Microstructuring of Mesoporous Titania Films Loaded with Silver Salts to Enhance the Photocatalytic Degradation of Methyl Blue under Visible Light
Abstract
:1. Introduction
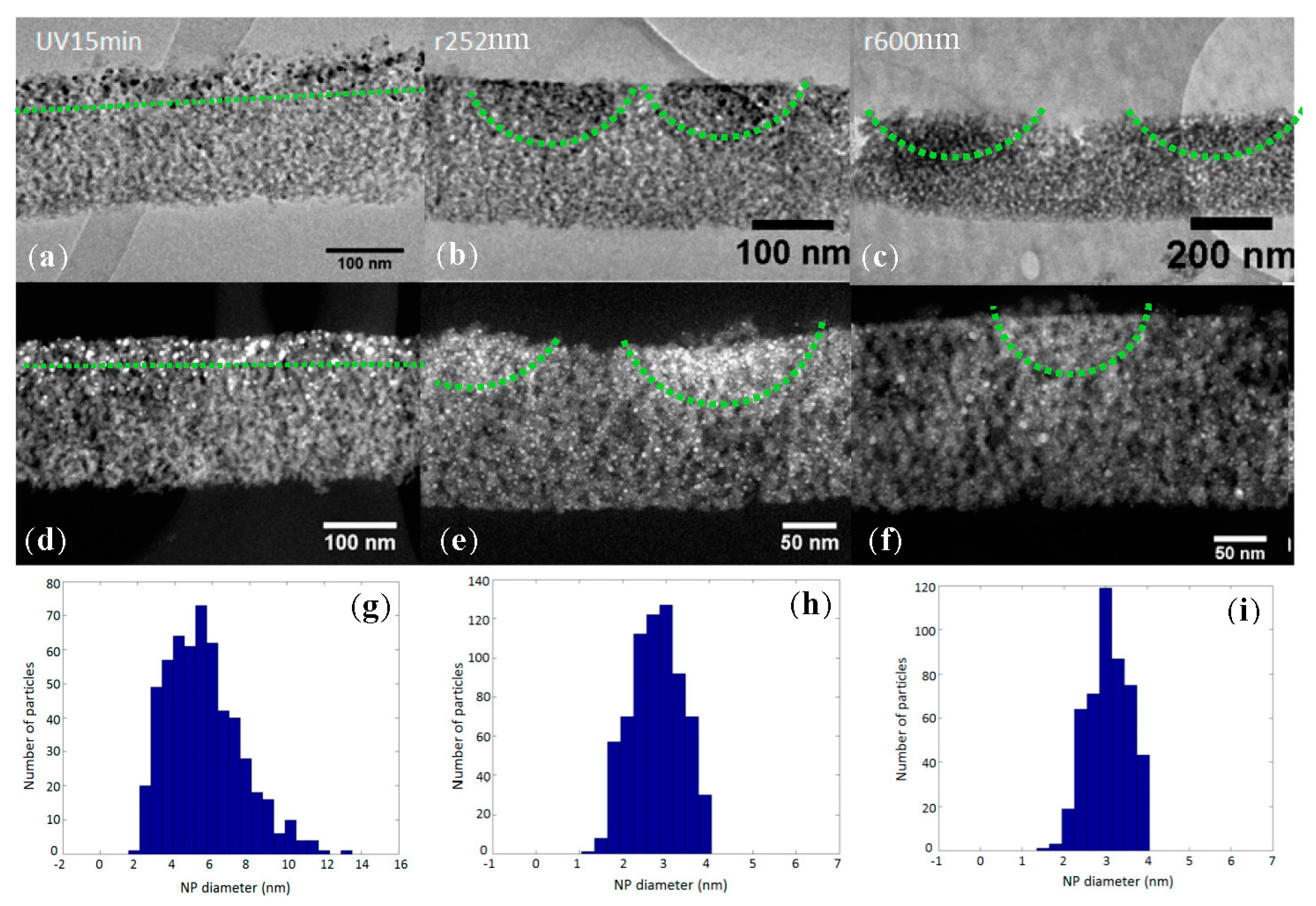
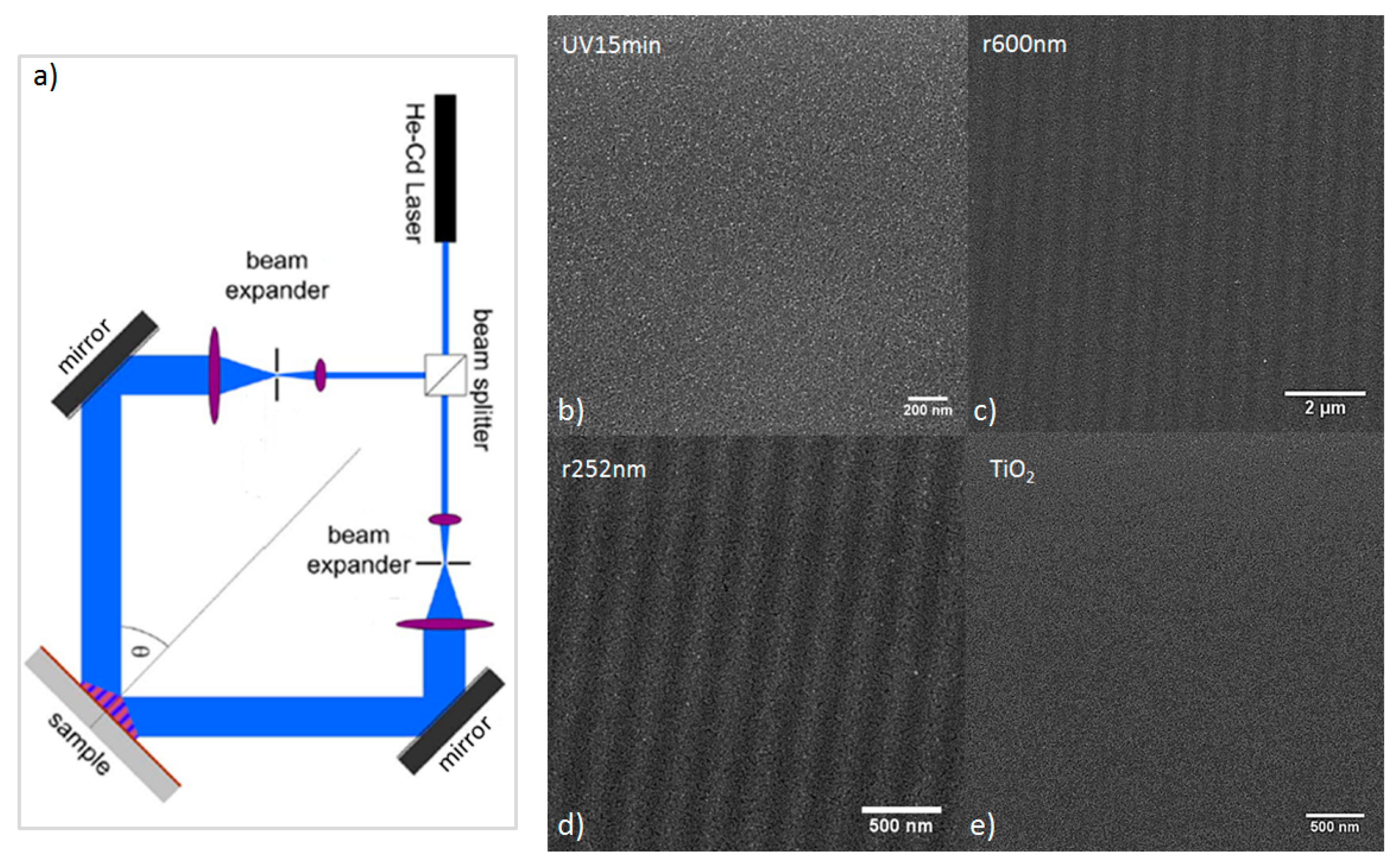
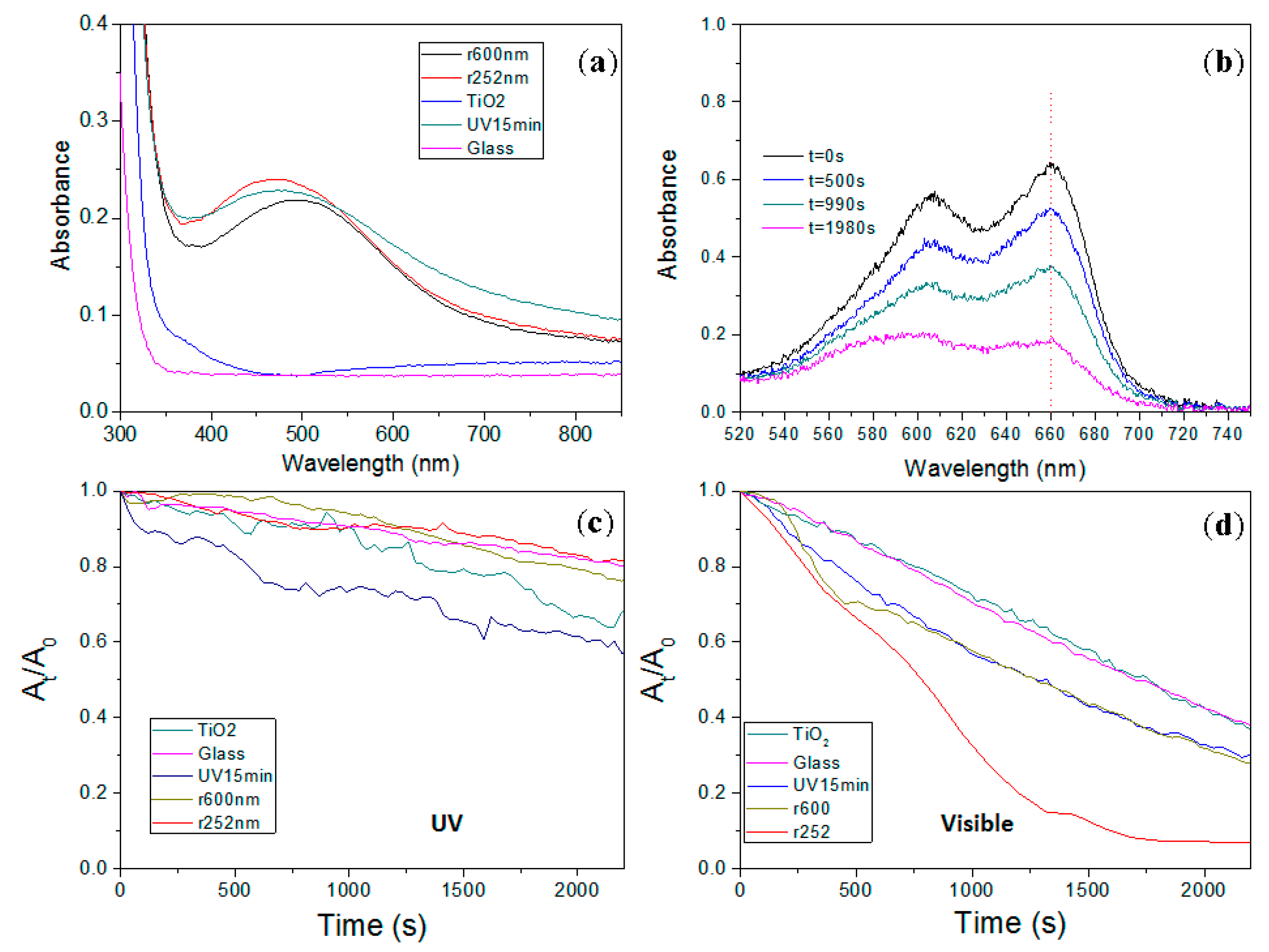
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Grabowska, E.; Zaleska, A. Noble metal modified TiO2 for photocatalytic air purification. Physicochem. Probl. Miner. Process. 2015, 51, 49–57. [Google Scholar]
- Ramírez, R.J.; Arellano, C.A.P.; Varia, J.; Martínez, S.S. Visible light-induced photocatalytic elimination of organic pollutants by TiO2: A review. Curr. Org. Chem. 2015, 19, 540–555. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Jalil, A.A.; Triwahyono, S. Visible-light photoactivity of plasmonic silver supported on mesoporous TiO2 nanoparticles (Ag-MTN) for enhanced degradation of 2-chlorophenol: Limitation of Ag-Ti interaction. Appl. Surf. Sci. 2017, 392, 1068–1077. [Google Scholar] [CrossRef]
- Kowalska, E.; Rau, S.; Ohtani, B.; Kowalska, E.; Rau, S.; Ohtani, B. Plasmonic Titania Photocatalysts Active under UV and Visible-Light Irradiation: Influence of Gold Amount, Size, and Shape. J. Nanotechnol. 2012, 2012, 361853. [Google Scholar] [CrossRef]
- Banerjee, A.N. The design, fabrication, and photocatalytic utility of nanostructured semiconductors: Focus on TiO2-based nanostructures. Nanotechnol. Sci. Appl. 2011, 4, 35–65. [Google Scholar] [CrossRef] [PubMed]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Crespo-Monteiro, N.; Destouches, N.; Bois, L.; Chassagneux, F.; Reynaud, S.; Fournel, T. Reversible and Irreversible Laser Microinscription on Silver-Containing Mesoporous Titania Films. Adv. Mater. 2010, 22, 3166–3170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Epicier, T.; Lefkir, Y.; Vitrant, G.; Destouches, N. HAADF-STEM characterization and simulation of nanoparticle distributions in an inhomogeneous matrix. J. Microsc. 2017, 266, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Monteiro, N.; Destouches, N.; Epicier, T.; Balan, L.; Vocanson, F.; Lefkir, Y.; Michalon, J.-Y. Changes in the Chemical and Structural Properties of Nanocomposite Ag: TiO2 Films during Photochromic Transitions. J. Phys. Chem. C 2014, 118, 24055–24061. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.C.; Yip, H.Y.; Li, Q.; Kwong, K.W.; Xu, A.-W.; Wong, P.K. Ambient Light Reduction Strategy to Synthesize Silver Nanoparticles and Silver-Coated TiO2 with Enhanced Photocatalytic and Bactericidal Activities. Langmuir 2003, 19, 10372–10380. [Google Scholar] [CrossRef]
- Sung-Suh, H.M.; Choi, J.R.; Hah, H.J.; Koo, S.M.; Bae, Y.C. Comparison of Ag deposition effects on the photocatalytic activity of nanoparticulate TiO2 under visible and UV light irradiation. J. Photochem. Photobiol. Chem. 2004, 163, 37–44. [Google Scholar] [CrossRef]
- Nadar, L.; Sayah, R.; Vocanson, F.; Crespo-Monteiro, N.; Boukenter, A.; Joao, S.S.; Destouches, N. Influence of reduction processes on the colour and photochromism of amorphous mesoporous TiO2 thin films loaded with a silver salt. Photochem. Photobiol. Sci. 2011, 10, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Monteiro, N.; Cazier, A.; Vocanson, F.; Lefkir, Y.; Reynaud, S.; Michalon, J.-Y.; Kämpfe, T.; Destouches, N.; Jourlin, Y. Microstructuring of Mesoporous Titania Films Loaded with Silver Salts to Enhance the Photocatalytic Degradation of Methyl Blue under Visible Light. Nanomaterials 2017, 7, 334. https://doi.org/10.3390/nano7100334
Crespo-Monteiro N, Cazier A, Vocanson F, Lefkir Y, Reynaud S, Michalon J-Y, Kämpfe T, Destouches N, Jourlin Y. Microstructuring of Mesoporous Titania Films Loaded with Silver Salts to Enhance the Photocatalytic Degradation of Methyl Blue under Visible Light. Nanomaterials. 2017; 7(10):334. https://doi.org/10.3390/nano7100334
Chicago/Turabian StyleCrespo-Monteiro, Nicolas, Anthony Cazier, Francis Vocanson, Yaya Lefkir, Stéphanie Reynaud, Jean-Yves Michalon, Thomas Kämpfe, Nathalie Destouches, and Yves Jourlin. 2017. "Microstructuring of Mesoporous Titania Films Loaded with Silver Salts to Enhance the Photocatalytic Degradation of Methyl Blue under Visible Light" Nanomaterials 7, no. 10: 334. https://doi.org/10.3390/nano7100334



