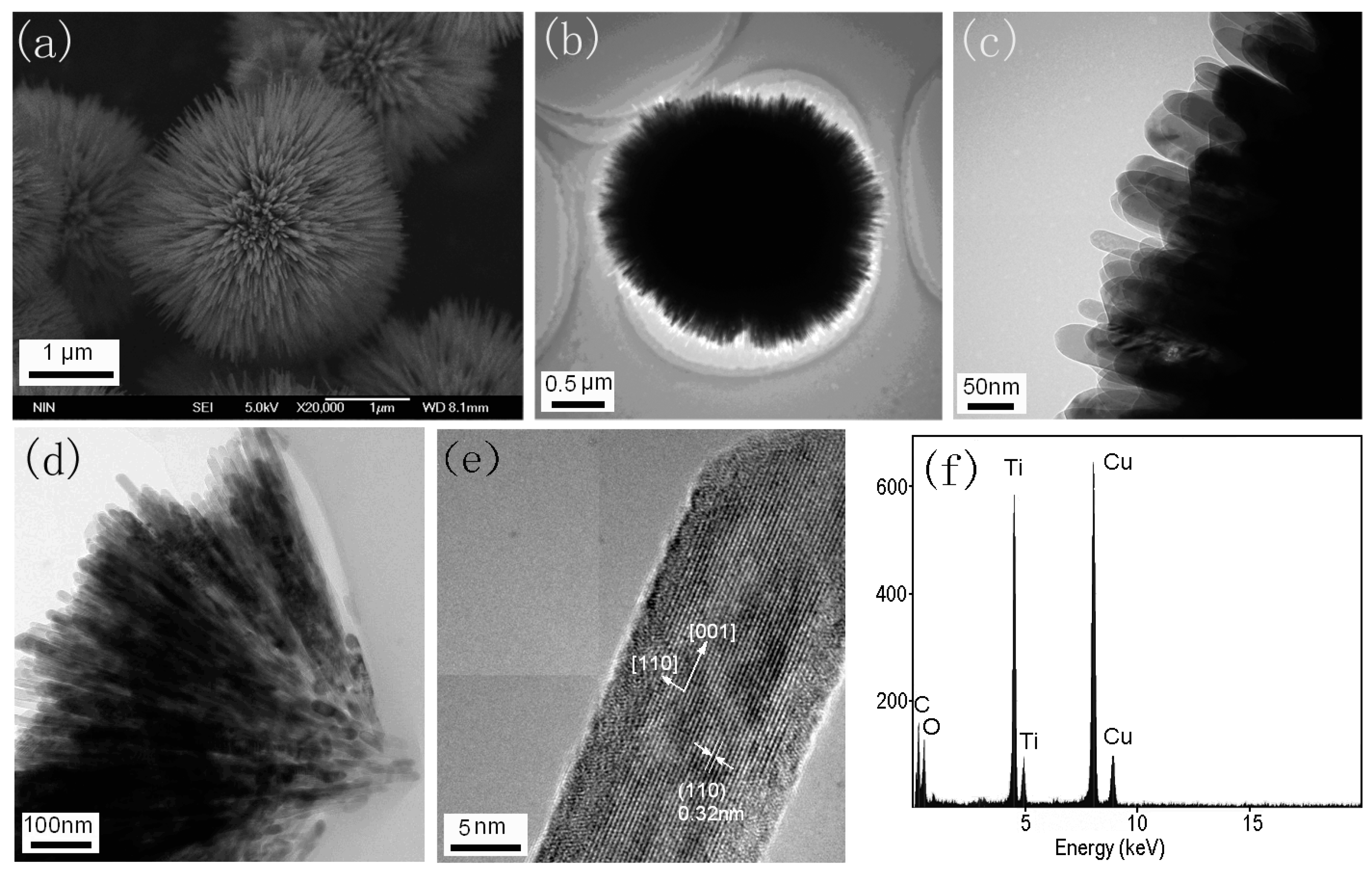
2.2.1. Urchin-Like Hierarchical TiO2 Particles
2.2.2. Cr Doped Urchin-Like Hierarchical TiO2 Particles
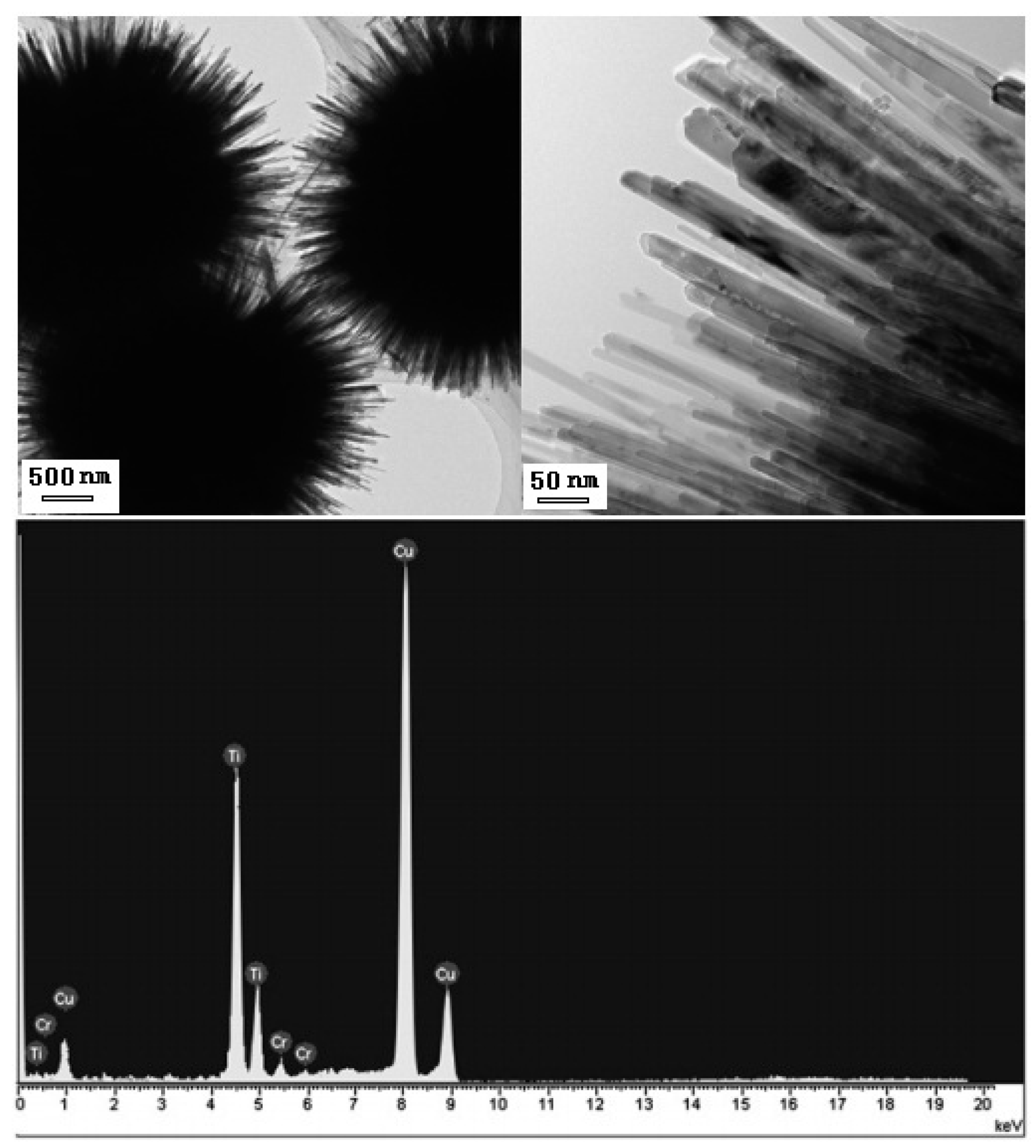
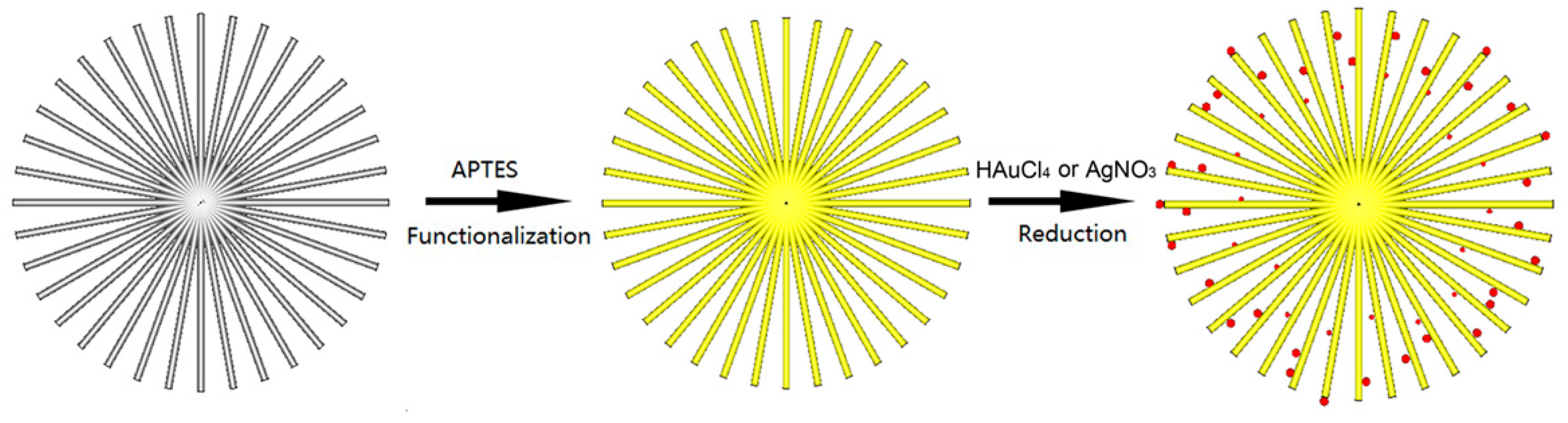
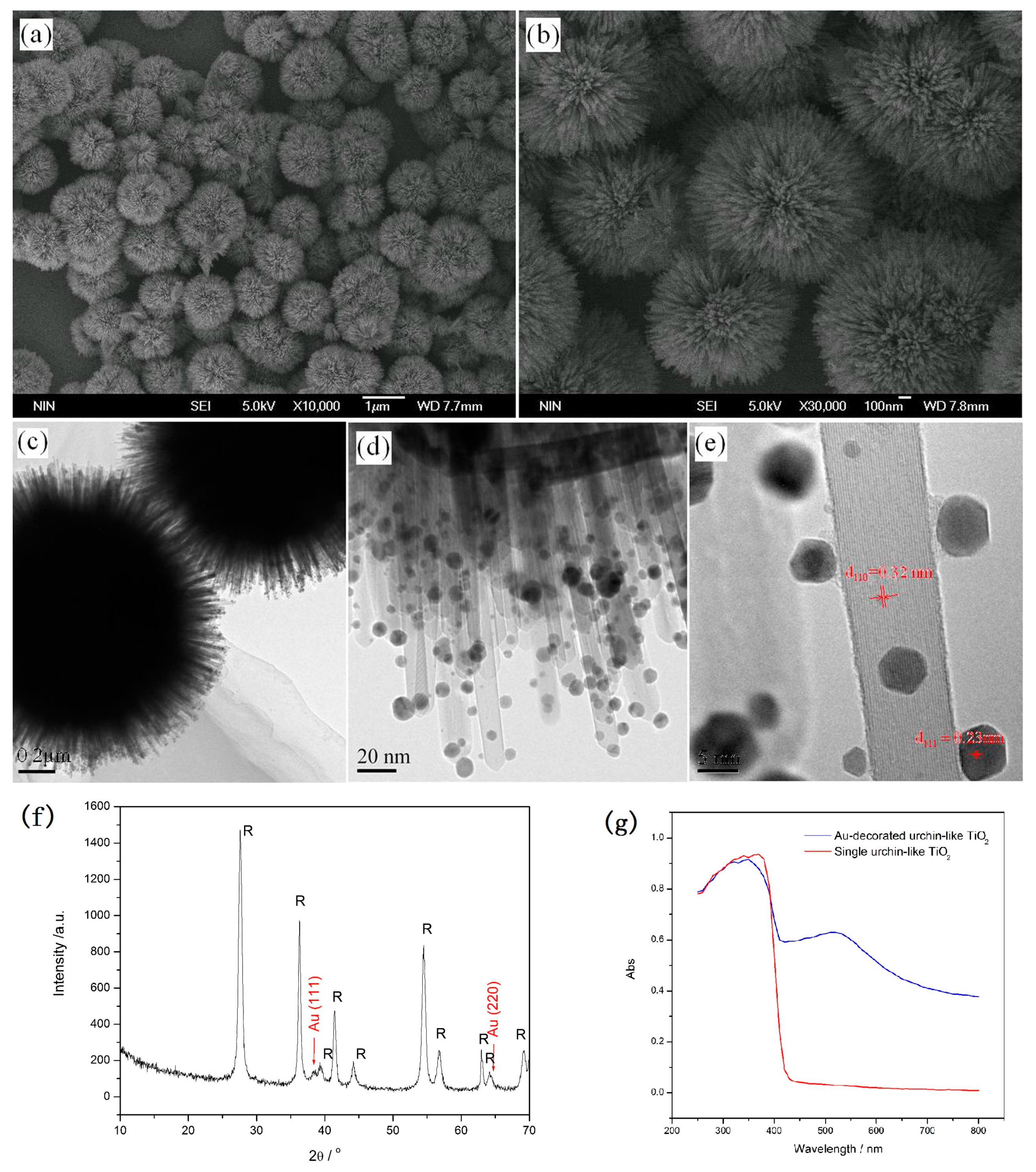
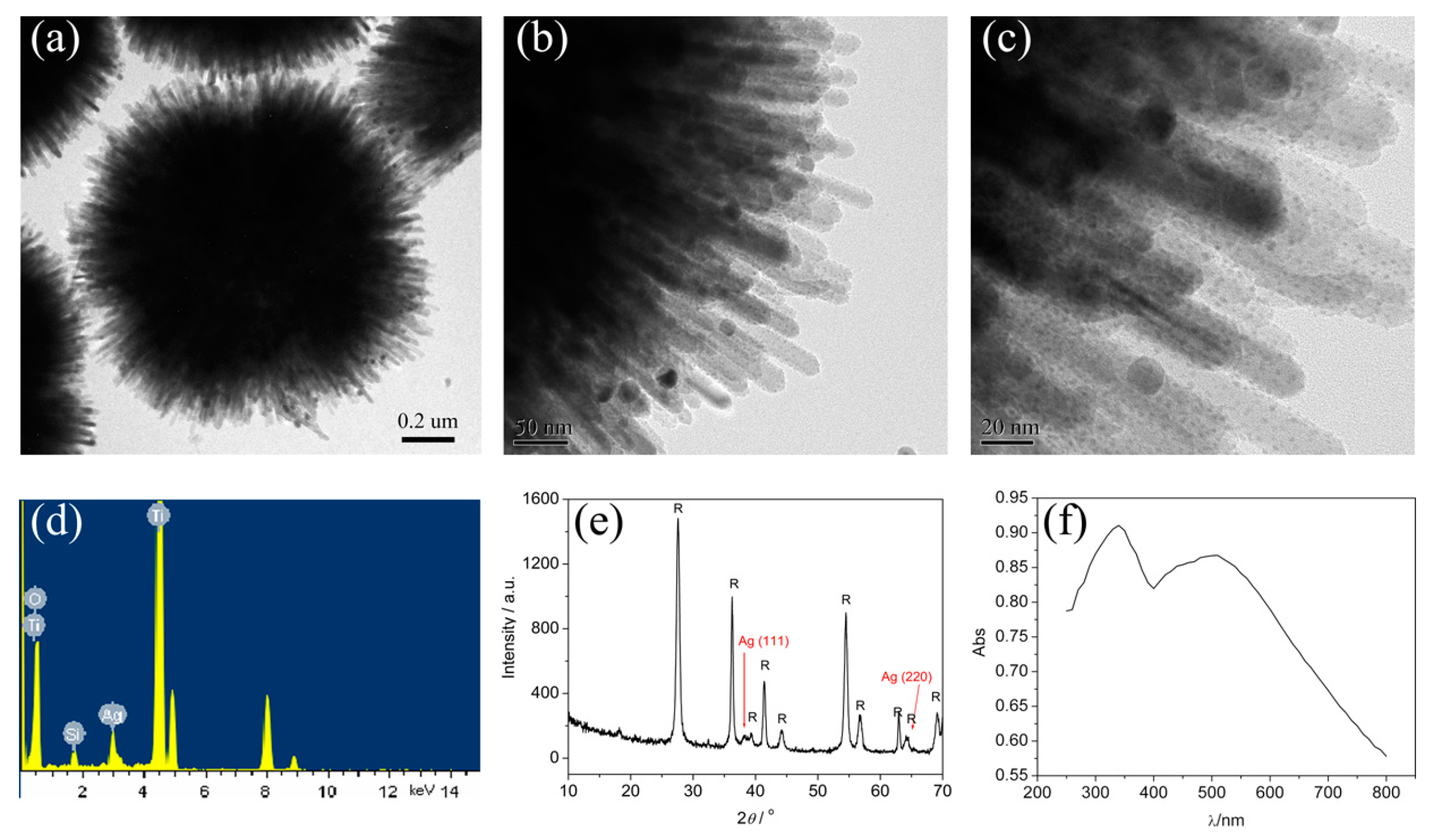
2.2.3. Noble Metal Nanoparticle-Decorated 3D Urchin-Like TiO2 Particles
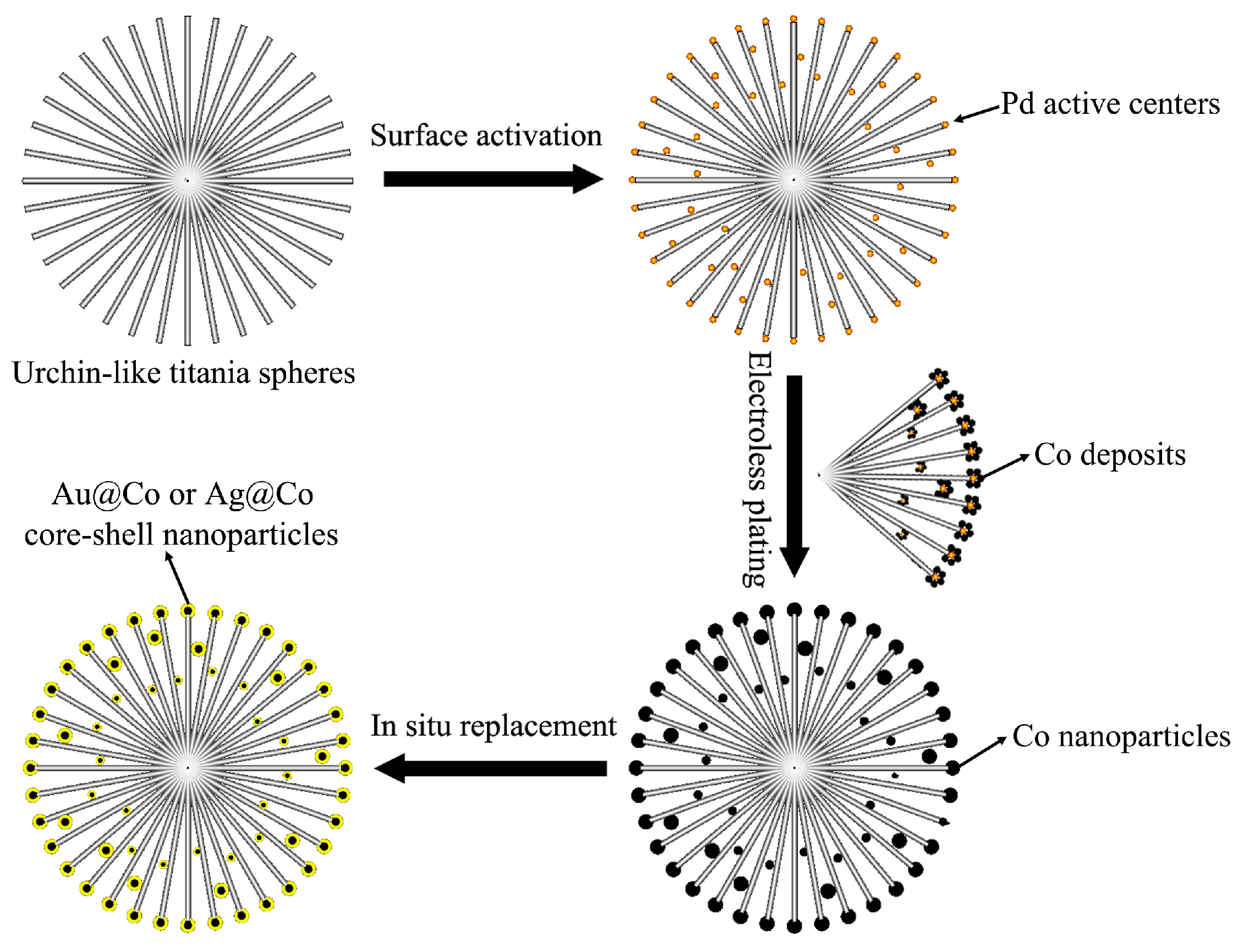
2.2.4. Core/Shell-Structured Bimetallic Nanoparticle-Decorated 3D Urchin-Like Hierarchical TiO2 Particles
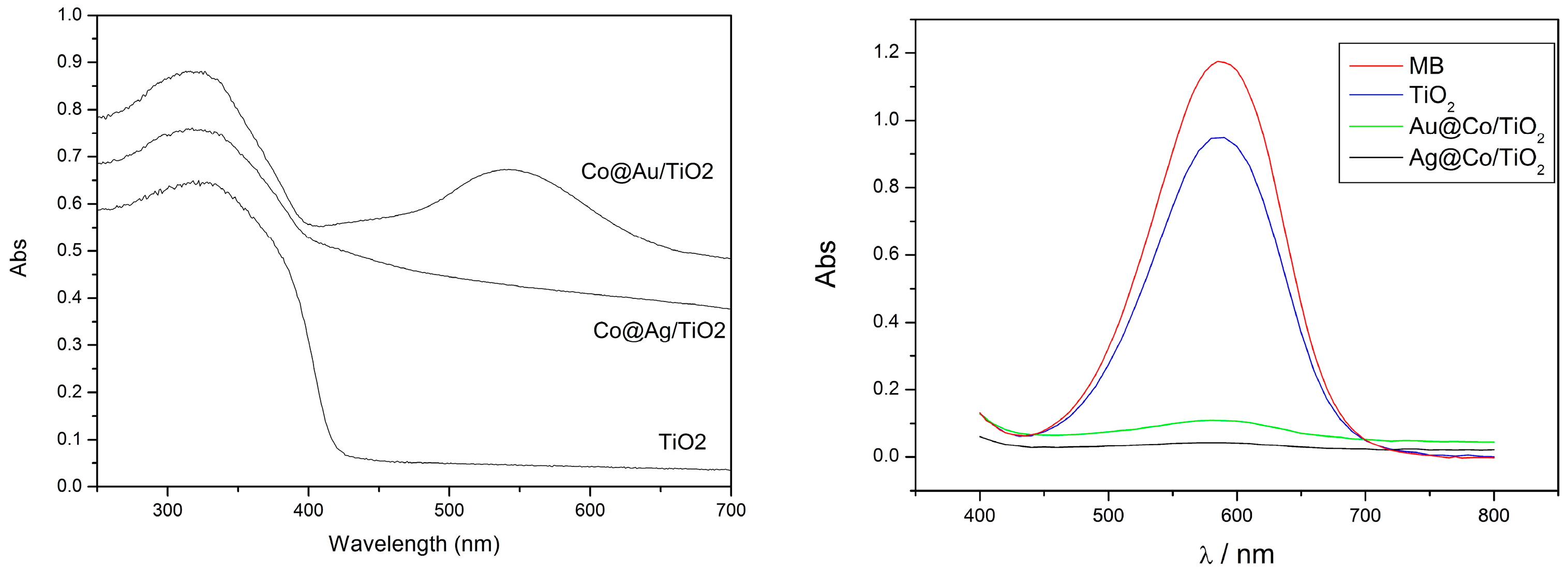
2.2.5. Photocatalytic Activity of Urchin-Like Hierarchical TiO2 and Their Composites
3. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fattakhova-Rohlfing, D.; Zaleska, A.; Bein, T. Three-Dimensional Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9487–9558. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Kamarudin, S.K. Titanium dioxide in fuel cell technology: An overview. J. Power Sources 2015, 278, 109–118. [Google Scholar] [CrossRef]
- Chen, X.B.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lan, Z.A.; Wang, X. Conjugated Polymers: Catalysts for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef] [PubMed]
- Kessler, F.K.; Zheng, Y.; Schwarz, D.; Merschjann, C.; Schnick, W.; Wang, X.; Bojdys, M.J. Functional carbon nitride materials-design strategies for electrochemical devices. Nat. Rev. Mater. 2017, 2, 17030. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.A.; Wang, X. Surface engineering of graphitic carbon nitride polymers with cocatalysts for photocatalytic overall water splitting. Chem. Sci. 2017, 8, 5261–5274. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.M.; Lu, G.Q. TiO2-based photocatalysts-crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Ruokolainen, M.; Ollikainen, E.; Sikanen, T.; Kotiaho, T.; Kostiainen, R. Oxidation of tyrosine-phosphopeptides by titanium dioxide photocatalysis. J. Am. Chem. Soc. 2016, 138, 7452–7455. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H. Semiconductor photocatalysis-mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically Ordered Macro-Mesoporous TiO2-Graphene Composite Films: Improved Mass Transfer, Reduced Charge Recombination, and Their Enhanced Photocatalytic Activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Liu, Z.P. Particle size, shape and activity for photocatalysis on TiO2 anatase nanoparticles in aqueous surroundings. J. Am. Chem. Soc. 2011, 133, 15743–15752. [Google Scholar] [CrossRef] [PubMed]
- Meulen, T.; Mattson, A.; Oesterlund, L. A comparative study of the photocatalytic oxidation of propane on anatase, rutile, and mixed-phase anatase-rutile TiO2 nanoparticles: Role of surface intermediates. J. Catal. 2007, 25, 131–138. [Google Scholar]
- Kang, X.W.; Chen, S.W. Photocatalytic reduction of methylene blue by TiO2 nanotube arrays: Effects of TiO2 crystalline phase. J. Mater. Sci. 2010, 45, 2696–2702. [Google Scholar] [CrossRef]
- Tanaka, S.; Nogami, D.; Tsuda, N.; Miyake, Y. Synthesis of highly-monodisperse spherical TiO2 particles with diameters in the submicron range. J. Colloid Interface Sci. 2009, 334, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M.; Ghayeb, Y. Fabrication, characterization and photoelectrochemical performance of chromium-sensitized TiO2 nanotubes as efficient photoanodes for solar water splitting. J. Solid State Electrochem. 2016, 20, 683–689. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 2791–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.; Tang, Y.; Müllen, K.; Feng, X. TiO2 Nanosheet-Mediated Construction of a Two-Dimensional TiO2/Cadmium Sulfide Heterostructure for High Hydrogen Evolution Activity. Adv. Mater. 2014, 26, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Bartl, M.H.; Boettcher, S.W.; Frindell, K.L.; Stucky, G.D. 3-D molecular assembly of function in TiO2-based composite material systems. Acc. Chem. Res. 2005, 38, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Li, J.; Wu, Q. Construction of TiO2 hierarchical nanostructures from nanocrystals and their photocatalytic properties. ACS Appl. Mater. Interfaces 2011, 3, 3448–3453. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bian, Z.; Zhu, J.; Zhang, D.; Li, G.; Huo, Y.; Li, H.; Lu, Y. Mesoporous TiO2 spheres with tunable chamber lasmon and enhanced photocatalytic activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Termin, A.; Hoffman, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Su, R.; Tiruvalam, R.; He, Q.; Dimitratos, N.; Kesavan, L.; Hammond, C.; Lopez-Sanchez, J.A.; Bechstein, R.; Kiely, C.J.; Hutchings, G.J.; et al. Promotion of phenol photodecomposition over TiO2 using Au, Pd, and AuPd nanoparticles. ACS Nano 2012, 6, 6284–6292. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, N.; Vorobets, V.; Linnik, O.; Manuilov, E.; Kolbasovb, G.; Eremenkoa, A. Photoelectrochemical and photocatalytic properties of mesoporous TiO2 films modified with silver and gold nanoparticles. Surf. Interface Anal. 2010, 42, 1205–1208. [Google Scholar] [CrossRef]
- Arabtzis, I.M.; Stergiopoulos, T.; Andreeva, D.; Kitova, S.; Neophytides, S.G.; Falaras, P. Charaterizarion and photocatalytic activity of Au/TiO2 thin films for azo-dye degradation. J. Catal. 2003, 220, 127–135. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Preparation and Enhanced Electrorheological Activity of TiO2 Doped with Chromium Ion. Chem. Mater. 2004, 16, 321–328. [Google Scholar] [CrossRef]
- Zhao, X.P.; Duan, X. In situ sol-gel preparation of polysaccharide/titanium oxide hybrid colloids and their electrorheological effect. J. Colloid Interface Sci. 2002, 251, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.B.; Xiang, L.Q.; Zhao, X.P. Monodisperse spherical mesoporous Eu-doped TiO2 phosphor particles and the luminescence properties. Appl. Phys. Lett. 2007, 90, 113112. [Google Scholar] [CrossRef]
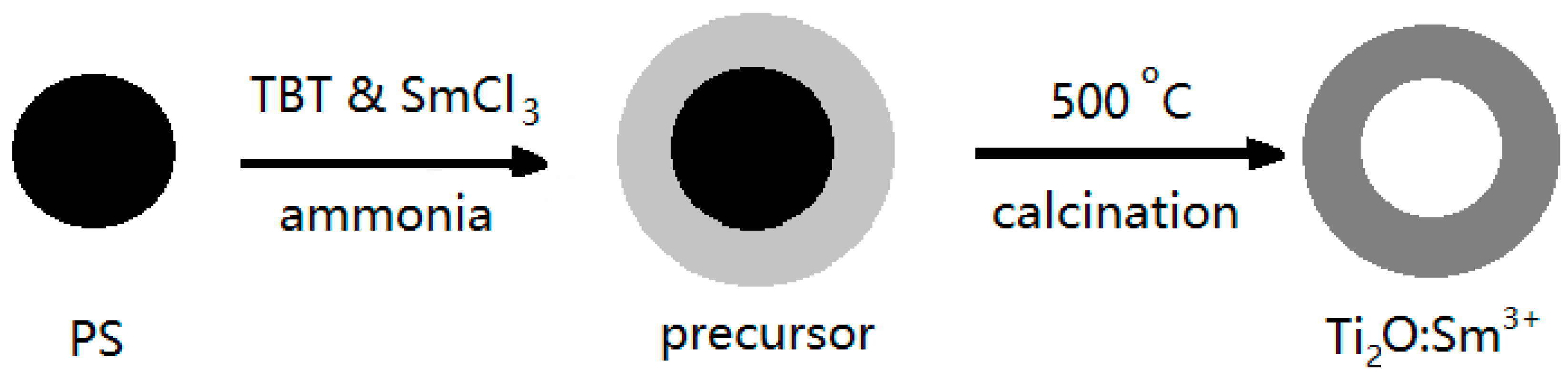
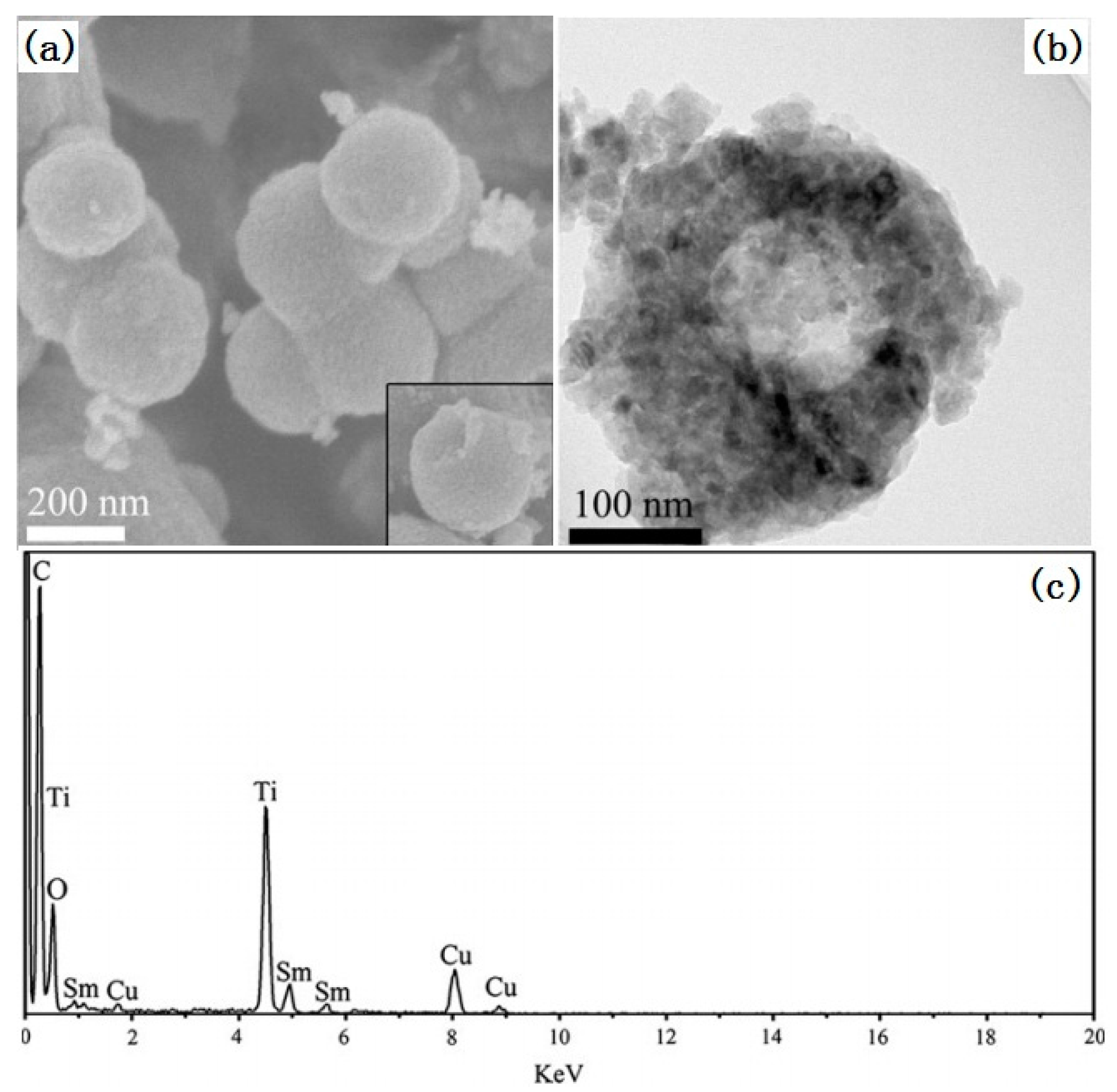
- An, G.F.; Yang, C.S.; Jin, S.H.; Chen, G.W.; Zhao, X.P. Hollow TiO2:Sm3+ spheres with enhanced photoluminescence fabricated by a facile method using polystyrene as template. J. Mater. Sci. 2013, 48, 5483–5488. [Google Scholar] [CrossRef]
- Xiang, L.Q.; Zhao, X.P.; Yin, J.B.; Fan, B.L. Well-organized 3-D urchin-like multilevel TiO2 microspheres with high photocatalytic activity. J. Mater. Sci. 2012, 47, 1436–1445. [Google Scholar] [CrossRef]
- Xiang, L.Q.; Zhao, X.P.; Shang, C.H.; Yin, J.B. Au or Ag nanoparticle-decorated 3D urchin-like TiO2 nanostructures: Synthesis, characterization, and enhanced photocatalytic activity. J. Colloid Interface Sci. 2013, 403, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.Q.; Liu, S.; Yin, J.B.; Zhao, X.P. Bimetallic core/shell nanoparticle-decorated 3D urchin-like hierarchical TiO2 nanostructures with magneto-responsive and decolorization characteristics. Nanoscale Res. Lett. 2015, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.B.; Zhao, X.P. Titanate nano-whisker electrorheological fluid with high suspended stability and ER activity. Nanotechnology 2006, 17, 192–196. [Google Scholar] [CrossRef]
- Guo, H.X.; Zhao, X.P.; Ning, G.H.; Liu, G.Q. Synthesis of Ni/Polystyrene/TiO2 multiply coated microsphere. Langmuir 2003, 19, 4884–4888. [Google Scholar] [CrossRef]
- Guo, H.X.; Zhao, X.P.; Guo, H.L.; Zhao, Q. Preparation of porous SiO2/Ni/TiO2 multiply microsphere responsive to electric and magnetic fields. Langmuir 2003, 19, 9799–9803. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Electrorheological fluids based on glycerol-activated TiO2 gel and silicone oil with high yield strength. J. Colloid Interface Sci. 2003, 257, 228–236. [Google Scholar] [CrossRef]
- Xiang, L.Q.; Yin, J.B.; Gao, W.S.; Zhao, X.P. Controllable Preparation of Quasi-monodispersed Spherical TiO2 Particles. J. Inorg. Mater. Chin. 2007, 22, 253–258. [Google Scholar]
- Ma, L.M.; Zheng, F.X.; Zhao, X.P. Sedimentation behaviour of hierarchical porous TiO2 microspheres electrorheological fluids. J. Intel. Mater. Syst. Struct. 2015, 26, 1936–1944. [Google Scholar] [CrossRef]
- Wang, J.H.; Chen, G.W.; Yin, J.B.; Luo, C.R.; Zhao, X.P. Enhanced electrorheological performance and antisedimentation property of mesoporous anatase TiO2 shell prepared by hydrothermal process. Smart Mater. Struct. 2017, 26, 035036. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhao, X.P. Core/Shell Nanocomposite Based on the Local Polarization and Its Electrorheological Behavior. Langmuir 2005, 21, 6553–6559. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.B.; Xia, X.; Wang, X.X.; Zhao, X.P. The electrorheological effect and dielectric properties of suspensions containing polyaniline@TiO2 nanocable-like particles. Soft Matter 2011, 7, 10978–10986. [Google Scholar] [CrossRef]
- Xiang, L.Q.; Zhao, X.P. Electrorheological activity of a composite of TiO2-Coated Montmorillonite. J. Mater. Chem. 2003, 13, 1529–1532. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhao, X.P. Preparation of kaolinite/TiO2 coated nanocomposite and their electro-rheological properties. J. Mater. Chem. 2003, 13, 2248–2253. [Google Scholar] [CrossRef]
- Zhao, X.P.; Yin, J.B. Preparation and electrorheological characteristics of rare-earth-doped TiO2 suspensions. Chem. Mater. 2002, 14, 2258–2263. [Google Scholar] [CrossRef]
- Zhao, X.P.; Yin, J.B.; Xiang, L.Q.; Zhao, Q. Electrorheological fluids containing Ce-doped TiO2. J. Mater. Sci. 2002, 37, 2569–2573. [Google Scholar] [CrossRef]
- Zhao, X.P.; Yin, J.B.; Xiang, L.Q.; Zhao, Q. Effect of rare earth substitution on electrorheological properties of TiO2. Int. J. Mod. Phys. B 2002, 16, 2371–2377. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Temperature effect of rare earth-doped TiO2 Electrorheological fluids. J. Phys. D Appl. Phys. 2001, 34, 2063–2067. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Electrorheological effect of Cerium-Doped TiO2. Chin. Phys. Lett. 2001, 18, 1144–1146. [Google Scholar]
- Yin, J.B.; Zhao, X.P. Enhanced electrorheological activity of mesoporous Cr-doped TiO2 from activated pore wall and high surface area. J. Phys. Chem. B 2006, 110, 12916–12925. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Zhao, X.P. A bionic nano-papilla particle and its super-oleophilic ability. Adv. Funct. Mater. 2005, 15, 1815–18212. [Google Scholar] [CrossRef]
- Xiang, L.Q.; Zhao, X.P.; Yin, J.B. Micro/nano-structured montmorillonite/TiO2 particles with high electrorheological activity. Rheol. Acta 2011, 50, 87–95. [Google Scholar] [CrossRef]
- Qiao, Y.P.; Zhao, X.P.; Su, Y.Y. Dielectric metamaterial particles with enhanced efficiency of mechanical/electrical energy transformation. J. Mater. Chem. 2011, 21, 394–399. [Google Scholar] [CrossRef]
- Eiden-Assmann, S.; Widoniak, J.; Maret, G. Synthesis and Characterization of Porous and Nonporous Monodisperse Colloidal TiO2 Particles. Chem. Mater. 2004, 16, 6–11. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, L. Hollow Micro/Nanomaterials with Multilevel Interior Structures. Adv. Mater. 2009, 21, 3621–3638. [Google Scholar] [CrossRef]
- Réti, B.; Kiss, G.I.; Gyulavári, T.; Baan, K.; Magyari, K.; Hernadi, K. Carbon sphere templates for TiO2 hollow structures: Preparation, characterization and photocatalytic activity. Catal. Today 2017, 284, 160–168. [Google Scholar] [CrossRef]
- Orellana, M.; Osiglio, L.; Arnal, P.M.; Pizzio, L.R. TiO2 hollow spheres modified with tungstophosphoric acid with enhanced visible light absorption for the photodegradation of 4-chlorophenol. Photochem. Photobiol. Sci. 2017, 16, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lan, K.; Bagabas, A.A.; Zhang, P.; Gao, W.; Wang, J.; Sun, Z.; Fan, J.; Elzatahry, A.A.; Zhao, D. Ordered Macro/Mesoporous TiO2 Hollow Microspheres with Highly Crystalline Thin Shells for High-Efficiency Photoconversion. Small 2016, 12, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, Z.Y.; Su, B.L. Hierarchically Structured Porous Materials for Energy Conversion and Storage. Adv. Funct. Mater. 2012, 22, 4634–4667. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, M.H.; Kim, H.J.; Lim, G.; Choi, Y.S.; Park, N.G.; Kim, K.; Lee, W.I. Formation of Highly Efficient Dye-Sensitized Solar Cells by Hierarchical Pore Generation with Nanoporous TiO2 Spheres. Adv. Mater. 2009, 21, 3668–3673. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. mesoporous TiO2 photocatalysts: Preparation, characterization and reaction mechanisms. J. Mater. Chem. 2011, 21, 11686–11707. [Google Scholar] [CrossRef]
- Li, S.; Zheng, J.; Zhao, Y.; Liu, Y. Preparation of a three-dimensional ordered macroporous titanium dioxide material with polystyrene colloid crystal as a template. J. Appl. Polym. Sci. 2008, 107, 3903–3908. [Google Scholar] [CrossRef]
- Hegazy, A.; Prouzet, E. Room temperature synthesis and thermal evolution of porous nanocrystalline TiO2 anatase. Chem. Mater. 2012, 24, 245–254. [Google Scholar] [CrossRef]
- Zheng, X.; Lv, Y.; Kuang, Q.; Zhu, Z.; Long, X.; Yang, S. Close-Packed Colloidal SiO2 as a Nanoreactor: Generalized Synthesis of Metal Oxide Mesoporous Single Crystals and Mesocrystals. Chem. Mater. 2014, 26, 5700–5709. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Preparation and Electrorheological Activity of Mesoporous Rare-Earth-Doped TiO2. Chem. Mater. 2002, 14, 4633–4640. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Wormhole-like mesoporous Ce-doped TiO2: A new electrorheological material with high activity. J. Mater. Chem. 2003, 13, 689–695. [Google Scholar] [CrossRef]
- Joo, J.B.; Lee, I.; Dahl, M.; Moon, G.D.; Zaera, F.; Yin, Y. Controllable Synthesis of Mesoporous TiO2 Hollow Shells: Toward an Efficient Photocatalyst. Adv. Funct. Mater. 2013, 23, 4246–4254. [Google Scholar] [CrossRef]
- Bian, Z.F.; Zhu, J.; Cao, F.L.; Huo, Y.N.; Lu, Y.F.; Li, H.X. Solvothermal synthesis of well-defined TiO2 mesoporous nanotubes with enhanced photocatalytic activity. Chem. Commun. 2010, 46, 8451–8453. [Google Scholar] [CrossRef] [PubMed]
- Seisenbaeva, G.A.; Moloney, M.P.; Tekoriute, R.; Hardy-Dessources, A.; Nedelec, J.M.; Gun’ko, Y.K.; Kessler, V.G. Biomimetic Synthesis of Hierarchically Porous Nanostructured Metal Oxide Microparticles-Potential Scaffolds for Drug Delivery and Catalysis. Langmuir 2010, 26, 9809–9817. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Qi, Y.; Lun, N.; Liu, N. Large scale synthesis and gas-sensing properties of anatase TiO2 Three-dimensional hierarchical nanostructures. Langmuir 2010, 26, 12841–12848. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Wang, L.; Zhao, J.; Wang, H.; Li, Y.; Zhang, Q.; Xu, J. Template-free synthesis of hierarchical TiO2 hollow microspheres as scattering layer for dye-sensitized solar cells. Appl. Surf. Sci. 2016, 369, 170–177. [Google Scholar] [CrossRef]
- Zhang, D.; Li, G.; Wang, F.; Yu, J.C. Green synthesis of a self-assembled rutile mesocrystalline photocatalyst. CrystEngComm 2010, 12, 1759–1763. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Sun, C.; Cheng, L.; Wang, L.; Lu, G.Q.; Cheng, H.M. Titania polymorphs derived from crystalline titanium diboride. CrystEngComm 2009, 11, 2677–2682. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P.; Xiang, L.Q.; Xia, X.; Zhang, Z.S. Enhanced electrorheology of suspensions containing sea-urchin-like hierarchical Cr-doped TiO2 particles. Soft Matter 2009, 5, 4687–4697. [Google Scholar] [CrossRef]
- Vaneski, A.; Susha, A.S.; Rodríguez-Fernández, J.; Berr, M.; Jäckel, F.; Feldmann, J.; Rogach, A.L. Hybrid colloidal heterostructures of anisotropic semiconductor nanocrystals decorated with noble metals: Synthesis and function. Adv. Funct. Mater. 2011, 21, 1547–1556. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Kim, D.P.; Kim, D.H. Surface lasmon induced visible light photocatalytic activity of TiO2 nanospheres decorated by Au nanoparticles with controlled configuration. J. Phys. Chem. C 2012, 116, 2500–2506. [Google Scholar] [CrossRef]
- Grigorieva, A.V.; Goodilin, E.A.; Derlyukova, L.E.; Anufrieva, T.A.; Tarasov, A.B.; Dobrovolskii, Y.A.; Tretyakov, Y.D. TiO2 nanotubes supported platinum catalyst in CO oxidation process. Appl. Catal. A Gen. 2009, 362, 20–25. [Google Scholar] [CrossRef]
- Kafizas, A.; Dunnil, C.W.; Parkin, I.P. The relationship between photocatalytic activity and Photochromic state of nanoparticulate silver surface loaded titanium dioxide thin-films. Phys. Chem. Chem. Phys. 2011, 13, 13827–13838. [Google Scholar] [CrossRef] [PubMed]
- Wodka, D.; Bielanska, E.; Socha, R.P.; Elzbieciak-Wodka, M.; Gurgul, J.; Nowak, P.; Warszynski, P.; Kumakiri, I. Photocatalytic activity of titanium dioxide modified by silver nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.J. Selective Pt deposition onto the face (110) of TiO2 assembled microspheres that substantially enhances the photocatalytic properties. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Feng, L.L.; Wu, X.C.; Ren, L.R.; Xiang, Y.J.; He, W.W.; Zhang, K.; Zhou, W.Y.; Xie, S.S. Well-controlled synthesis of Au@Pt nanostructures by gold-nanorod-seeded growth. Chem. Eur. J. 2008, 14, 9764–9771. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S.; Hofmann, C.; Ali, T.A.; Kelly, A.T.; Morosan, E.; Nordlander, P.; Whitmire, K.H.; Halas, N.J. Magnetic-plasmonic core-shell nanoparticles. J. Am. Chem. Soc. 2009, 3, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Fujita, T.; Ding, Y.; Chen, M.W. A Three-dimensional gold-decorated nanoporous copper core-shell composite for electrocatalysis and nonenzymatic biosensing. Adv. Funct. Mater. 2010, 20, 2279–2285. [Google Scholar] [CrossRef]
- Xuan, S.; Xiang, Y.; Wang, J.; Yu, J.C.; Leung, K.C. Preparation, Characterization, and Catalytic Activity of Core/Shell Fe3O4@Polyaniline@Au Nanocomposites. Langmuir 2009, 25, 11835–11843. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Clavero, C.; Huba, Z.; Carroll, K.J.; Carpenter, E.E.; Gu, D.F.; Lukaszew, R.A. Plasmonics and Enhanced Magneto-Optics in Core-Shell Co-Ag Nanoparticles. Nano Lett. 2011, 11, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, Y.; Yu, L.; Dong, L.; Shi, C.; Hu, M.J.; Xu, Y.J.; Wen, L.P.; Yu, S.H. Hydrophilic Co@Au Yolk/Shell Nanospheres: Synthesis, Assembly, and Application to Gene Delivery. Adv. Mater. 2010, 22, 1407–1411. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, L.; Zhao, X. Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials 2017, 7, 310. https://doi.org/10.3390/nano7100310
Xiang L, Zhao X. Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials. 2017; 7(10):310. https://doi.org/10.3390/nano7100310
Chicago/Turabian StyleXiang, Liqin, and Xiaopeng Zhao. 2017. "Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties" Nanomaterials 7, no. 10: 310. https://doi.org/10.3390/nano7100310



