Regulation of the Electroanalytical Performance of Ultrathin Titanium Dioxide Nanosheets toward Lead Ions by Non-Metal Doping
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Pure and Non-Metal Doped Ultrathin 2D TiO2 Nanosheets
2.3. Electrode Fabrication
2.4. Materials Characterization Techniques
2.5. Electrochemical Experiments
2.6. Computational Details
3. Results and Discussion
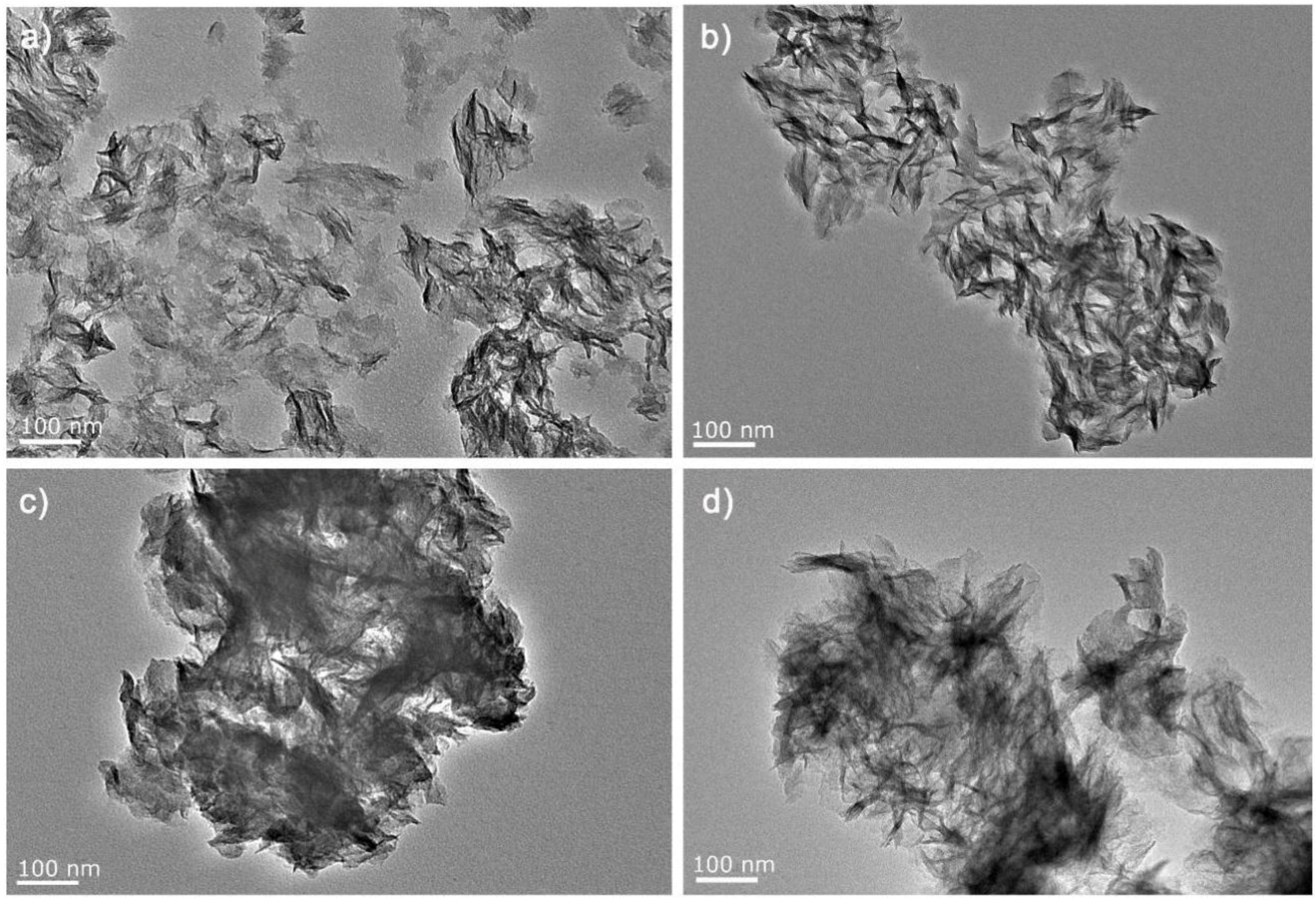
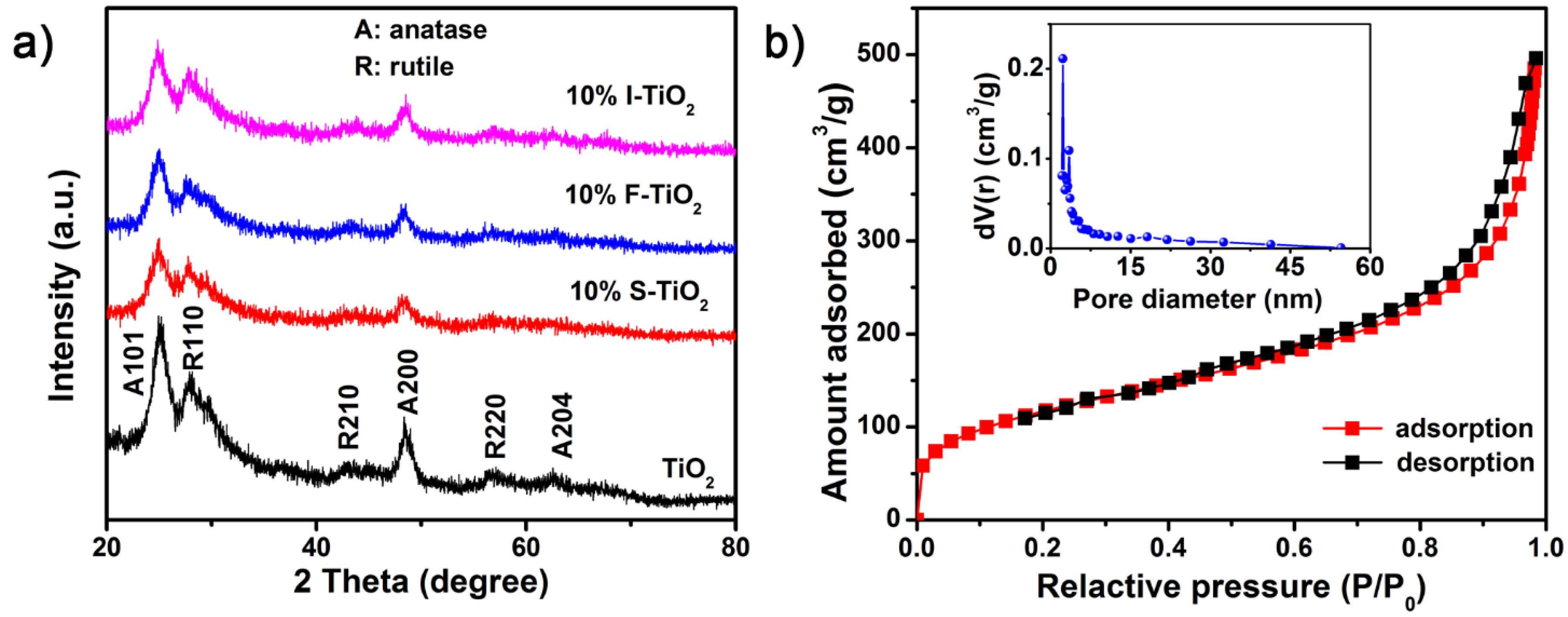
3.1. Material Characterization
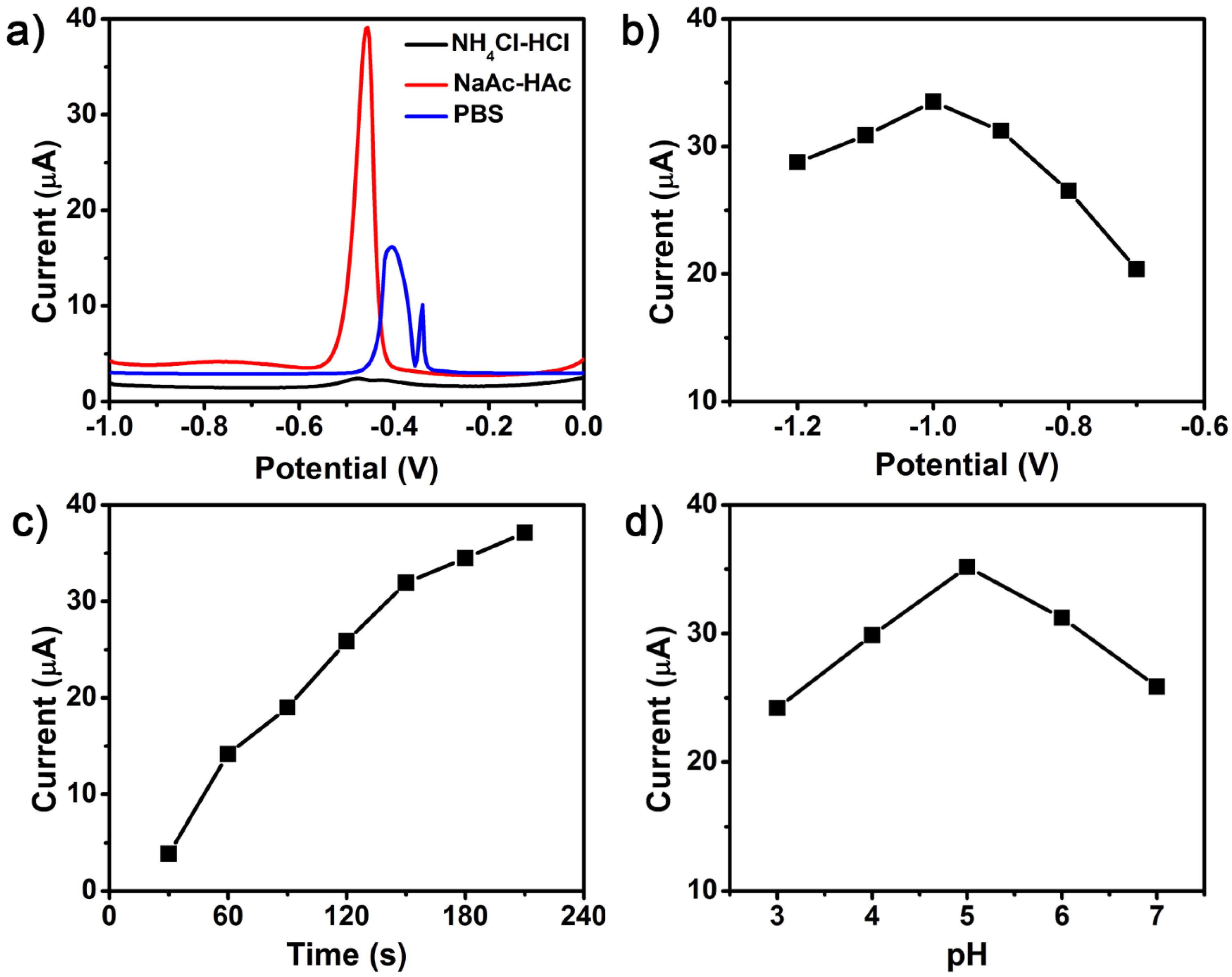
3.2. Experimental Parameters Optimization
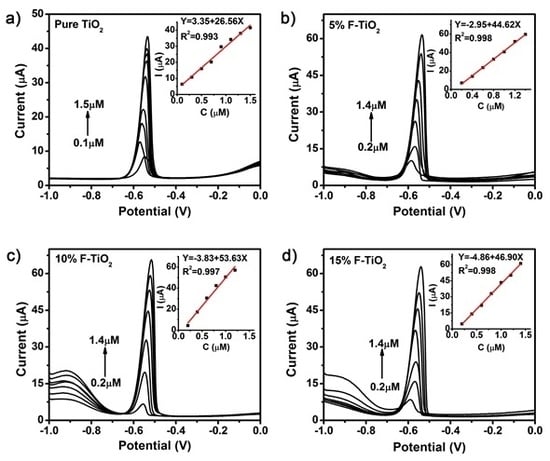
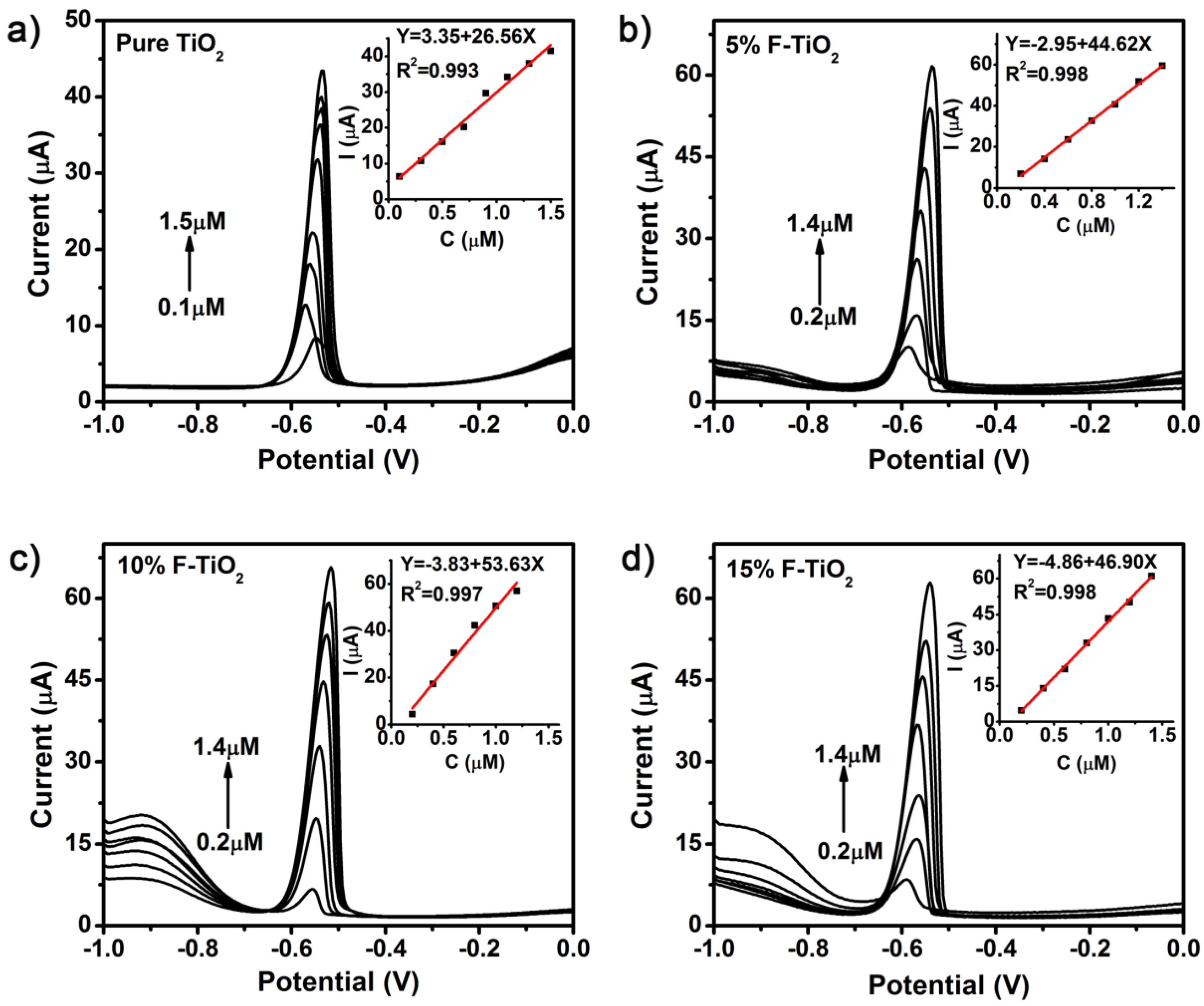
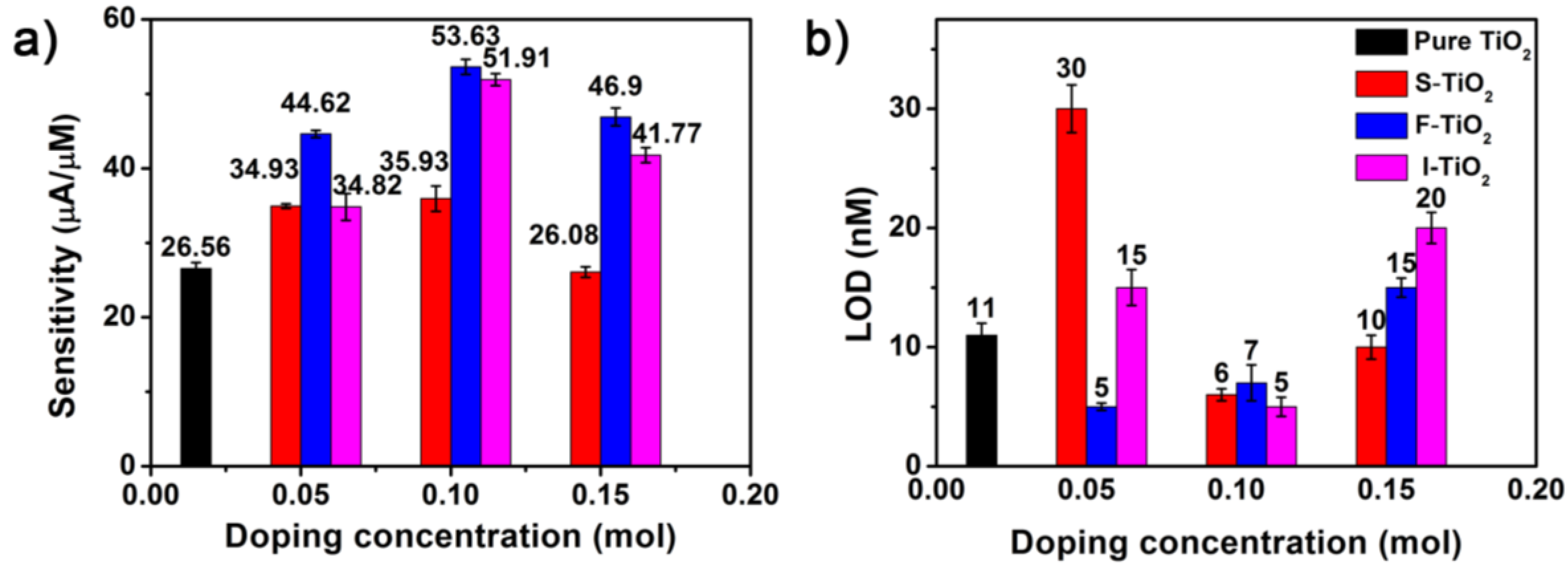
3.3. Comparison of Electroanalytical Performance of Non-Metal Doped TiO2 NSs
3.4. Study on the Reasons for Improved Electrochemical Properties of F-Doped TiO2
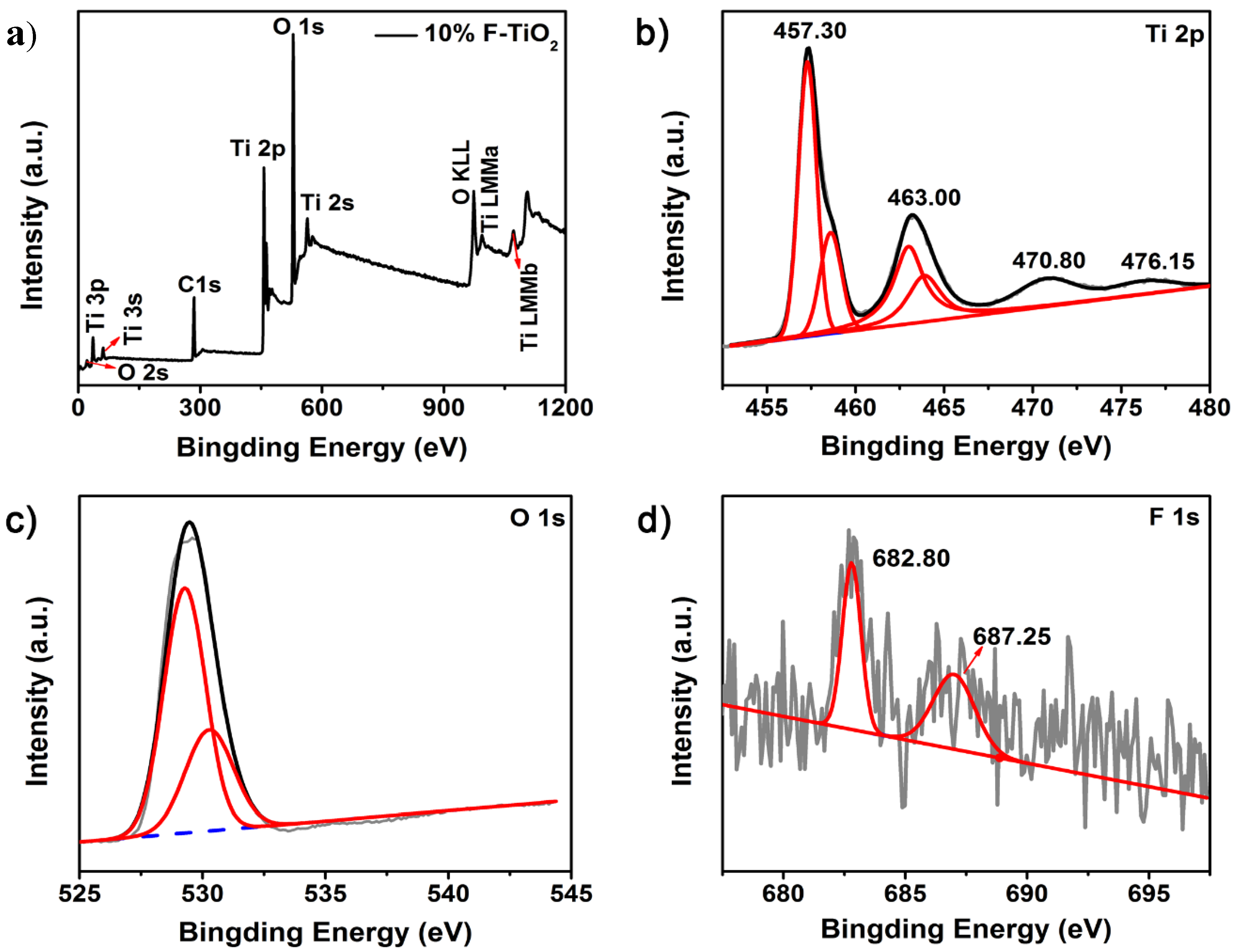
3.4.1. Surface Chemical States Investigation
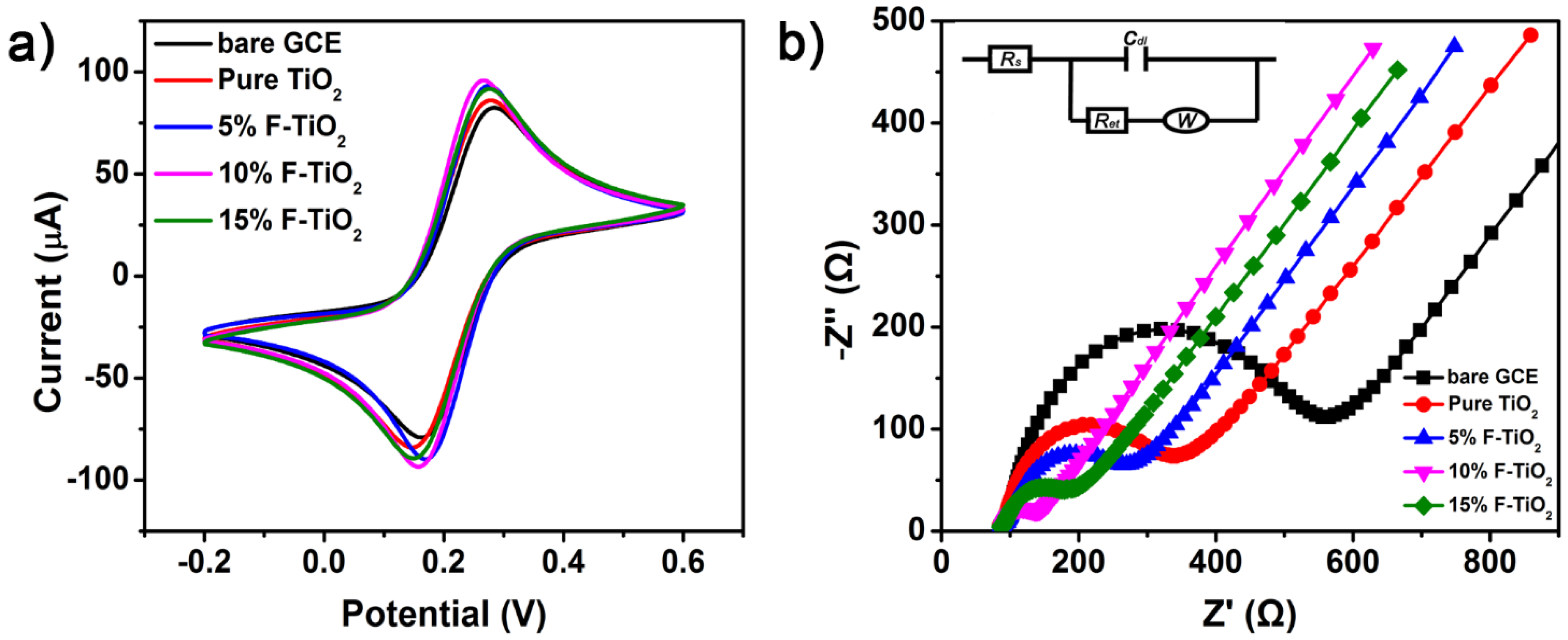
3.4.2. Further Electrochemical Characterization
3.4.3. Theoretical Calculations
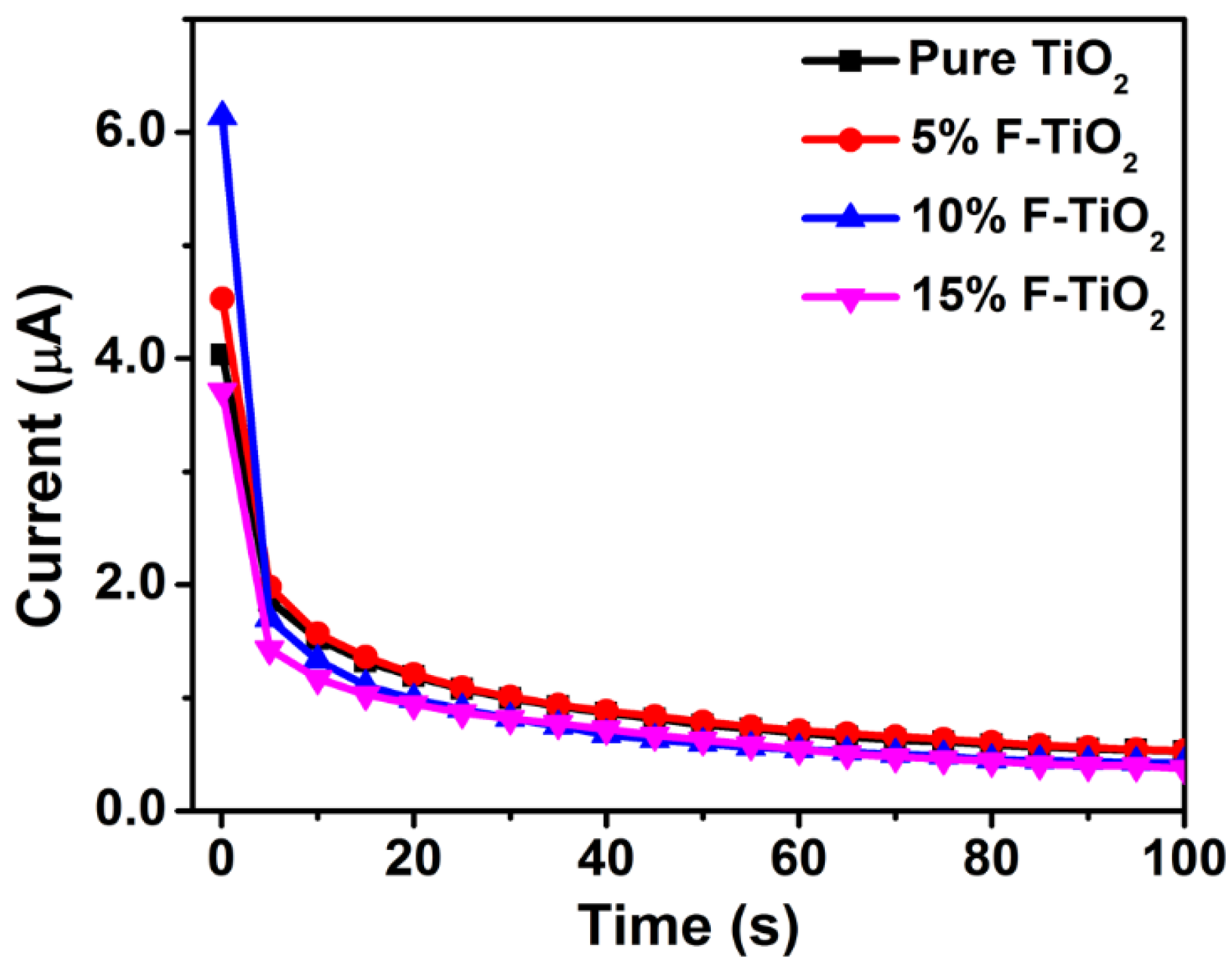
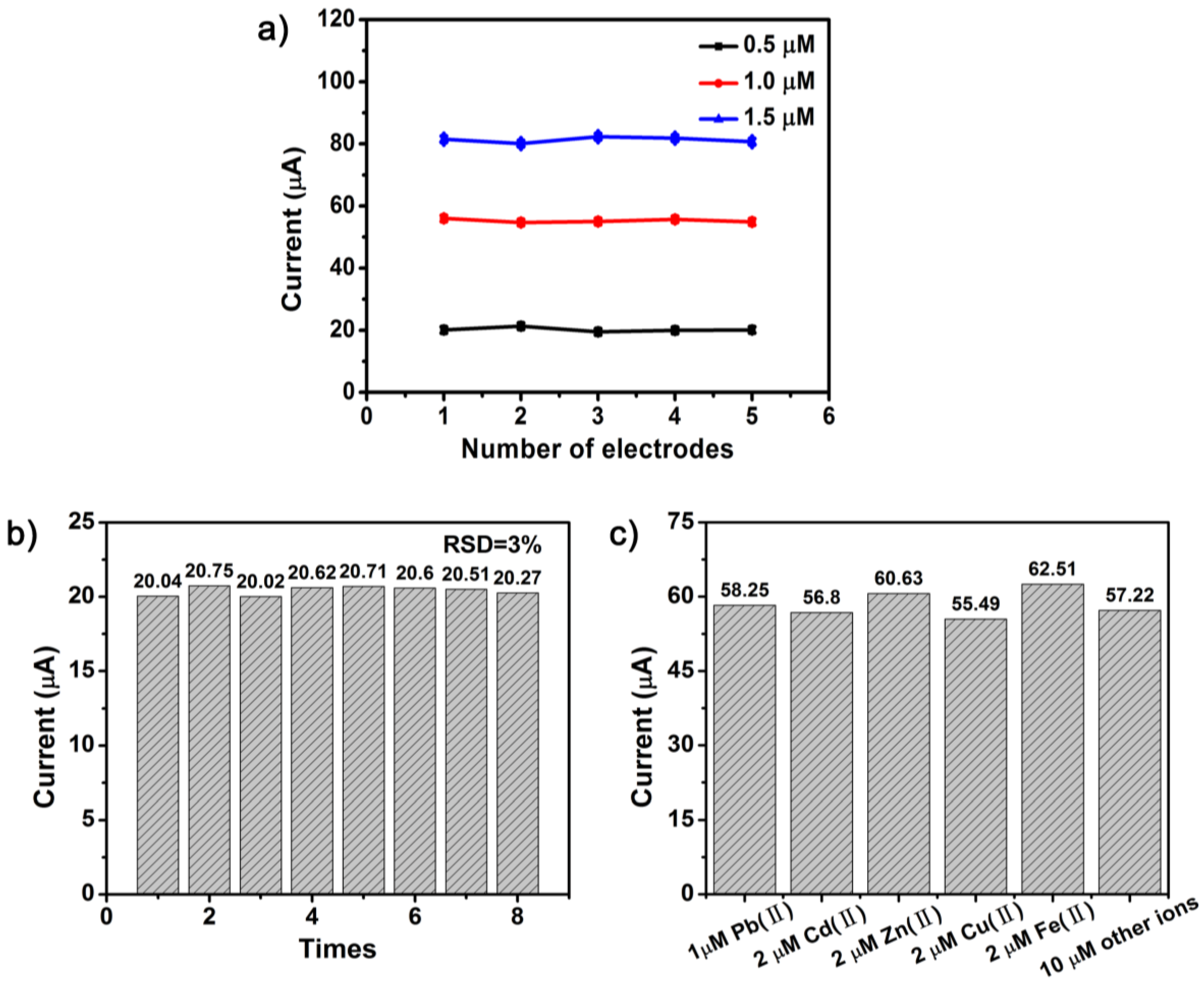
3.4.4. Stability and Interference Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yao, X.Z.; Guo, Z.; Yuan, Q.H.; Liu, Z.G.; Liu, J.H.; Huang, X.J. Exploiting differential electrochemical stripping behaviors of Fe3O4 nanocrystals toward heavy metal ions by crystal cutting. ACS Appl. Mater. Interfaces 2014, 6, 12203–12213. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duan, G.; Li, Y.; Liu, G.; Wang, J.; Zhang, H.; Dai, Z.; Cai, W. Fabrication of Gold Nanoparticles by Laser Ablation in Liquid and Their Application for Simultaneous Electrochemical Detection of Cd2+, Pb2+, Cu2+, Hg2+. ACS Appl. Mater. Interfaces 2014, 6, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P. Determination of metal content in honey by atomic absorption and emission spectrometries. TrAC Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Liu, H.-W.; Jiang, S.-J.; Liu, S.-H. Determination of cadmium, mercury and lead in seawater by electrothermal vaporization isotope dilution inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1999, 54, 1367–1375. [Google Scholar] [CrossRef]
- Wan, Z.; Xu, Z.; Wang, J. Flow injection on-line solid phase extraction for ultra-trace lead screening with hydride generation atomic fluorescence spectrometry. Analyst 2006, 131, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, M.D.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Direct determination of toxic trace metals in honey and sugars using inductively coupled plasma atomic emission spectrometry. Talanta 2005, 65, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Agnes, M.; Nitti, A.; Vander Griend, D.A.; Dondi, D.; Merli, D.; Pasini, D. A chiroptical molecular sensor for ferrocene. Chem. Commun. 2016, 52, 11492–11495. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Leza, N.J.; Roy, K.; Dondi, D.; Gattuso, G.; Shimizu, L.S.; Pasini, D. A chiroptical probe for sensing metal ions in water. Eur. J. Org. Chem. 2013, 2013, 6078–6083. [Google Scholar] [CrossRef]
- Economou, A.; Fielden, P.R. Selective determination of Ni(II) and Co(II) by flow injection analysis and adsorptive cathodic stripping voltammetry on a wall jet mercury film electrode. Talanta 1998, 46, 1137–1146. [Google Scholar] [CrossRef]
- Wang, J. Stripping analysis at bismuth electrodes: A review. Electroanalysis 2005, 17, 1341–1346. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Hočevar, S.B.; Ogorevc, B.; Wang, J. Characterization and applications of a bismuth bulk electrode. Electroanalysis 2004, 16, 719–723. [Google Scholar] [CrossRef]
- Guzsvány, V.; Nakajima, H.; Soh, N.; Nakano, K.; Imato, T. Antimony-film electrode for the determination of trace metals by sequential-injection analysis/anodic stripping voltammetry. Anal. Chim. Acta 2010, 658, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, H.; Fu, C.; Wang, F.; Ding, Y. Sensors and Actuators B: Chemical A novel design of engineered multi-walled carbon nanotubes material and its improved performance in simultaneous detection of Cd (II) and Pb (II) by square wave anodic stripping voltammetry. Sens. Actuators B. Chem. 2016, 236, 144–152. [Google Scholar] [CrossRef]
- Zhao, D.; Guo, X.; Wang, T.; Alvarez, N.; Shanov, V.N.; Heineman, W.R. Simultaneous Detection of Heavy Metals by Anodic Stripping Voltammetry Using Carbon Nanotube Thread. Electroanalysis 2014, 26, 488–496. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Tang, L.; Zeng, G.; Zhang, J.; Peng, B.; Xie, X.; Lai, C.; Long, B.; Zhu, J. Determination of Cd2+ and Pb2+ Based on Mesoporous Carbon Nitride/Self-Doped Polyaniline Nanofibers and Square Wave Anodic Stripping Voltammetry. Nanomaterials 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Renedo, O.D.; Martínez, M.J.A. A novel method for the anodic stripping voltammetry determination of Sb(III) using silver nanoparticle-modified screen-printed electrodes. Electrochem. Commun. 2007, 9, 820–826. [Google Scholar] [CrossRef]
- Liu, Z.G.; Sun, Y.F.; Chen, W.K.; Kong, Y.; Jin, Z.; Chen, X.; Zheng, X.; Liu, J.H.; Huang, X.J.; Yu, S.H. Facet-Dependent Stripping Behavior of Cu2O Microcrystals Toward Lead Ions: A Rational Design for the Determination of Lead Ions. Small 2015, 11, 2493–2498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Chen, X.; Liu, J.H.; Huang, X.J. Well-arranged porous Co3O4 microsheets for electrochemistry of Pb(II) revealed by stripping voltammetry. Electrochem. Commun. 2013, 30, 59–62. [Google Scholar] [CrossRef]
- Gong, T.Z.J.; Song, D.; Zhang, L.; Hu, X. Stripping Voltammetric Detection of Mercury(II) Based on a Bimetallic Au-Pt Inorgani-Organic Hybrid Nanocomposite Modified Glassy Carbon Electrode. Anal. Chem. 2010, 82, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.K.; Raj, C.R. Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal. Chem. 2008, 80, 4836–4844. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Jin, J.; Wang, C.; Sun, Y.; Zhang, Y.; Liu, Y. Ag Nanopaticles-Modified 3D Graphene Foam for Binder-Free Electrodes of Electrochemical Sensors. Nanomaterials 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liao, T.; Dou, Y.; Hwang, S.M.; Park, M.-S.; Jiang, L.; Kim, J.H.; Dou, S.X. Generalized self-assembly of scalable two-dimensional transition metal oxide nanosheets. Nat. Commun. 2014, 5, 3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Huang, H.; Kubota, M.; He, Z.; Kobayashi, N.; Zhou, X.; Jin, B.; Luo, J. Synergetic effect of MoS2 and g-C3N4 as cocatalysts for enhanced photocatalytic H2 production activity of TiO2. Mater. Res. Bull. 2016, 76, 79–84. [Google Scholar] [CrossRef]
- Chen, C.; Wen, Y.; Hu, X.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan, B.; Huang, Y. Na+ intercalation pseudocapacitance in graphene-coupled titanium oxide enabling ultra-fast sodium storage and long-term cycling. Nat. Commun. 2015, 6, 6929. [Google Scholar] [CrossRef] [PubMed]
- Hoshide, T.; Zheng, Y.; Hou, J.; Wang, Z.; Li, Q.; Zhao, Z.; Ma, R.; Sasaki, T.; Geng, F. Flexible Lithium-Ion Fiber Battery by the Regular Stacking of Two-Dimensional Titanium Oxide Nanosheets Hybridized with Reduced Graphene Oxide. Nano Lett. 2017, 17, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Asahi, A.R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y.; Lee, V.; Lee, M. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2016, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal. A Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, D.; Jiang, Z.; Li, J. Visible-light photocatalytic regeneration of NADH using P-doped TiO2 nanoparticles. J. Mol. Catal. B Enzym. 2006, 43, 44–48. [Google Scholar] [CrossRef]
- Han, T.; Fan, T.; Chow, S.K.; Zhang, D. Biogenic N-P-codoped TiO2: Synthesis, characterization and photocatalytic properties. Bioresour. Technol. 2010, 101, 6829–6835. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 1994, 6, 8245–8257. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Search, H.; Journals, C.; Contact, A.; Iopscience, M.; Address, I.P. Ab initio Force Constant Approach to Phonon Dispersion Relations of Diamond and Graphite. Europhys. Lett. 1995, 32, 729. [Google Scholar]
- Qiao, X.-Q.; Zhang, Z.-W.; Tian, F.-Y.; Hou, D.-F.; Tian, Z.-F.; Li, D.-S.; Zhang, Q. Enhanced Catalytic Reduction of p-Nitrophenol on Ultrathin MoS2 Nanosheets Decorated with Noble Metal Nanoparticles. Cryst. Growth Des. 2017, 17, 3538–3547. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, Y.; Qin, R.; Mo, S.; Chen, G.; Gu, L.; Chevrier, D.M.; Zhang, P.; Guo, Q.; Zang, D.; et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 2016, 352, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Liao, T.; Ma, Z.; Tian, D.; Liu, Q.; Xiao, F.; Sun, Z.; Ho Kim, J.; Xue Dou, S. Graphene-like holey Co3O4 nanosheets as a highly efficient catalyst for oxygen evolution reaction. Nano Energy 2016, 30, 267–275. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Xu, H.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y. Green synthetic approach for Ti3+ self-doped TiO(2-x) nanoparticles with efficient visible light photocatalytic activity. Nanoscale 2013, 5, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wu, J.; Ju, H. Nitrogen-Doped Porous Carbon Derived from Metal-Organic Gel for Electrochemical Analysis of Heavy-Metal Ion. ACS Appl. Mater. Interfaces 2014, 6, 16210–16216. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, H.; Zhou, S.; Song, T.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Electrochimica Acta Simultaneous detection of Cd (II) and Pb (II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/Nafion/bismuth-film electrode. Electrochim. Acta 2014, 143, 143–151. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, C.; Meng, F.; Li, H.; Wang, L.; Liu, J.; Huang, X. SnO2/Reduced Graphene Oxide Nanocomposite for the Simultaneous Electrochemical Detection of Cadmium (II), Lead (II), Copper (II), and Mercury (II): An Interesting Favorable Mutual Interference. J. Phys. Chem. C 2012, 116, 1034–1041. [Google Scholar] [CrossRef]
- Zeng, A.; Liu, E.; Tan, S.N.; Zhang, S.; Gao, J. Stripping Voltammetric Analysis of Heavy Metals at Nitrogen Doped Diamond-Like Carbon Film Electrodes. Electroanalysis 2002, 14, 1294–1298. [Google Scholar]
- Cerutti, S.; Silva, M.F.; Gásquez, J.A.; Olsina, R.A.; Martinez, L.D. On-line preconcentration/determination of cadmium in drinking water on activated carbon using 8-hydroxyquinoline in a flow injection system coupled to an inductively coupled plasma optical emission spectrometer. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 43–50. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Lin, X.; Rong, F.; Fu, D.; Yuan, C. Enhanced photocatalytic activity of fluorine doped TiO2 by loaded with Ag for degradation of organic pollutants. Powder Technol. 2012, 219, 173–178. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, Z.; Asif, M.; Wang, H.; Yu, Y.; Hu, Y.; Liu, H.; Xiao, F. Inkjet Printing Synthesis of Sandwiched Structured Ionic Liquid-Carbon Nanotube-Graphene Film: Toward Disposable Electrode for Sensitive Heavy Metal Detection in Environmental Water Samples. Ind. Eng. Chem. Res. 2017, 56, 1696–1703. [Google Scholar] [CrossRef]
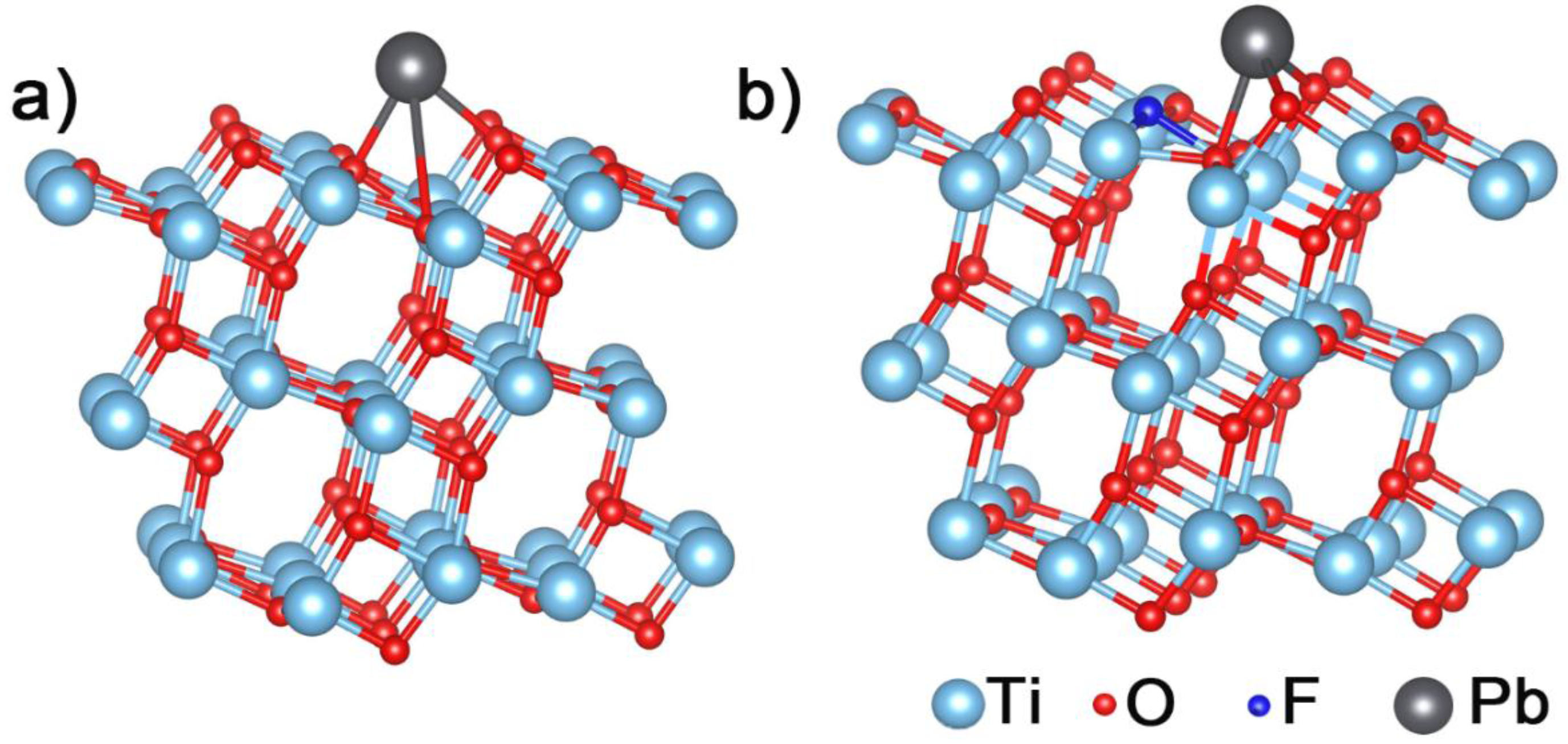
| Cyrstal Surface | Bond Length (Å) | Ead (eV) |
|---|---|---|
| TiO2-101 | Pb–O 2.28 2.34 3.42 | −2.2361 |
| TiO2(F)-101 | Pb–O 2.22 2.23 2.40 | −2.6517 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liao, J.; Yang, F.; Xu, M.; Lin, S. Regulation of the Electroanalytical Performance of Ultrathin Titanium Dioxide Nanosheets toward Lead Ions by Non-Metal Doping. Nanomaterials 2017, 7, 327. https://doi.org/10.3390/nano7100327
Zhang J, Liao J, Yang F, Xu M, Lin S. Regulation of the Electroanalytical Performance of Ultrathin Titanium Dioxide Nanosheets toward Lead Ions by Non-Metal Doping. Nanomaterials. 2017; 7(10):327. https://doi.org/10.3390/nano7100327
Chicago/Turabian StyleZhang, Junping, Jianjun Liao, Fan Yang, Ming Xu, and Shiwei Lin. 2017. "Regulation of the Electroanalytical Performance of Ultrathin Titanium Dioxide Nanosheets toward Lead Ions by Non-Metal Doping" Nanomaterials 7, no. 10: 327. https://doi.org/10.3390/nano7100327