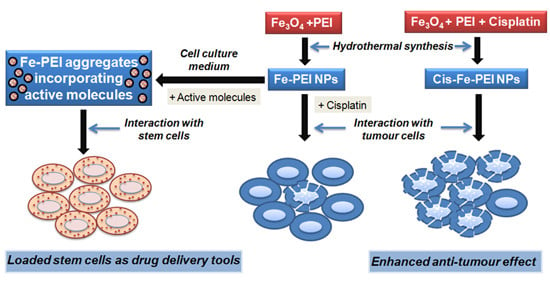
Evaluation of the Ability of Nanostructured PEI-Coated Iron Oxide Nanoparticles to Incorporate Cisplatin during Synthesis
Abstract
:1. Introduction
2. Results
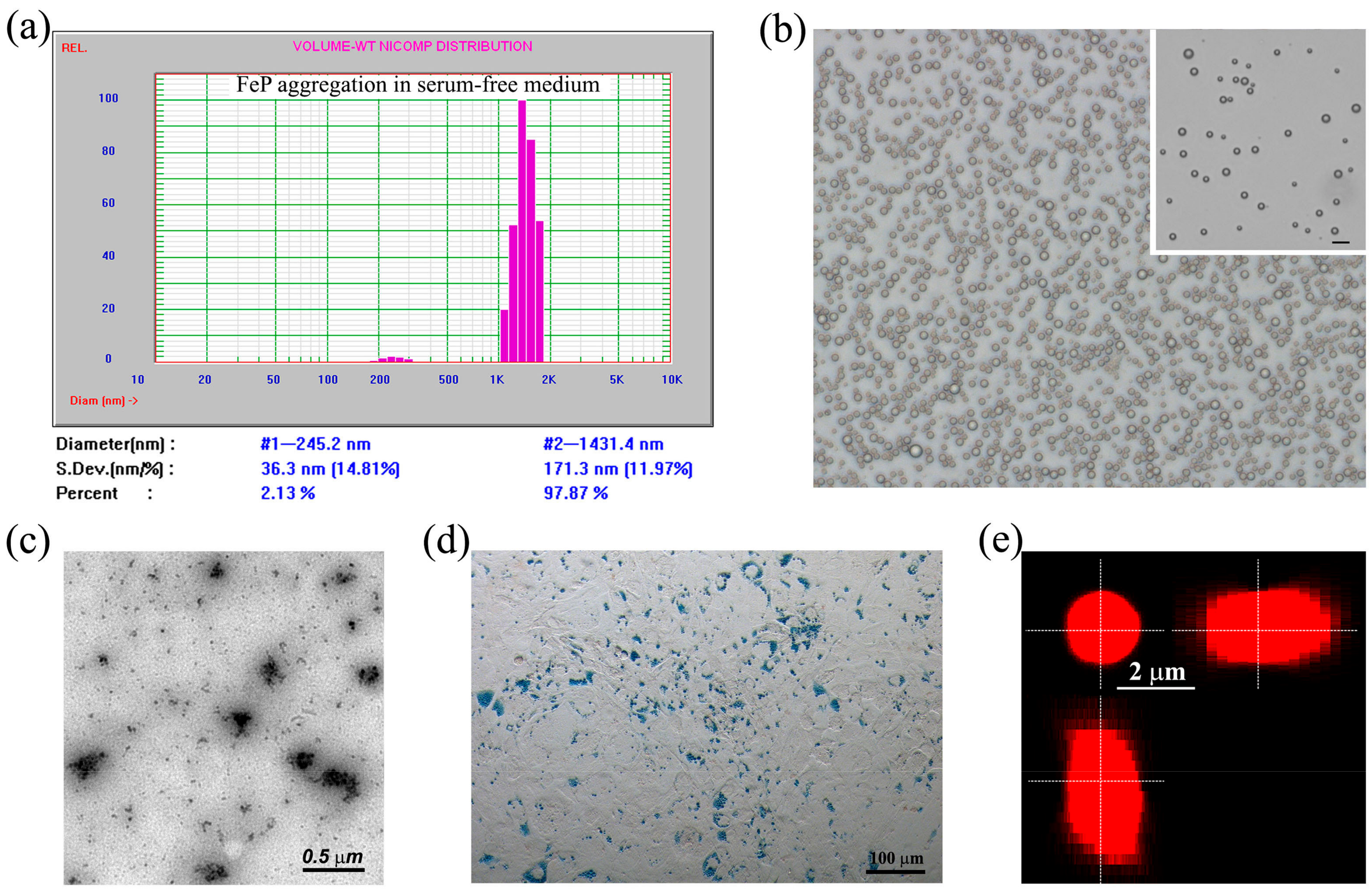
2.1. Selection and Characterization of Fe-PEI NPs
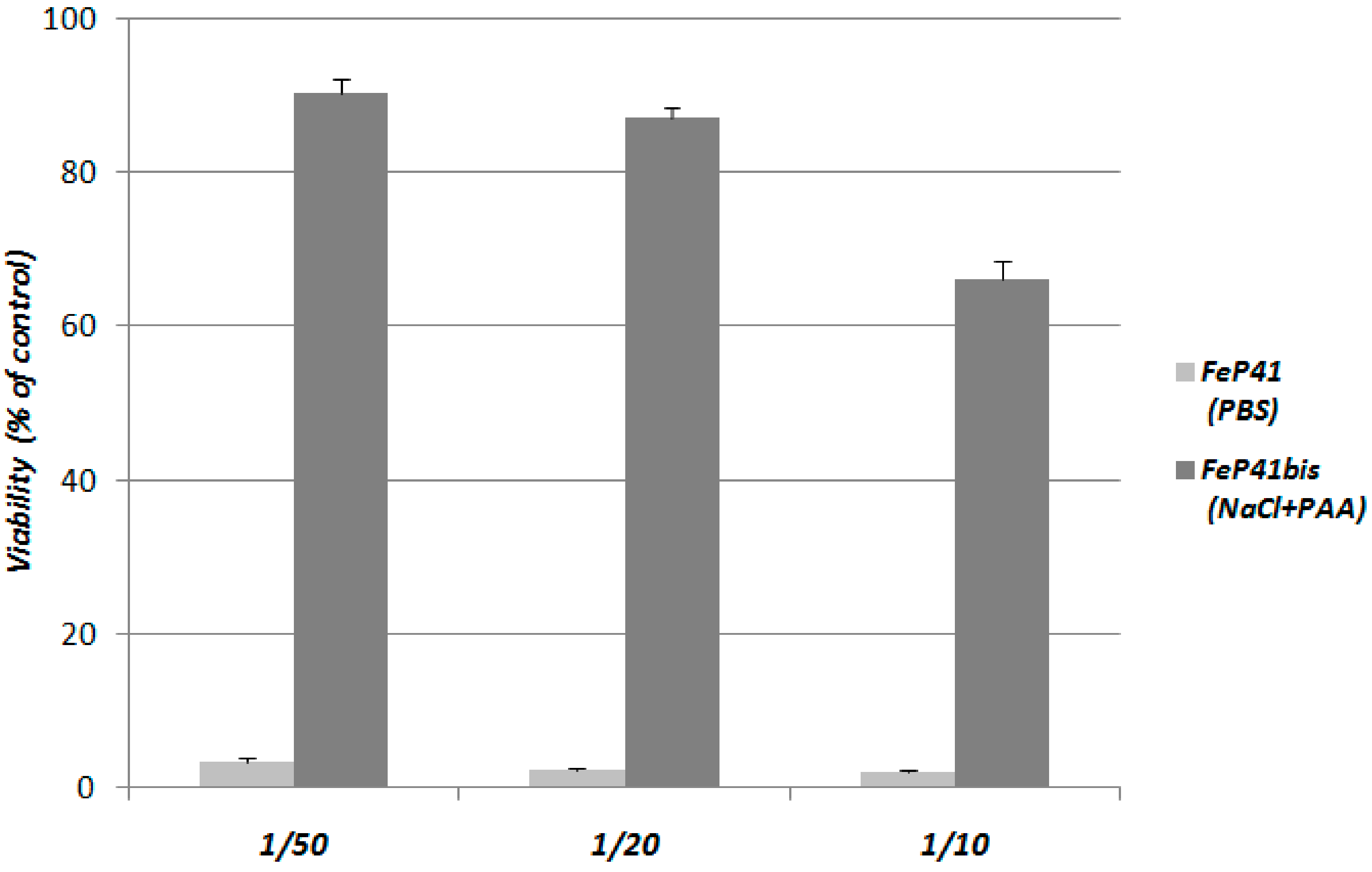
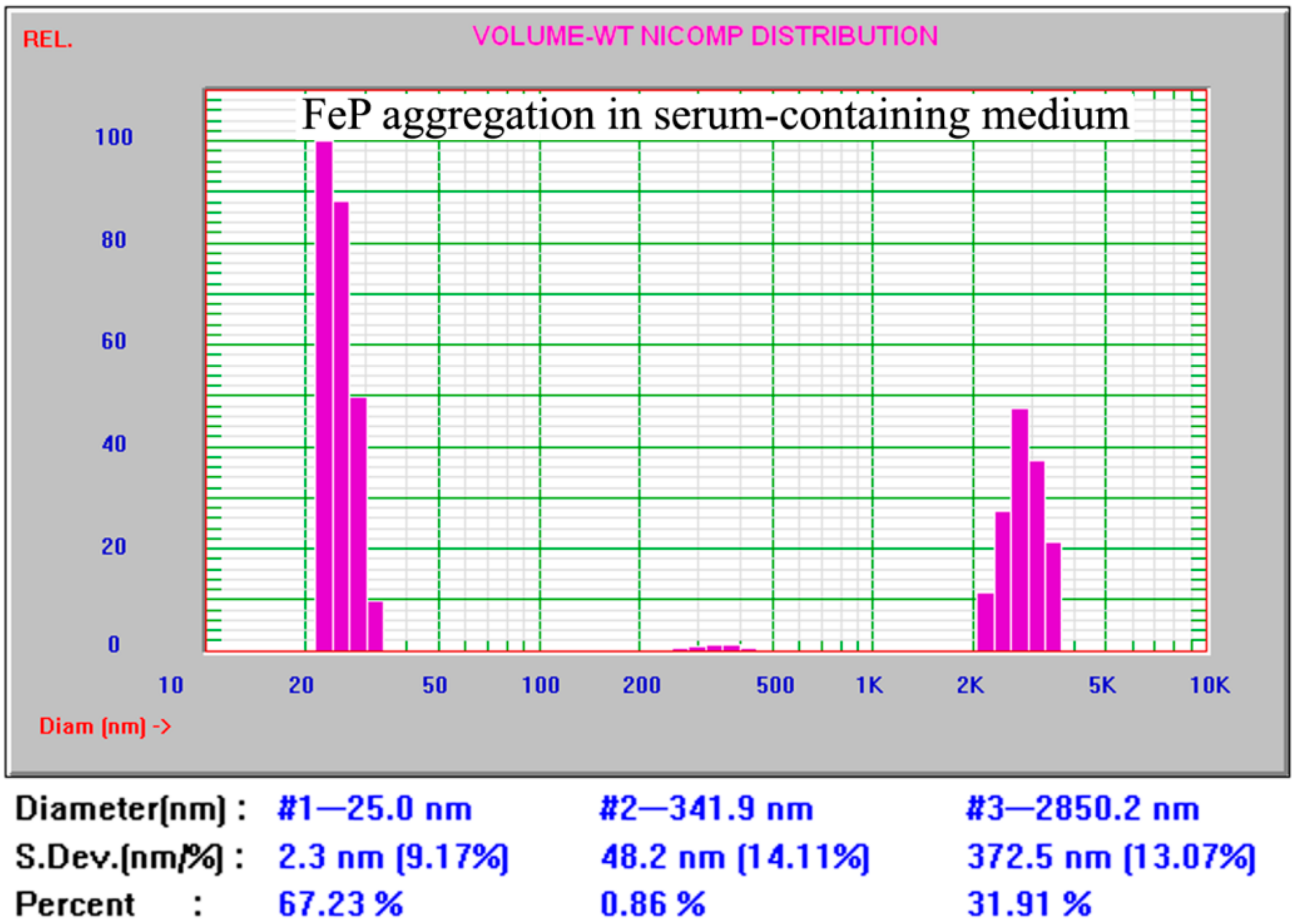
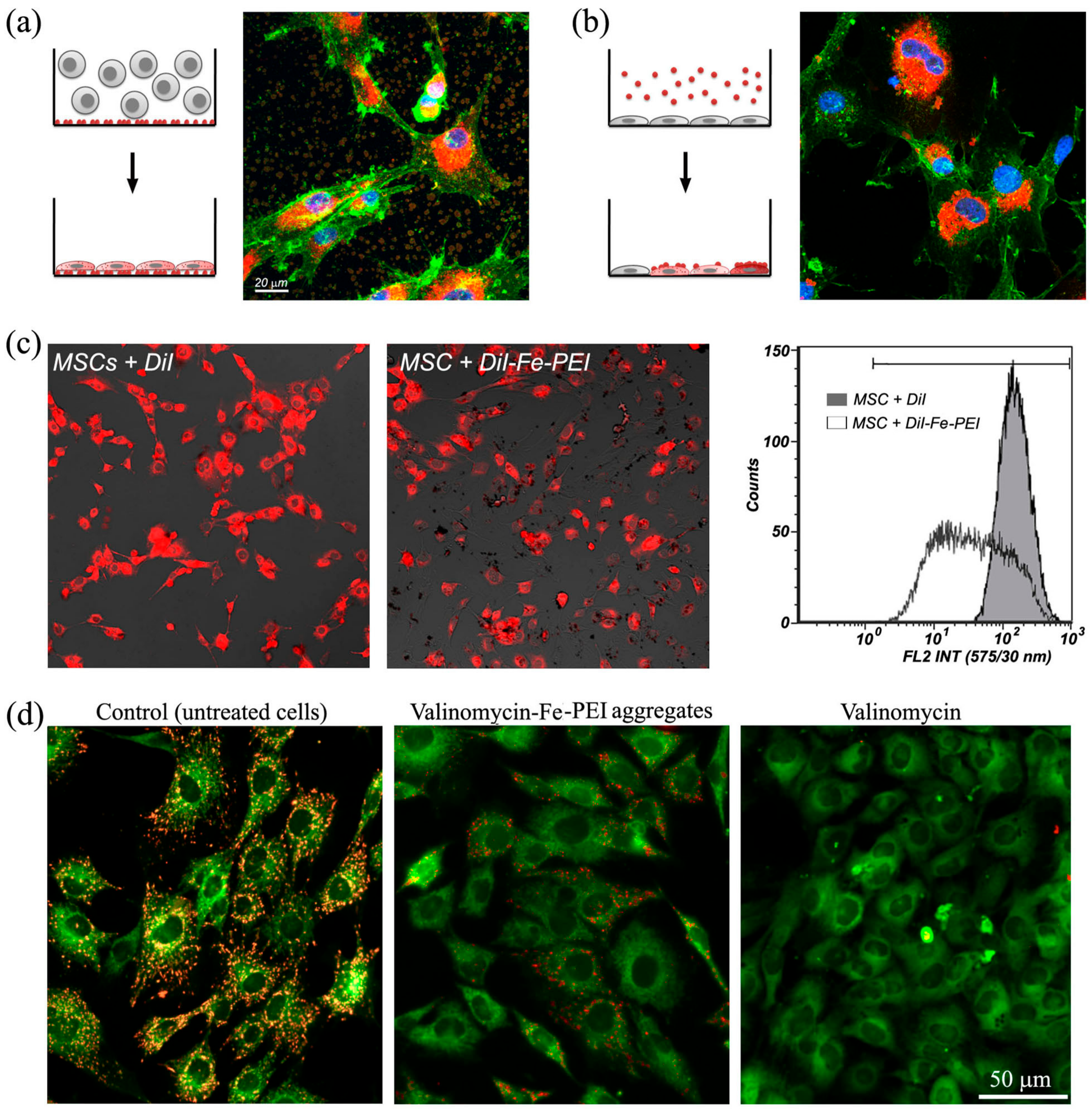
2.2. Aggregation of Fe-PEI NPs in the Culture Medium
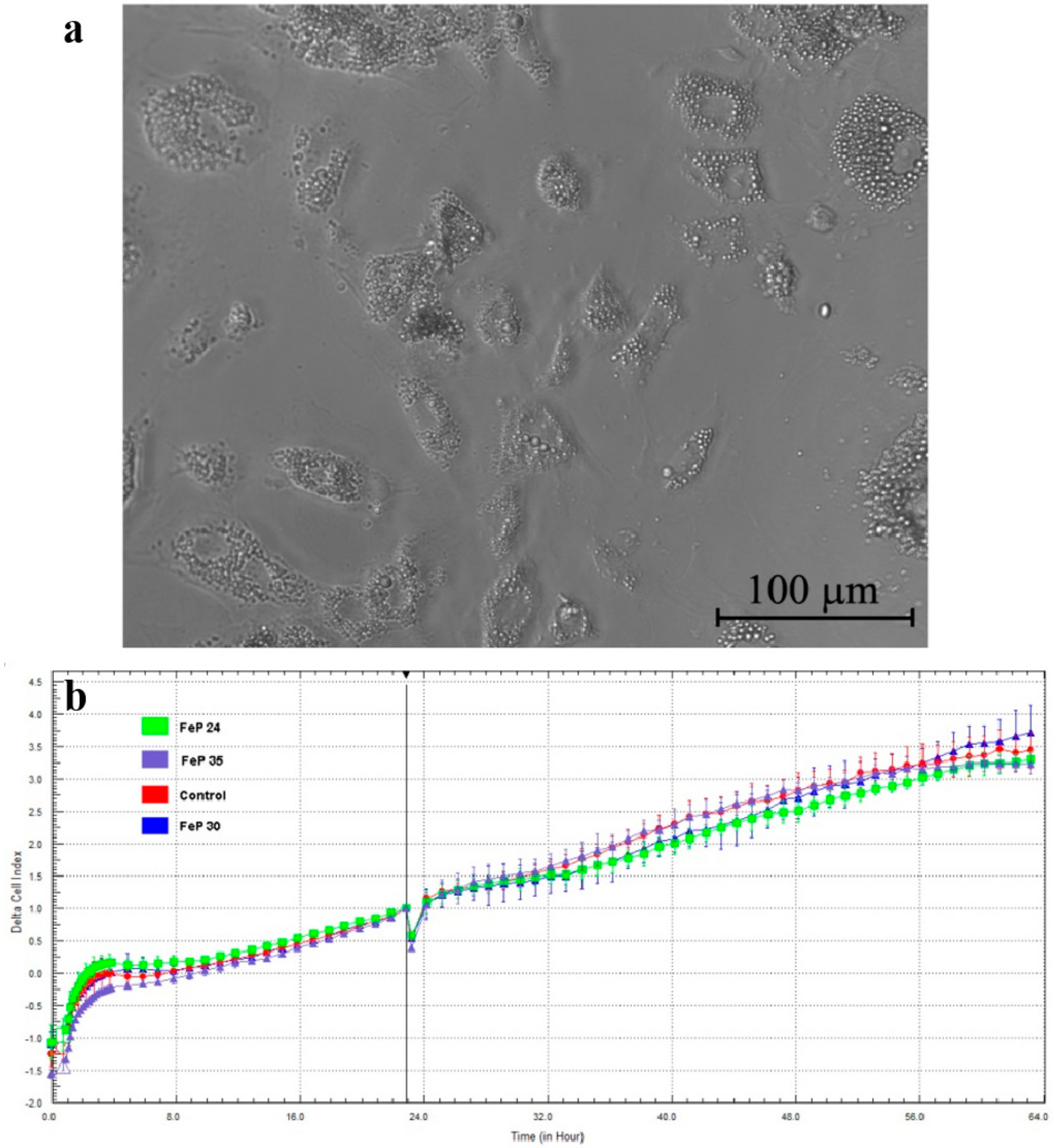
2.3. Fe-PEI NPs Incorporate Fluorescent Tracer DiI during Aggregation to Be Delivered into Adjoining Cells
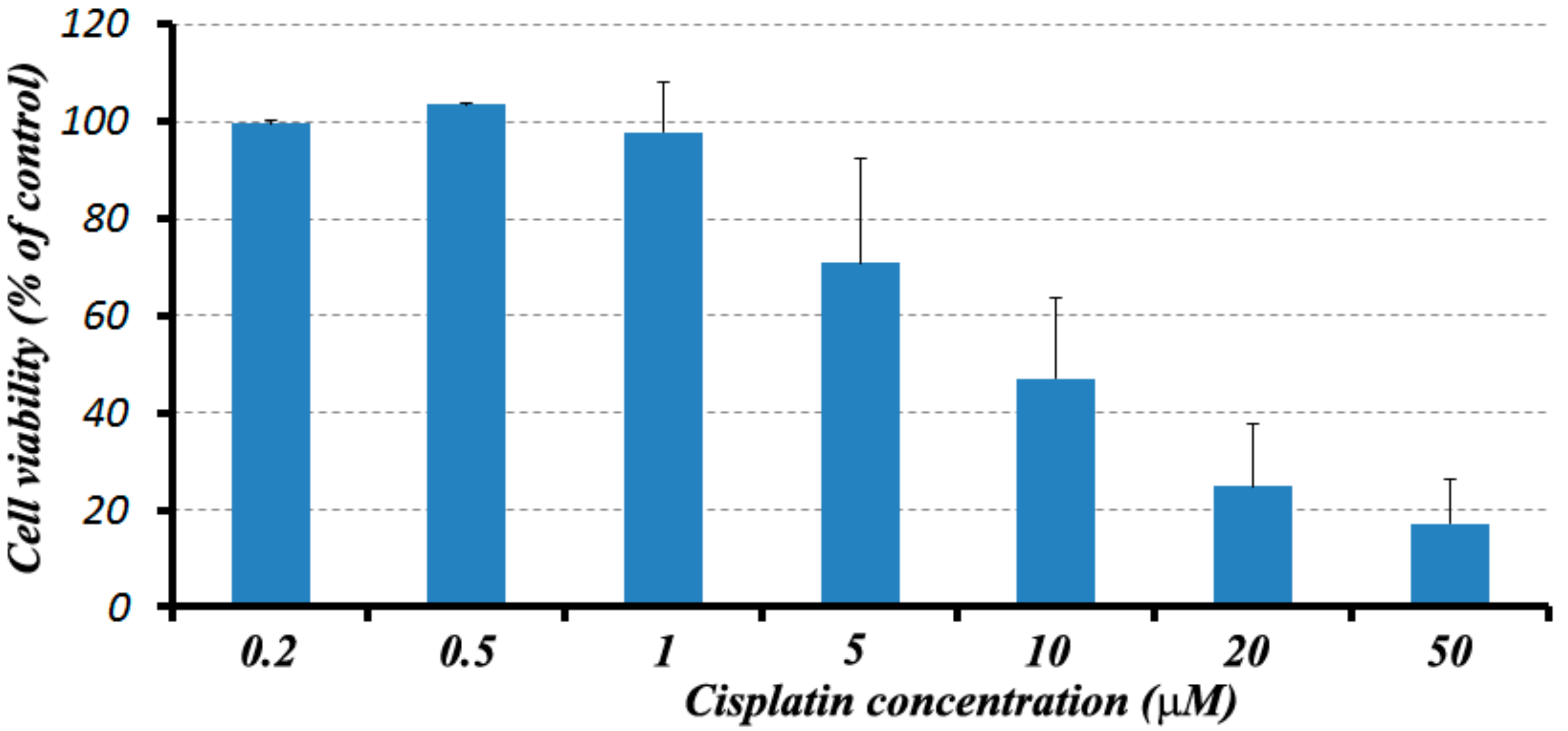
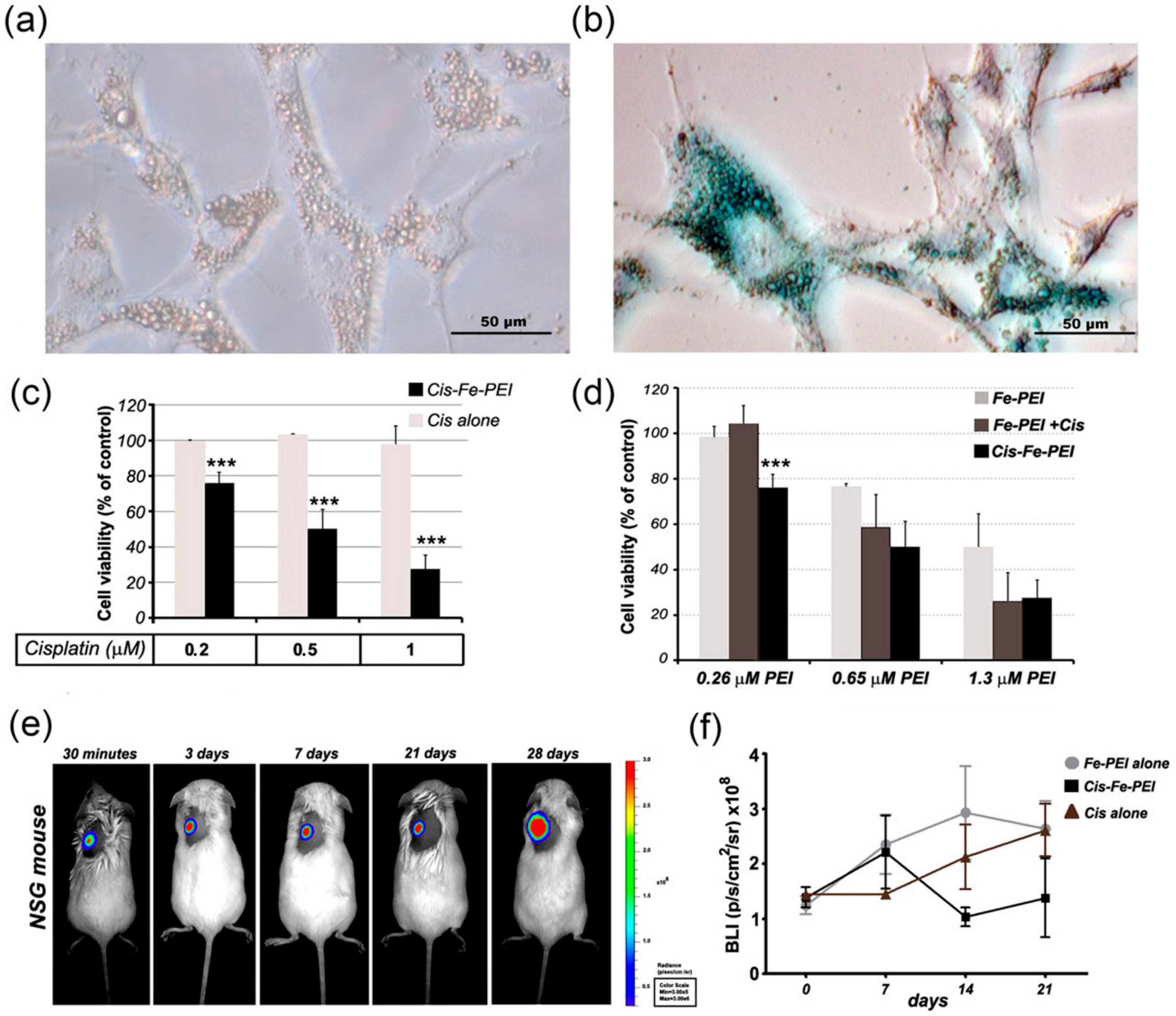
2.4. Fe-PEI NPs Increase the Efficiency of Intracellular Delivery of Cisplatin Incorporated during NPsSynthesis in Hydrothermal Conditions
3. Discussions
4. Materials and Methods
4.1. Preparation of Iron Oxide-PEI (Fe-PEI) Nanopowders
4.2. Preparation of Fe-PEI Stable Aqueous Suspensions
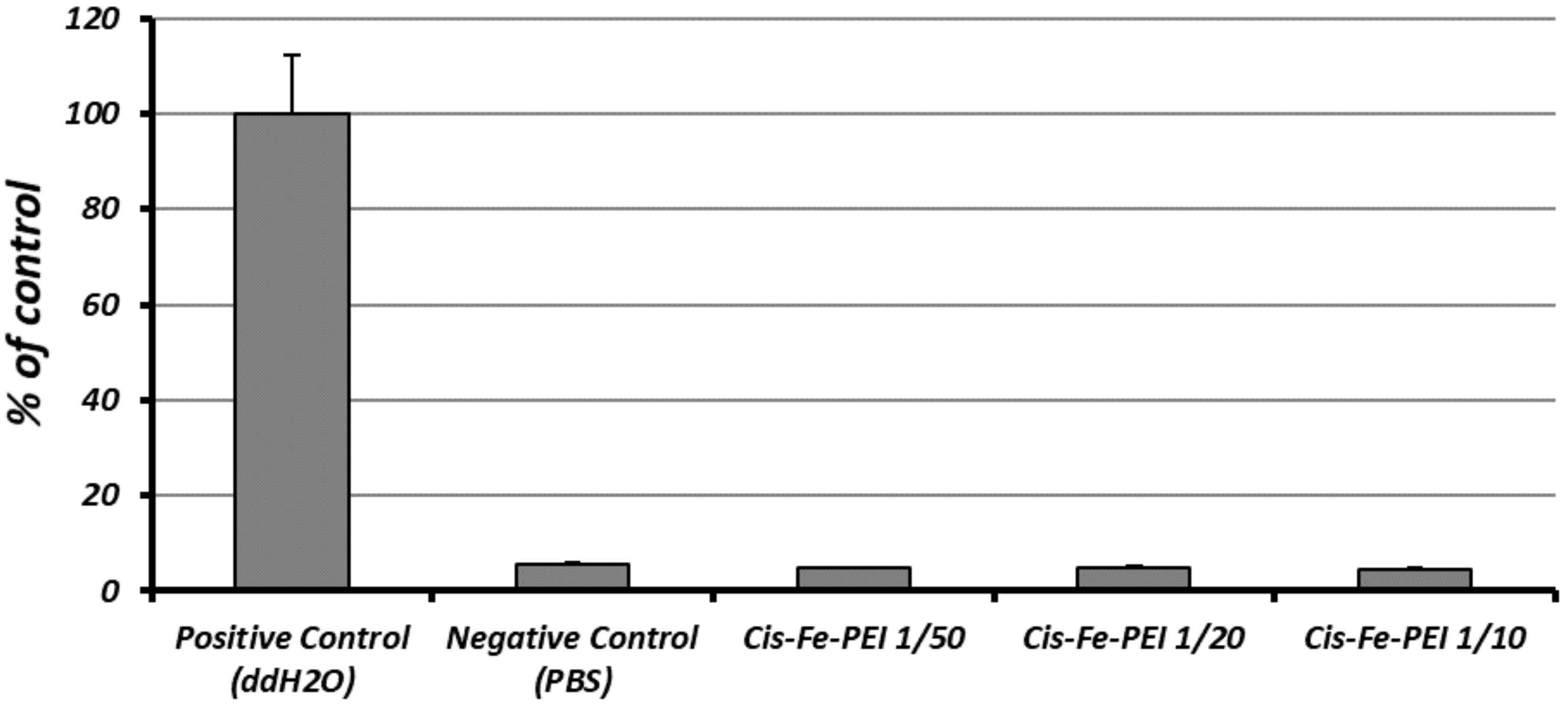
4.3. Incorporation of Cisplatin in Fe-PEI NPs
4.4. Characterization of Fe-PEI Nanopowders
4.5. Cells
4.6. Prussian Blue Staining for Iron
4.7. The Interaction of Fluorescently-Labelled Fe-PEI NPs with MSCs
4.8. Bioluminescence Imaging on Cells in Culture
4.9. Transmission Electron Microscopy (TEM)
4.10. Measurement of Mitochondrial Membrane Potential (ΔΨm)
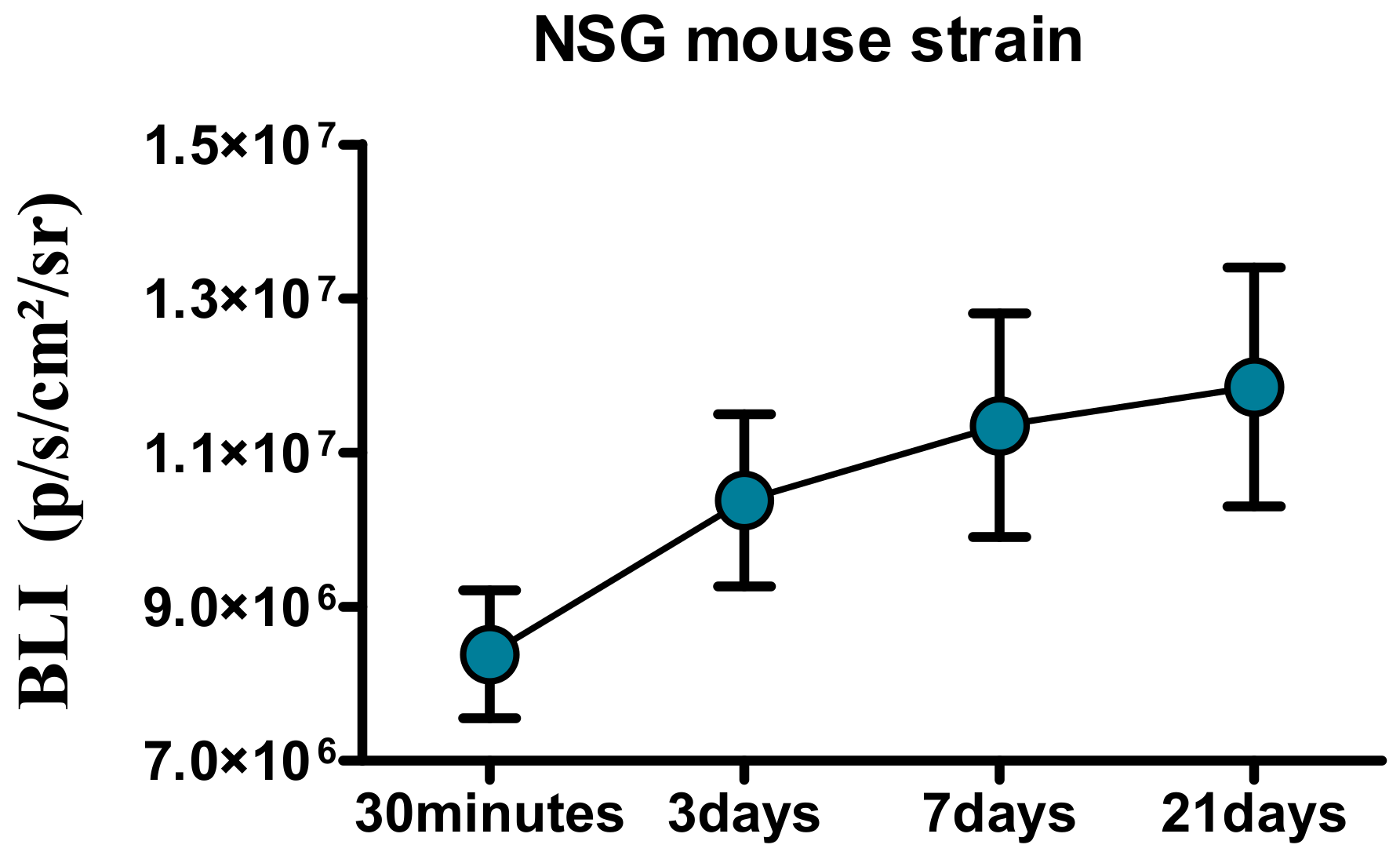
4.11. In Vivo U87 Cell Implantation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cis | Cisplatin |
| Cis-Fe-PEI | poly(ethylenimine) (PEI)-coated iron oxide nanoparticles with cisplatin incorporated during synthesis |
| DiI | fluorescent molecule-1′-Dioctadecyl-3,3,3′,3′-Tetramethylindocarbocyanine Perchlorate |
| DiI-Fe-PEI | poly(ethylenimine) (PEI)-coated iron oxide nanoparticle aggregates incorporating DiI |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EPC | endothelial progenitor cells |
| Fe-PEI NPs | poly(ethylenimine) (PEI)-coated iron oxide nanoparticles |
| Fe-PEI+Cis | poly(ethylenimine) (PEI)-coated iron oxide nanoparticles with cisplatin added post-synthesis |
| MSCs | mesenchymal stem cells |
| NP | nanoparticle |
| PAA | poly(acrylic acid) |
| PBS | phosphate buffer saline |
| U87 | human glioblastoma cell line U87 (Luciferase positive cells) |
Appendix A
| Sample | Peak 1, °C | ΔH, J/g | Δm, % | Peak 2, °C | ΔH, J/g | m, % | Peak 3, °C | ΔH, J/g | Δm, % | ΔmTotal, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe-PEI | 74.6 | 60 | −8 | 390.5 | 34 | 30 | 547 | 179.5 | −12.7 | −71.7 |
| Cis-Fe-PEI | 74.9 | 49.5 | −8 | 362.6 | 37.4 | 25 | 567 | 166 | −14.6 | −66 |
References
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (spions) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.H.; Swanson, S.D.; Ge, S.; Cao, Z.; Van Antwerp, M.E.; Landmark, K.J.; Baker, J.R. Dendrimer functionalized shell crosslinked iron oxide nanoparticles for in vivo magnetic resonance imaging of tumors. Adv. Mater. 2008, 20, 1671–1678. [Google Scholar] [CrossRef]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cristea, C.; Tertis, M.; Galatus, R. Magnetic Nanoparticles for Antibiotics Detection. Nanomaterials 2017, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cervadoro, A.; Ramirez, M.R.; Stigliano, C.; Brazdeikis, A.; Colvin, V.L.; Civera, P.; Key, J.; Decuzzi, P. Assembly of Iron Oxide Nanocubes for Enhanced Cancer Hyperthermia and Magnetic Resonance Imaging. Nanomaterials 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Hafeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, T.; Schopf, B.; Hofmann, H.; Hofmann, M.; von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Popescu, L.M.; Piticescu, R.M.; Petriceanu, M.; Ottaviani, M.F.; Cangiotti, M.; Vasile, E.; Dîrtu, M.M.; Wolff, M.; Garcia, Y.; Schinteie, G.; et al. Hydrothermal synthesis of nanostructured hybrids based on iron oxide and branched pei polymers. Influence of high pressure on structure and morphology. Mater. Chem. Phys. 2015, 161, 84–95. [Google Scholar] [CrossRef]
- Nemati, Z.; Salili, S.M.; Alonso, J.; Ataie, A.; Das, R.; Phan, M.H.; Srikanth, H. Superparamagnetic iron oxide nanodiscs for hyperthermia therapy: Does size matter? J. Alloys Compd. 2017, 714, 709–714. [Google Scholar] [CrossRef]
- Tran, D.L.; Pham, G.D.; Nguyen, X.P.; Vu, D.H.; Nguyen, N.T.; Tran, V.H.; Trang Mai, T.T.; Nguyen, H.B.; Le, Q.D.; Nguyen, T.N.; et al. Some biomedical applications of chitosan-based hybrid nanomaterials. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 45004. [Google Scholar] [CrossRef]
- Devkota, J.; Mai, T.T.T.; Stojak, K.; Ha, P.T.; Pham, H.N.; Nguyen, X.P.; Mukherjee, P.; Srikanth, H.; Phan, M.H. Synthesis, inductive heating, and magnetoimpedance-based detection of multifunctional Fe3O4 nanoconjugates. Sens. Actuators B 2014, 190, 715–722. [Google Scholar] [CrossRef]
- Lin, B.-L.; Zhang, J.-Z.; Lu, L.-J.; Mao, J.-J.; Cao, M.-H.; Mao, X.-H.; Zhang, F.; Duan, X.-H.; Zheng, C.-S.; Zhang, L.-M.; et al. Superparamagnetic Iron Oxide Nanoparticles-Complexed Cationic Amylose for In Vivo Magnetic Resonance Imaging Tracking of Transplanted Stem Cells in Stroke. Nanomaterials 2017, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Petri-Fink, A.; Steitz, B.; Finka, A.; Salaklang, J.; Hofmann, H. Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (spions): Colloidal stability, cytotoxicity, and cellular uptake studies. Eur. J. Pharm. Biopharm. 2008, 68, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Campbell, A.; Monopoli, M.P.; Lynch, I.; Salvati, A.; Dawson, K.A. Serum heat inactivation affects protein corona composition and nanoparticle uptake. Biomaterials 2010, 31, 9511–9518. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Deriu, M.A.; Popescu, L.M.; Ottaviani, M.F.; Danani, A.; Piticescu, R.M. Iron oxide/PAMAM nanostructured hybrids: Combined computational and experimental studies. J. Mater. Sci. 2016, 51, 1996–2007. [Google Scholar] [CrossRef]
- Cai, H.; An, X.; Cui, J.; Li, J.; Wen, S.; Li, K.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Facile hydrothermal synthesis and surface functionalization of polyethyleneimine-coatediron oxide nanoparticles for biomedical applications. ACS Appl. Mater. Interfaces 2013, 5, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Piticescu, R.M.; Appelhans, D.; Schöneich, M.; Meyer, M.; Burlacu, A.; Preda, B.; Schinteie, G.; Kuncser, V.; Vasile, E. High pressure hydrothermal procedure: A tool for surface modification of super-paramagnetic nanostructured materials for medical applications. In Hybrid Organic-Inorganic Interfaces—Towards Advanced Functional Materials; Marie, H.D., Andreas, T., Eds.; Wiley-VCH Verlag GmBH: Weinheim, Germany, 2017; Volume 2, pp. 695–727. ISBN 978-3-527-34255-6. [Google Scholar]
- Pangon, A.; Tashiro, K.; Chirachanchai, S. Polyethylenimine containing benzimidazole branching: A model system providing a balance of hydrogen bond network or chain mobility enhances proton conductivity. J. Phys. Chem. B 2011, 115, 11359–11367. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, C.; Pietzonka, C.; Heverhagen, J.; Kissel, T. Novel magnetic iron oxide nanoparticles coated with poly(ethylene imine)-g-poly(ethylene glycol) for potential biomedical application: Synthesis, stability, cytotoxicity and MR imaging. Int. J. Pharm. 2011, 15, 130–137. [Google Scholar] [CrossRef] [PubMed]
- El Ghandoor, H.; Zidan, H.M.; Khalil, M.M.H.; Ismail, M.I.M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Rosca, A.M.; Matei, C.; Dragan, E.; Burlacu, A. Cardiomyocyte apoptosis in ischaemia-reperfusion due to the exogenous oxidants at the time of reperfusion. Cell Biol. Int. 2012, 36, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, R.; Ozawa, T.; Dinca, E.B.; Banerjee, A.; Prados, M.D.; James, C.D.; Gupta, N. A human brainstem glioma xenograft model enabled for bioluminescence imaging. J. Neurooncol. 2010, 96, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Steitz, B.; Hofmann, H.; Kamau, S.W.; Hassa, P.O.; Hottiger, M.O.; von Rechenberg, B.; Hofmann-Amtenbrink, M.; Petri-Fink, A. Characterization of PEI-coated superparamagnetic iron oxide nanoparticles for transfection: Size distribution, colloidal properties and DNA interaction. J. Magn. Magn. Mater. 2007, 311, 300–305. [Google Scholar] [CrossRef]
- Do, M.A.; Yoon, G.J.; Yeum, J.H.; Han, M.; Chang, Y.; Choi, J.H. Polyethyleneimine-mediate synthesis of superparamagnetic iron oxide nanoparticles with enhanced sensitivity in T2 magnetic resonance imaging. Colloids Surf. B Biointerfaces 2014, 122, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Al-Deen, F.M.; Xiang, S.D.; Ma, C.; Wilson, K.; Coppel, R.L.; Selomulya, C.; Plebanski, M. Magnetic nanovectors for the development of DNA blood-stage malaria vaccines. Nanomaterials 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Rinaldi-Montes, N.; Alonso, J.; Amghouz, Z.; Garaio, E.; García, J.A.; Gorria, P.; Blanco, J.A.; Phan, M.H.; Srikanth, H. Boosted Hyperthermia Therapy by Combined AC Magnetic and Photothermal Exposures in Ag/Fe3O4 Nanoflowers. ACS Appl. Mater. Interfaces 2016, 8, 25162–25169. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Alonso, J.; Porshokouh, Z.N.; Kalappattil, V.; Torres, D.; Phan, M.-H.; Garaio, E.; García, J.Á.; Sanchez Llamazares, J.L.; Srikanthmmm, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.M.; Khurshid, H.; Garaio, E.; Garcia, J.A.; Phan, M.H.; Srikanth, H. Enhanced Magnetic Hyperthermia in Iron Oxide Nano-Octopods: Size and Anisotropy Effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Rosca, A.M.; Burlacu, A. Effect of 5-azacytidine: Evidence for alteration of the multipotent ability of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.B.; Ronningen, T.; Burlacu, A.; Simionescu, M.; Moskaug, J.O.; Valen, G. Remote transplantation of mesenchymal stem cells protects the heart against ischemia-reperfusion injury. Stem Cells 2014, 32, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Odent Grigorescu, G.; Preda, M.B.; Radu, E.; Rosca, A.M.; Tutuianu, R.; Mitroi, D.N.; Simionescu, M.; Burlacu, A. Combinatorial approach for improving the outcome of angiogenic therapy in ischemic tissues. Biomaterials 2015, 60, 72–81. [Google Scholar] [CrossRef] [PubMed]

| Sample Name | Iron Oxide:PEI Mass Ratio | CisplatinPresence |
|---|---|---|
| Fe-PEI | 0.5 | no |
| Cis-Fe-PEI | 0.5 | Incorporated before hydrothermal synthesis |
| Fe-PEI+Cis | 0.5 | Incorporated after hydrothermal synthesis |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutuianu, R.; Popescu, L.M.; Preda, M.B.; Rosca, A.-M.; Piticescu, R.M.; Burlacu, A. Evaluation of the Ability of Nanostructured PEI-Coated Iron Oxide Nanoparticles to Incorporate Cisplatin during Synthesis. Nanomaterials 2017, 7, 314. https://doi.org/10.3390/nano7100314
Tutuianu R, Popescu LM, Preda MB, Rosca A-M, Piticescu RM, Burlacu A. Evaluation of the Ability of Nanostructured PEI-Coated Iron Oxide Nanoparticles to Incorporate Cisplatin during Synthesis. Nanomaterials. 2017; 7(10):314. https://doi.org/10.3390/nano7100314
Chicago/Turabian StyleTutuianu, Raluca, Laura Madalina Popescu, Mihai Bogdan Preda, Ana-Maria Rosca, Roxana Mioara Piticescu, and Alexandrina Burlacu. 2017. "Evaluation of the Ability of Nanostructured PEI-Coated Iron Oxide Nanoparticles to Incorporate Cisplatin during Synthesis" Nanomaterials 7, no. 10: 314. https://doi.org/10.3390/nano7100314