Advances in Single-Chain Nanoparticles for Catalysis Applications
Abstract
:1. Introduction
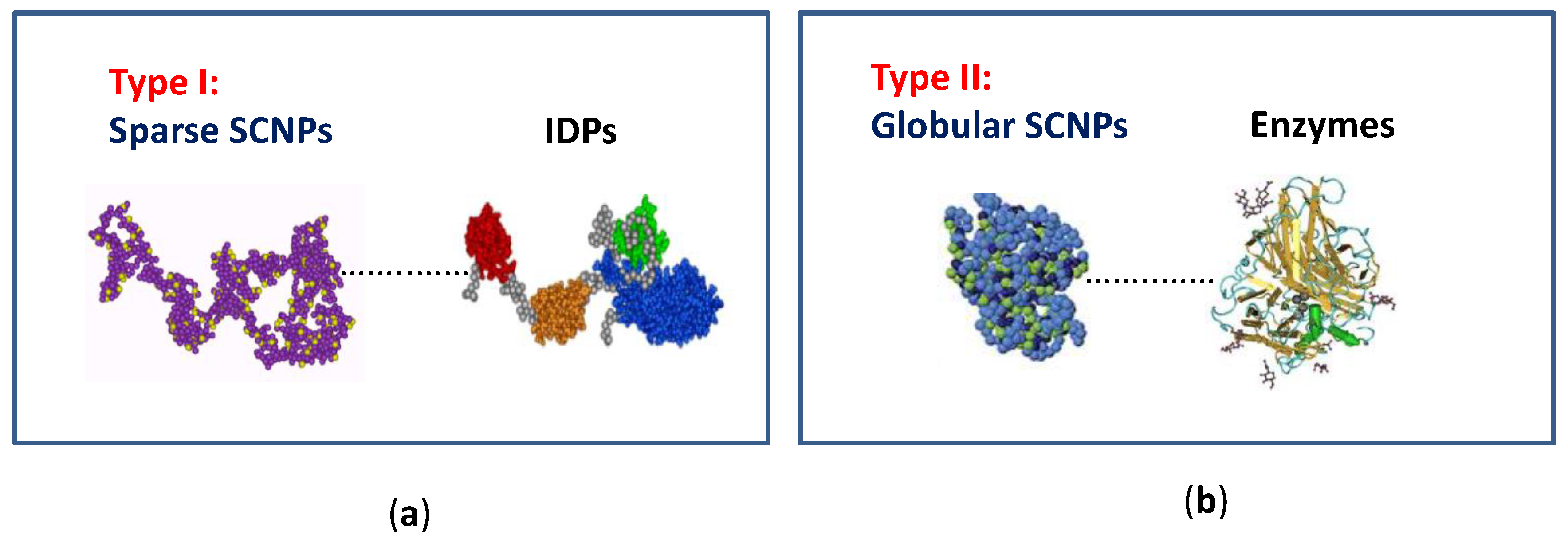
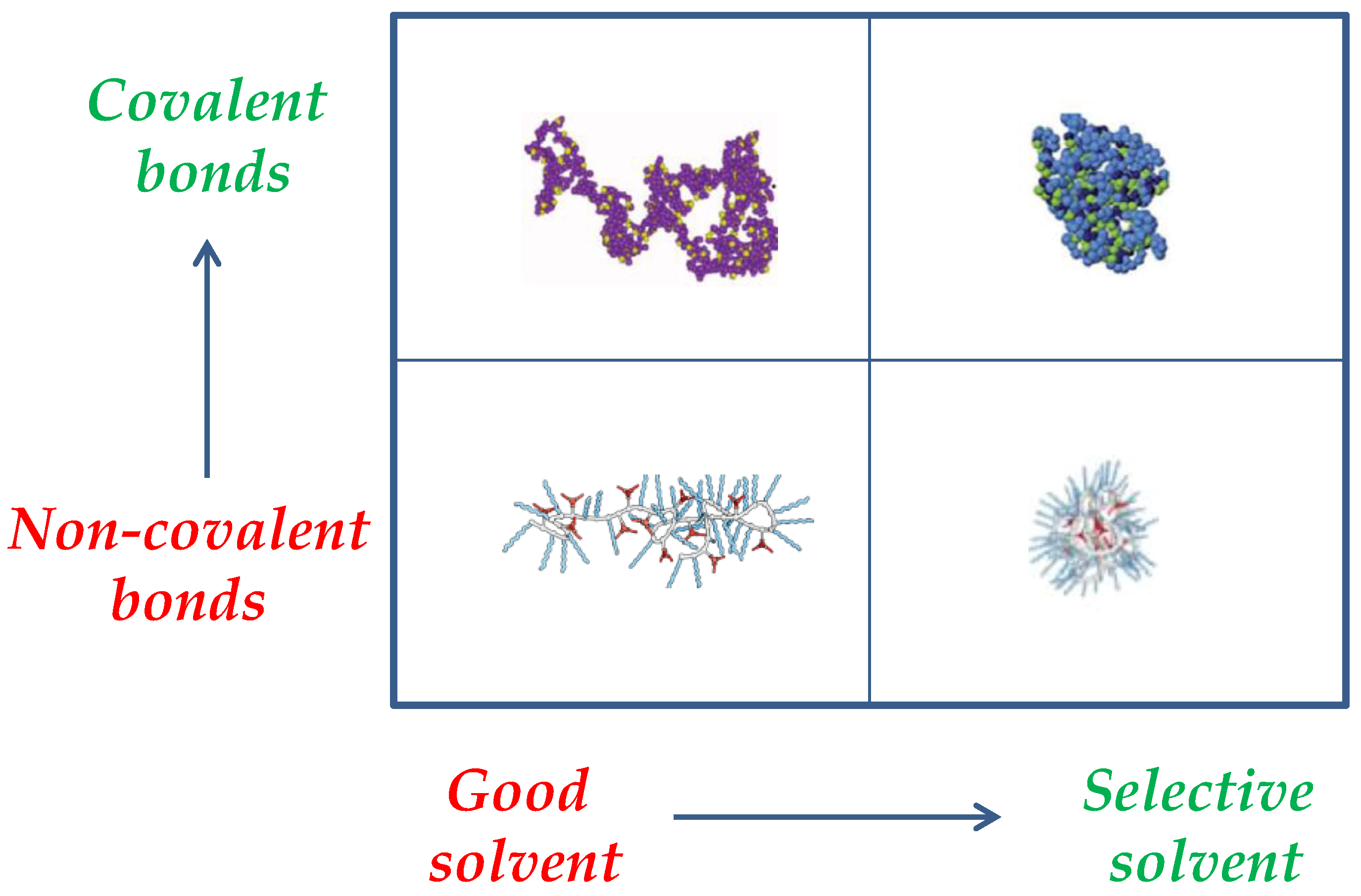
2. Single-Chain Nanoparticles as Bioinspired Nanoreactors/Nanocontainers
2.1. Synthesis of Nanomaterials
2.2. Synthesis of Polymers
2.3. Synthesis of Chemical Compounds
2.4. CO2 Capture and Release
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Copeland, R.A. Structural Components of Enzymes. In Enzymes: A Practical Introduction to Structure, Mechanism and Data Analysis, 2nd ed.; Wiley-VCH: New York, USA, 2000; pp. 42–75. [Google Scholar]
- Breslow, R.; Dong, S.D. Biomimetic Reactions Catalyzed by Cyclodextrins and Their Derivatives. Chem. Rev. 1998, 98, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamada, T.; Nagata, Y.; Suginome, M. High-Molecular-Weight Polyquinoxaline-Based Helically Chiral Phosphine (PQXphos) as Chirality-Switchable, Reusable, and Highly Enantioselective Monodentate Ligand in Catalytic Asymmetric Hydrosilylation of Styrenes. J. Am. Chem. Soc. 2010, 132, 7899–7901. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Fréchet, J.M.J. Applying key concepts from nature: Transition state stabilization, pre-concentration and cooperativity effects in dendritic biomimetics. Prog. Polym. Sci. 2005, 30, 385–402. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Ma, N.; Wang, Z.; Zhang, X.; Liu, J.; Shen, J. Block Copolymer Micelles as Matrixes for Incorporating Diselenide Compounds: A Model System for a Water-Soluble Glutathione Peroxidase Mimic Fine-Tuned by Ionic Strength. Langmuir 2006, 22, 5552–5555. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Meng, F.; Zhong, Z. Reversibly crosslinked temperature-responsive nano-sized polymersomes: Synthesis and triggered drug release. J. Mater. Chem. 2009, 19, 4183–4190. [Google Scholar] [CrossRef]
- Latorre-Sanchez, A.; Pomposo, J.A. Recent bioinspired applications of single-chain nanoparticles. Polym. Int. 2016, 65, 855–860. [Google Scholar] [CrossRef]
- Huo, M.; Wang, N.; Fang, T.; Sun, M.; Wei, Y.; Yuan, J. Single-chain polymer nanoparticles: Mimic the proteins. Polymer 2015, 66, A11–A21. [Google Scholar] [CrossRef]
- Pomposo, J.A. Bioinspired single-chain polymer nanoparticles. Polym. Int. 2014, 63, 589–592. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, M.; Latorre-Sanchez, A.; Pomposo, J.A. Advances in Single Chain Technology. Chem. Soc. Rev. 2015, 44, 6122–6142. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Feng, X.; Xing, H.; Xu, Y.; Kim, B.K.; Baig, N.; Zhou, T.; Gewirth, A.A.; Lu, Y.; Oldfield, E.; et al. A Highly Efficient Single-Chain Metal-Organic Nanoparticle Catalyst for Alkyne-Azide “Click” Reactions in Water and in Cells. J. Am. Chem. Soc. 2016, 138, 11077–11080. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, A.; Arbe, A.; Colmenero, J.; Pomposo, J.A. Metallo-Folded Single-Chain Nanoparticles with Catalytic Selectivity. ACS Macro Lett. 2014, 3, 439–443. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Akbari, S.; Moreno, A.J.; Lo Verso, F.; Arbe, A.; Colmenero, J.; Pomposo, J.A. Design and Preparation of Single-Chain Nanocarriers Mimicking Disordered Proteins for Combined Delivery of Dermal Bioactive Cargos. Macromol. Rapid Commun. 2013, 34, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Piyapakorn, P.; Akashi, M. Formation of Unimer Nanoparticles by Controlling the Self-Association of Hydrophobically Modified Poly(amino acid)s. Langmuir 2012, 28, 5249–5256. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Sugita, T.; Fukae, K.; Sawamoto, M. Synthesis and Single-Chain Folding of Amphiphilic Random Copolymers in Water. Macromolecules 2014, 47, 589–600. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Arbe, A.; Kohlbrecher, J.; Colmenero, J.; Pomposo, J.A. Efficient Synthesis of Single-Chain Globules Mimicking the Morphology and Polymerase Activity of Metalloenzymes. Macromol. Rapid Commun. 2015, 36, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Riddles, C.J.; Van De Mark, M.R. Electroviscous Contribution to the Rheology of Colloidal Unimolecular Polymer (CUP) Particles in Water. Langmuir 2013, 29, 14034–14043. [Google Scholar] [CrossRef] [PubMed]
- Perez-Baena, I.; Asenjo-Sanz, I.; Arbe, A.; Moreno, A.J.; Lo Verso, F.; Colmenero, J.; Pomposo, J.A. Efficient Route to Compact Single-Chain Nanoparticles: Photoactivated Synthesis via Thiol–Yne Coupling Reaction. Macromolecules 2014, 47, 8270–8280. [Google Scholar] [CrossRef]
- Pomposo, J.A.; Rubio-Cervilla, J.; Moreno, A.J.; Lo Verso, F.; Bacova, P.; Arbe, A.; Colmenero, J. Folding Single Chains to Single-Chain Nanoparticles via Reversible Interactions: What Size Reduction Can One Expect? Macromolecules 2017, 50, 1732–1739. [Google Scholar] [CrossRef]
- De-La-Cuesta, J.; González, E.; Moreno, A.J.; Arbe, A.; Colmenero, J.; Pomposo, J.A. Size of Elastic Single-Chain Nanoparticles in Solution and on Surfaces. Macromolecules 2017, 50, 6323–6331. [Google Scholar] [CrossRef]
- Gillissen, M.A.J.; Terashima, T.; Meijer, E.W.; Palmans, A.R.A.; Voets, I.K. Sticky Supramolecular Grafts Stretch Single Polymer Chains. Macromolecules 2013, 46, 4120–4125. [Google Scholar] [CrossRef]
- Mavila, S.; Eivgi, O.; Berkovich, I.; Lemcoff, N.G. Intramolecular Cross-Linking Methodologies for the Synthesis of Polymer Nanoparticles. Chem. Rev. 2016, 116, 878–961. [Google Scholar] [CrossRef] [PubMed]
- Pomposo, J.A. Single-Chain Polymer Nanoparticles: Synthesis, Characterization, Simulations and Applications; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Wulff, G.; Chong, B.-O.; Kolb, U. Soluble Single-Molecule Nanogels of Controlled Structure as a Matrix for Efficient Artificial Enzymes. Angew. Chem. Int. Ed. 2006, 45, 2955–2958. [Google Scholar] [CrossRef] [PubMed]
- Njikang, G.; Liu, G.; Hong, L. Chiral Imprinting of Diblock Copolymer Single-Chain Particles. Langmuir 2011, 27, 7176–7184. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Mes, T.; De Greef, T.F.A.; Gillissen, M.A.J.; Besenius, P.; Palmans, A.R.A.; Meijer, E.W. Single-Chain Folding of Polymers for Catalytic Systems in Water. J. Am. Chem. Soc. 2011, 133, 4742–4745. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Terashima, T.; Sawamoto, M. Self-Folding Polymer Iron Catalysts for Living Radical Polymerization. ACS Macro Lett. 2017, 6, 830–835. [Google Scholar] [CrossRef]
- Perez-Baena, I.; Barroso-Bujans, F.; Gasser, U.; Arbe, A.; Moreno, A.J.; Colmenero, J.; Pomposo, J.A. Endowing Single-Chain Polymer Nanoparticles with Enzyme-Mimetic Activity. ACS Macro Lett. 2013, 2, 775–779. [Google Scholar] [CrossRef]
- Thanneeru, S.; Duay, S.S.; Jin, L.; Fu, Y.; Angeles-Boza, A.M.; He, J. Single Chain Polymeric Nanoparticles to Promote Selective Hydroxylation Reactions of Phenol Catalyzed by Copper. ACS Macro Lett. 2017, 6, 652–656. [Google Scholar] [CrossRef]
- He, J.; Tremblay, L.; Lacelle, S.; Zhao, Y. Preparation of polymer single chain nanoparticles using intramolecular photodimerization of coumarin. Soft Matter 2011, 7, 2380–2386. [Google Scholar] [CrossRef]
- Qian, G.; Zhu, B.; Wang, Y.; Deng, S.; Hu, A. Size-Tunable Polymeric Nanoreactors for One-Pot Synthesis and Encapsulation of Quantum Dots. Macromol. Rapid Commun. 2012, 33, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sun, S.; Wang, Y.; Deng, S.; Qian, G.; Wang, M.; Hu, A. Preparation of carbon nanodots from single chain polymeric nanoparticles and theoretical investigation of the photoluminescence mechanism. J. Mater. Chem. C 2013, 1, 580–586. [Google Scholar] [CrossRef]
- Kwon, W.; Rhee, S.W. Facile synthesis of graphitic carbon quantum dots with size tunability and uniformity using reverse micelles. Chem. Commun. 2012, 48, 5256–5258. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.-H.; di Lena, F.; Chai, C.L.L. PolyPEGA with predetermined molecular weights from enzyme-mediated radical polymerization in water. Chem. Commun. 2011, 47, 6464–6466. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.-H.; di Lena, F.; Chai, C.L.L. Metalloenzymatic radical polymerization using alkyl halides as initiators. Polym. Chem. 2011, 2, 589–594. [Google Scholar] [CrossRef]
- Sigg, S.J.; Seidi, F.; Renggli, K.; Silva, T.B.; Kali, G.; Bruns, N. Horseradish Peroxidase as a Catalyst for Atom Transfer Radical Polymerization. Macromol. Rapid Commun. 2011, 32, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.B.; Spulber, M.; Kocik, M.K.; Seidi, F.; Charan, H.; Rother, M.; Sigg, S.J.; Renggli, K.; Kali, G.; Bruns, N. Hemoglobin and Red Blood Cells Catalyze Atom Transfer Radical Polymerization. Biomacromolecules 2013, 14, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Tooley, C.A.; Pazicni, S.; Berda, E.B. Toward a tunable synthetic [FeFe] hydrogenase mimic: Single-chain nanoparticles functionalized with a single diiron cluster. Polym. Chem. 2015, 6, 7646–7651. [Google Scholar] [CrossRef]
- Greb, L.; Mutlu, H.; Barner-Kowollik, C.; Lehn, J.-M. Photo- and Metallo-responsive N-Alkyl α-Bisimines as Orthogonally Addressable Main-Chain Functional Groups in Metathesis Polymers. J. Am. Chem. Soc. 2016, 138, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Berkovich, I.; Mavila, S.; Iliashevsky, O.; Kozucha, S.; Lemcoff, N.G. Single-chain polybutadiene organometallic nanoparticles: An experimental and theoretical study. Chem. Sci. 2016, 7, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pu, H.; Jin, M.; Wan, D. Supramolecular Nanoparticles via Single-Chain Folding Driven by Ferrous Ions. Macromol. Rapid Commun. 2016, 37, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, G.M.; Perevyazko, I.; Happ, B.; Schubert, U.S. Intra- and inter-supramolecular complexation of poly(butyl methacrylate)-co-2-(1,2,3-triazol-4-yl)pyridine copolymers induced by CoII, FeII, and EuIII ions monitored by molecular hydrodynamics methods. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2632–2639. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. “Turning Over” Definitions in Catalytic Cycles. ACS Catal. 2012, 2, 2787–2794. [Google Scholar] [CrossRef]
- Artar, M.; Palmans, A.R.A. Catalysis Inside Folded Single Macromolecules in Water. In Effects of Nanoconfiement on Catalysis; Poli, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 105–123. [Google Scholar]
- Artar, M.; Terashima, T.; Sawamoto, M.; Meijer, E.W.; Palmans, A.R.A. Understanding the catalytic activity of single-chain polymeric nanoparticles in water. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 12–20. [Google Scholar] [CrossRef]
- Artar, M.; Souren, E.R.J.; Terashima, T.; Meijer, E.W.; Palmans, A.R.A. Single Chain Polymeric Nanoparticles as Selective Hydrophobic Reaction Spaces in Water. ACS Macro Lett. 2015, 4, 1099–1103. [Google Scholar] [CrossRef]
- Huerta, E.; Stals, P.J.M.; Meijer, E.W.; Palmans, A.R.A. Consequences of Folding a Water-Souble Polymer Around an Organocatalyst. Angew. Chem. Int. Ed. 2013, 52, 2906–2910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pauloehrl, T.; Presolski, S.; Albertazzi, L.; Palmans, A.R.A.; Meijer, E.W. Modular Synthetic Platform for the Construction of Functional Single-Chain Polymeric Nanoparticles: From Aqueous Catalysis to Photsensitization. J. Am. Chem. Soc. 2015, 137, 13096–13405. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.; Wirotius, A.-L.; Taton, D. Intramolecular Quaternization as Folding Strategy for the Synthesis of Catalytically Active Imidazolium-Based Single Chain Nanoparticles. ACS Macro Lett. 2017, 6, 489–494. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, R.; Gao, M.; Hao, P.; Yin, D. Bio-inspired single-chain polymeric nanoparticles containing a chiral salen TiIV complex for highly enantioselective sulfoxidation in water. Green Chem. 2017, 19, 1182–1193. [Google Scholar] [CrossRef]
- Mavila, S.; Rozenberg, I.; Lemcoff, N.-G. A general approach to mono- and bimetallic organometallic nanoparticles. Chem. Sci. 2014, 5, 4196–4203. [Google Scholar] [CrossRef]
- Willenbacher, J.; Altintas, O.; Trouillet, V.; Knöfel, N.; Monteiro, M.J.; Roesky, P.W.; Barner-Kowollik, C. Pd-complex driven formation of single-chain nanoparticles. Polym. Chem. 2015, 6, 4358–4365. [Google Scholar] [CrossRef]
- Thanneeru, S.; Nganga, J.K.; Amin, A.S.; Liu, B.; Jin, L.; Angeles-Boza, A.M.; He, J. “Enzymatic” Photoreduction of Carbon Dioxide using Polymeric Metallofoldamers Containing Nickel-Thiolate Cofactors. ChemCatChem 2017, 9, 1157–1162. [Google Scholar] [CrossRef]
- Knöfel, N.D.; Rothfuss, H.; Willenbacher, J.; Barner-Kowollik, C.; Roesky, P.W. Platinum(II)-Crosslinked Single-Chain Nanoparticles: An Approach towards Recyclable Homogeneous Catalysts. Angew. Chem. Int. Ed. 2017, 56, 4950–4954. [Google Scholar] [CrossRef] [PubMed]
- Huerta, E.; van Genabeek, B.; Stals, P.J.M.; Meijer, E.W.; Palmans, A.R.A. A Modular Approach to Introduce Function into Single-Chain Polymeric Nanoparticles. Macromol. Rapid Commun. 2014, 35, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Stals, P.J.M.; Cheng, C.-Y.; van Beek, L.; Wauters, A.C.; Palmans, A.R.A.; Han, S.; Meijer, E.W. Surface water retardation around single-chain polymeric nanoparticles: Critical for catalytic function? Chem. Sci. 2016, 7, 2011–2015. [Google Scholar] [CrossRef]
- Basasoro, S.; Gonzalez-Burgos, M.; Moreno, A.J.; Lo Verso, F.; Arbe, A.; Colmenero, J.; Pomposo, J.A. A Solvent-Based Strategy for Tuning the Internal Structure of Metallo-Folded Single-Chain Nanoparticles. Macromol. Rapid Commun. 2016, 37, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Henzler-Wildman, K.; Kern, D. Dynamic personalities of proteins. Nature 2007, 450, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Tong, X.; Farnia, F.; Yu, B.; Zhao, Y. CO2-Responsive Polymeric Single-Chain Nanoparticles and Self-Assembly for Gas-Tunable Nanoreactors. Chem. Mater. 2017, 29, 5693–5701. [Google Scholar] [CrossRef]
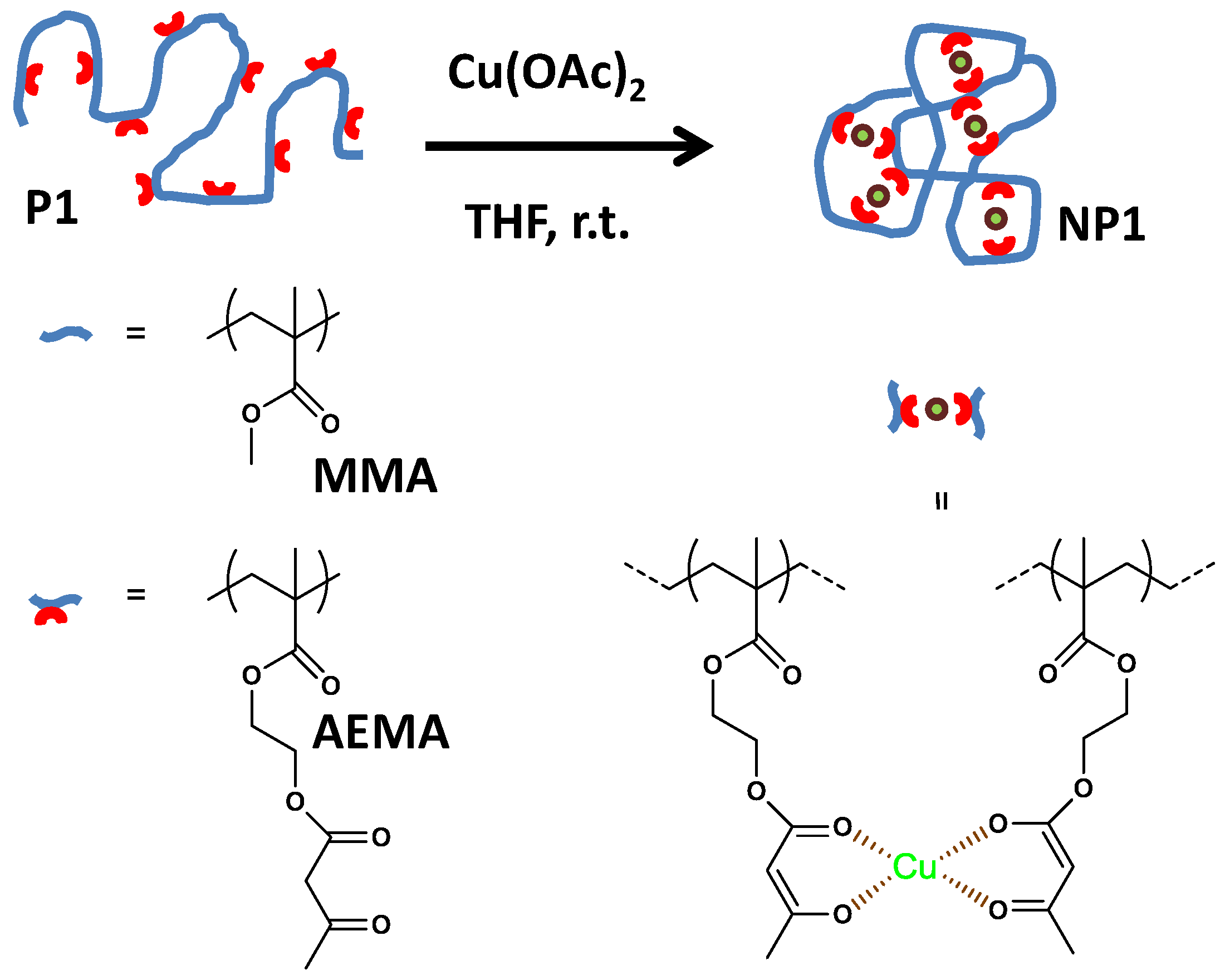
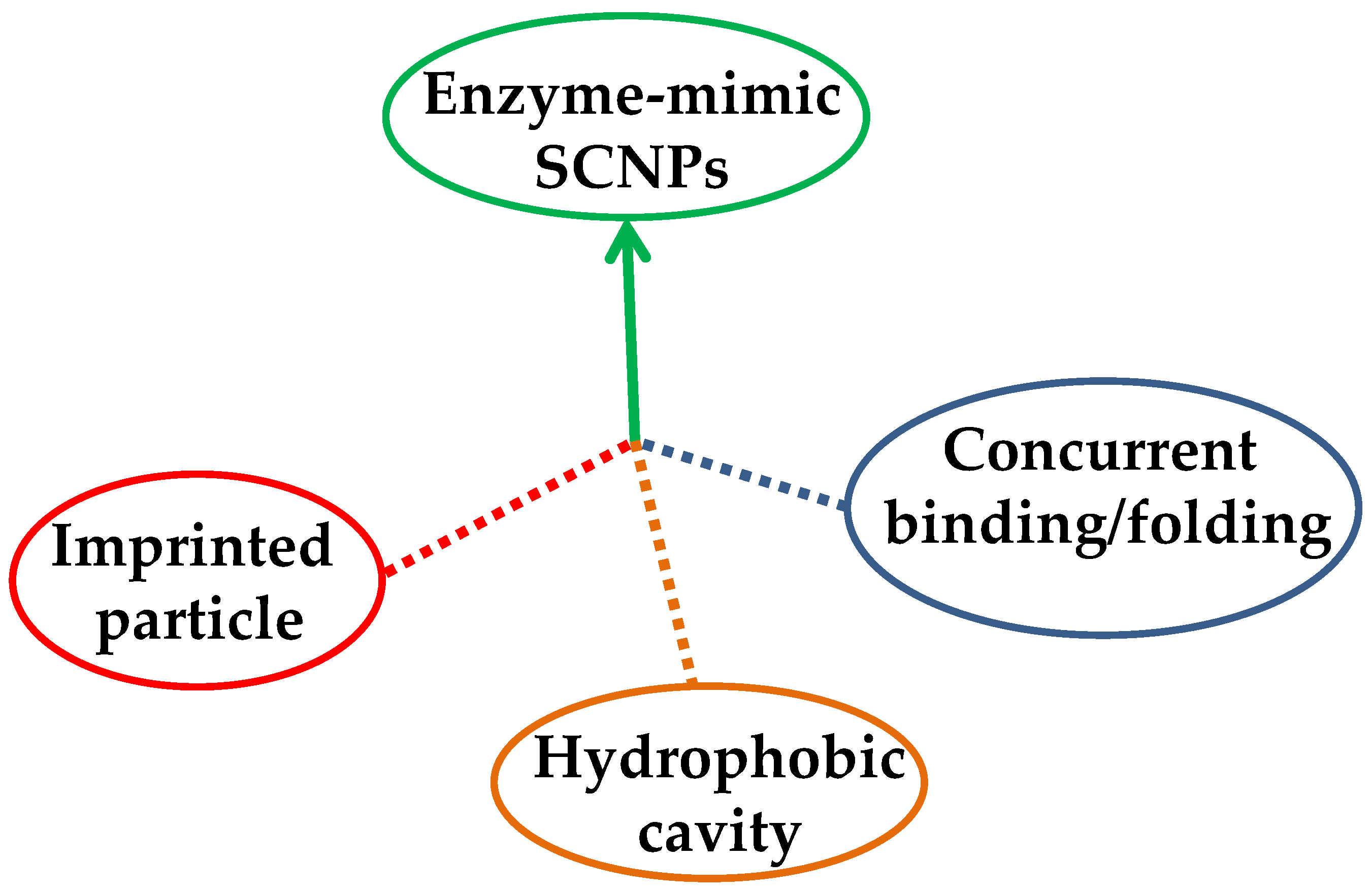

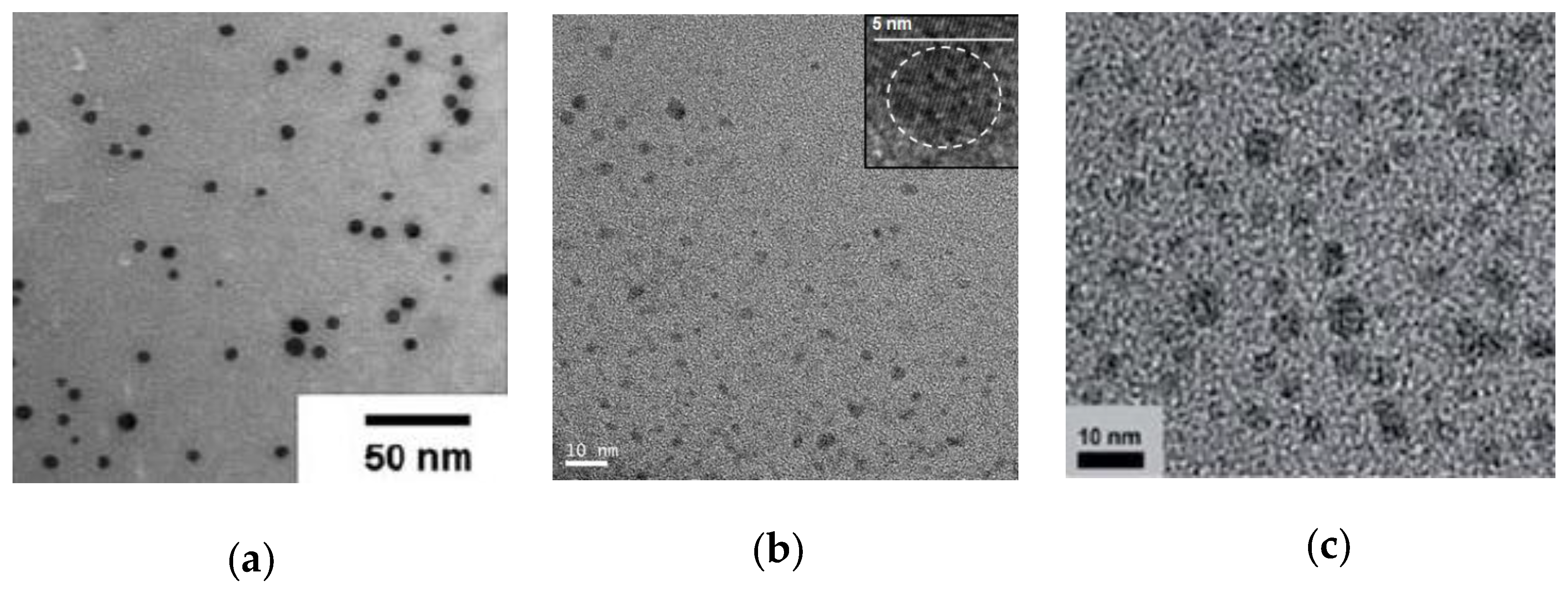
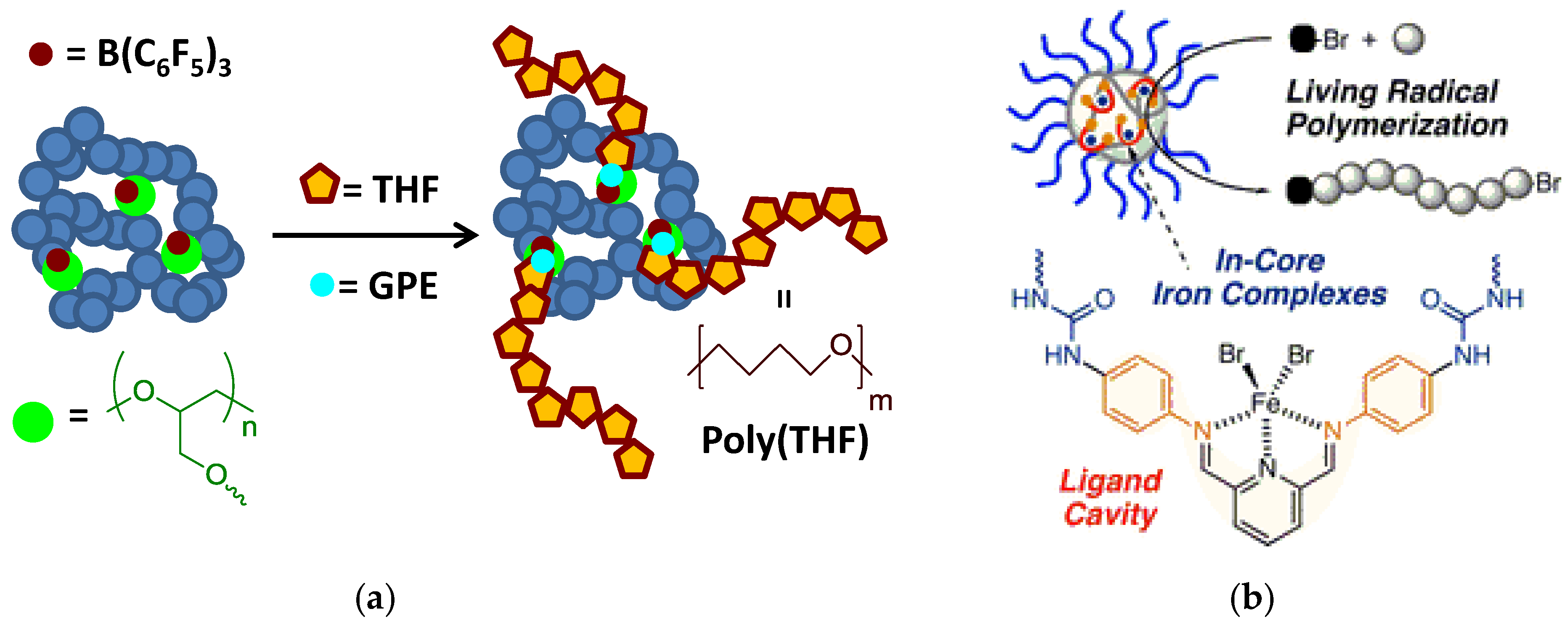
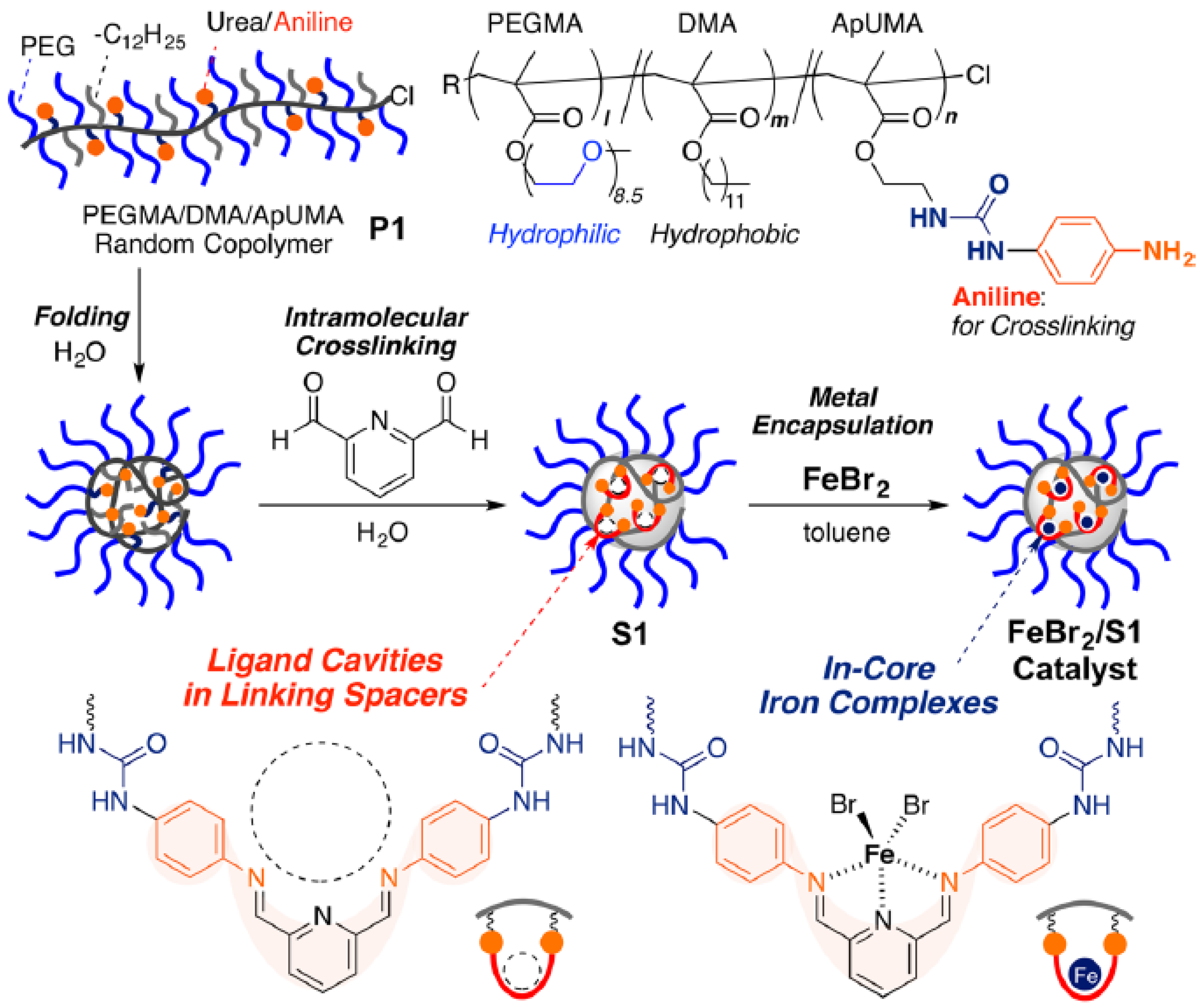

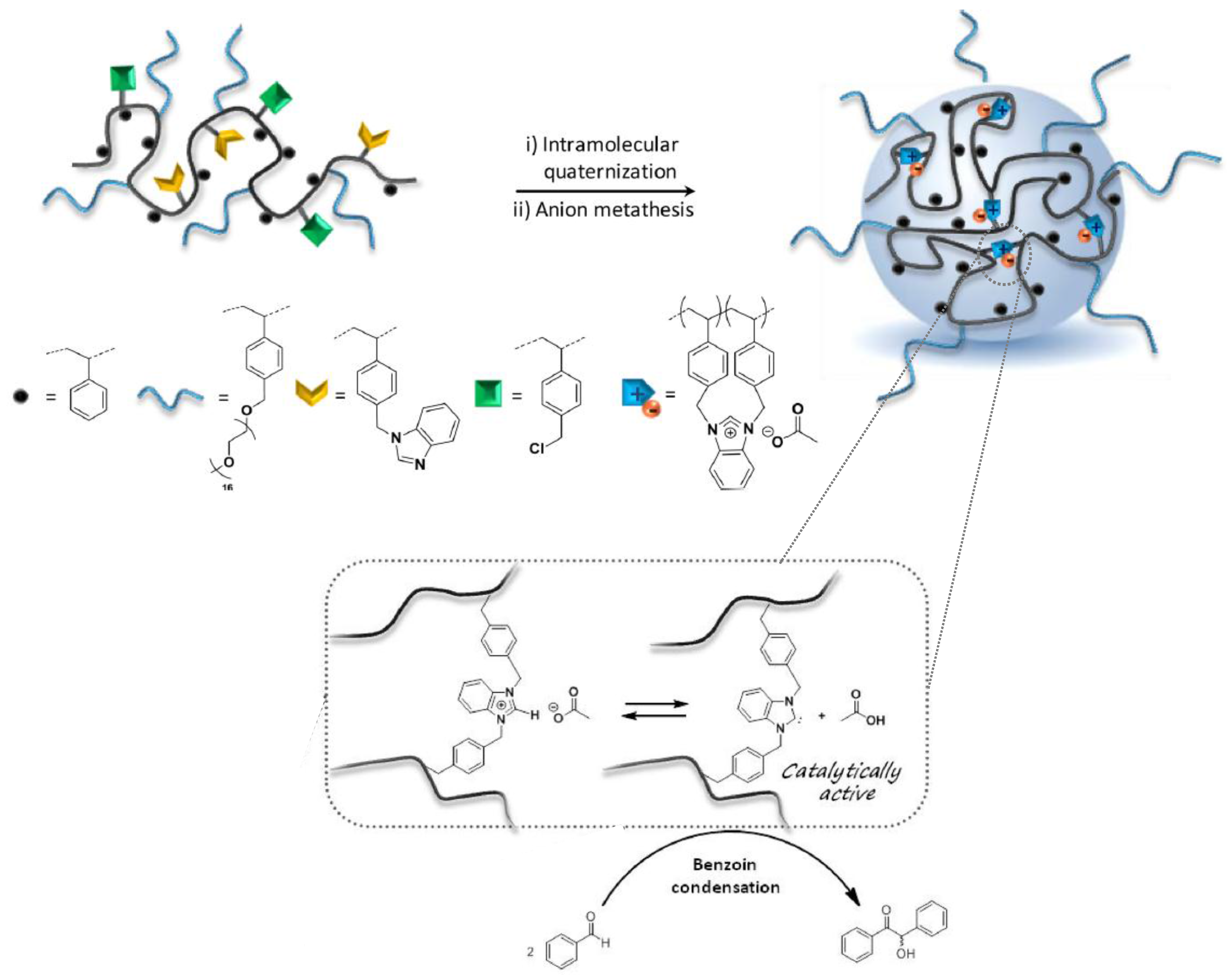
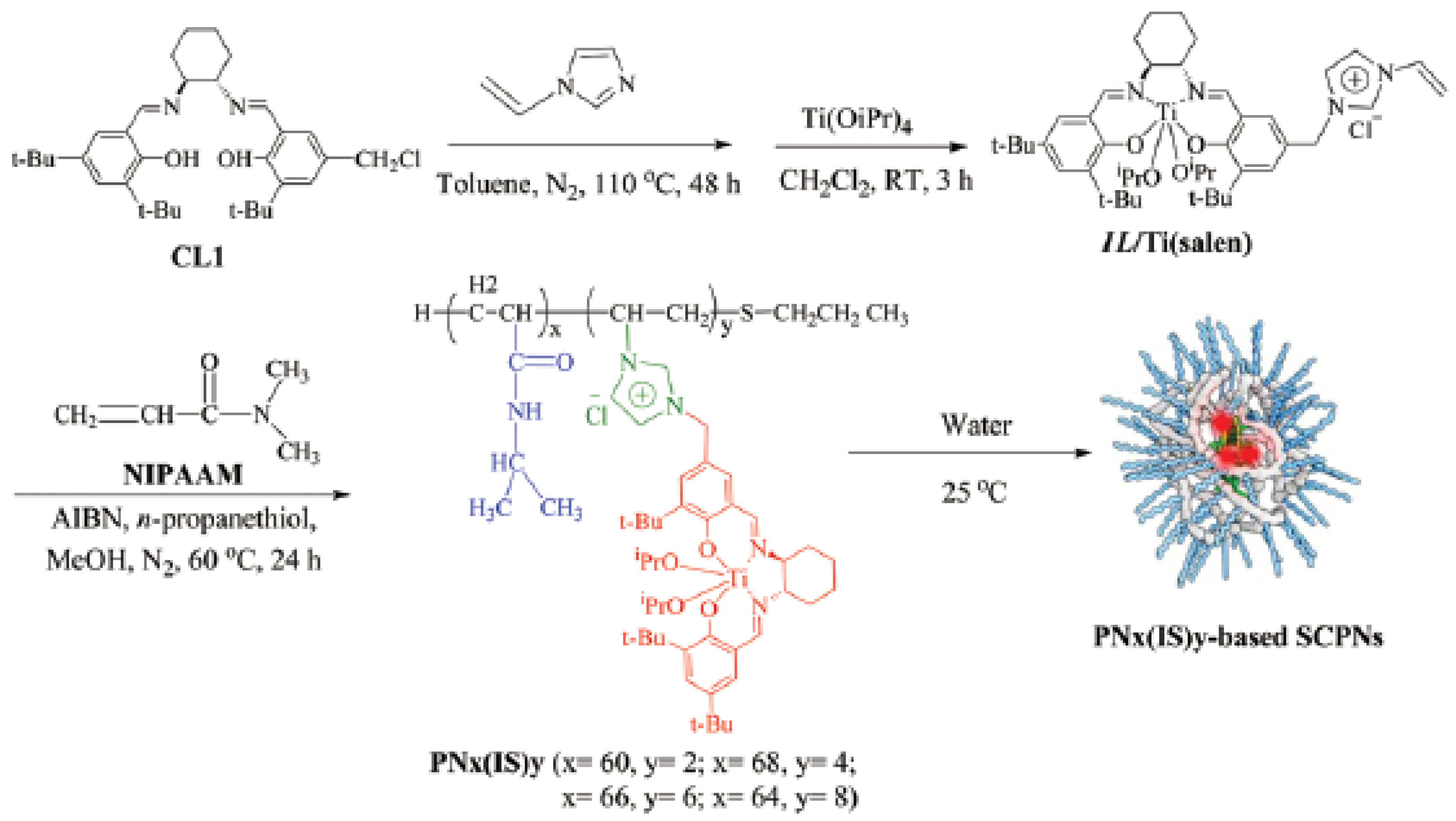
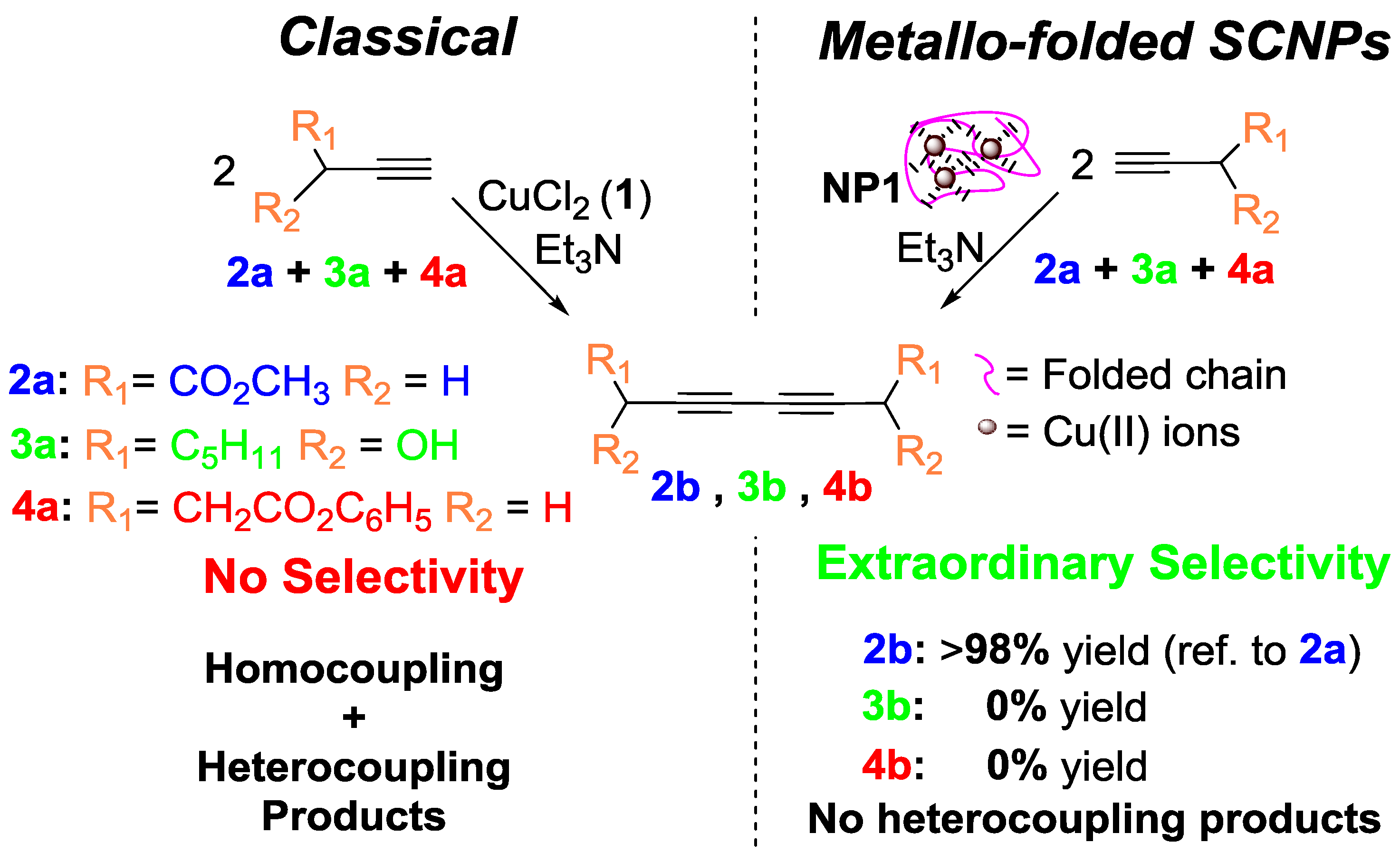
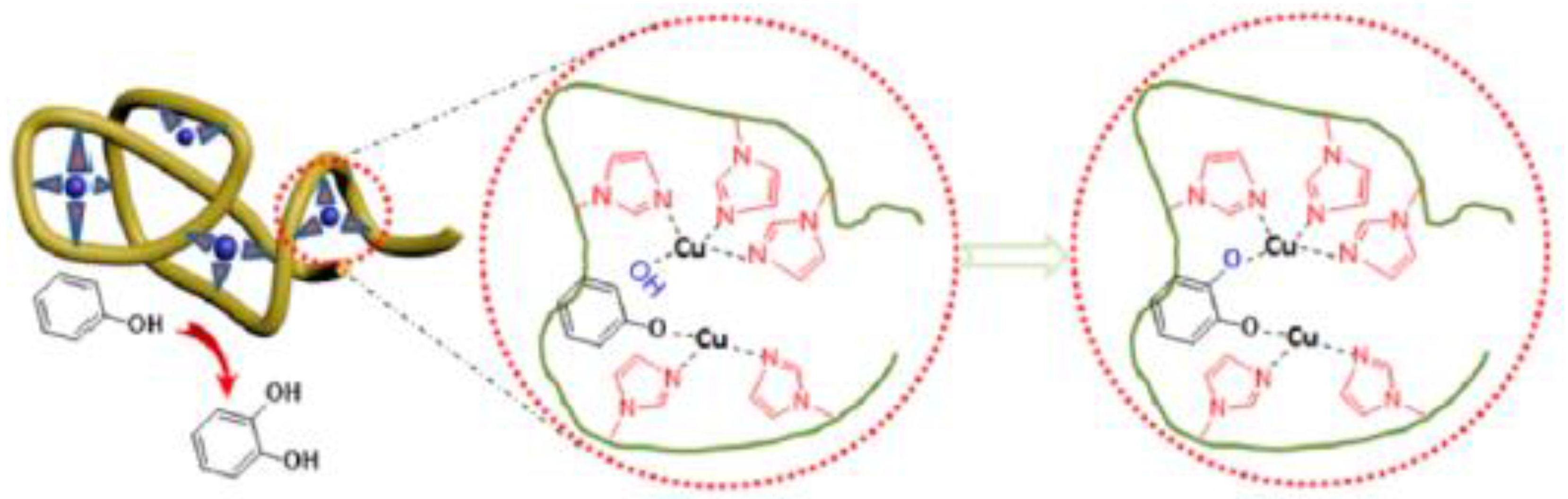

| Catalyst Type | c 1 | Mn (kDa) 2 | Ð 3 | Ieff (%) 4 | Reference |
|---|---|---|---|---|---|
| Laccase | 0.87 | 36.2 | 1.8 | 36 | [37] |
| SCNPs | 0.97 | 60.9 | 1.1 | 24 | [18] |
| Catalyst Type | c | Mn (kDa) | Ð | Ieff (%) | Reference |
|---|---|---|---|---|---|
| Hemoglobin | 0.73 | 200 | 2.2 | 3.2 | [39] |
| SCNPs | 0.63 | 50.7 | 1.8 | 6.4 | [18] |
| Reaction type 1 | Solvent 2 | T (°C) 3 | t (h) | c (%) 4 | TOF (h−1) 5 | Reference |
|---|---|---|---|---|---|---|
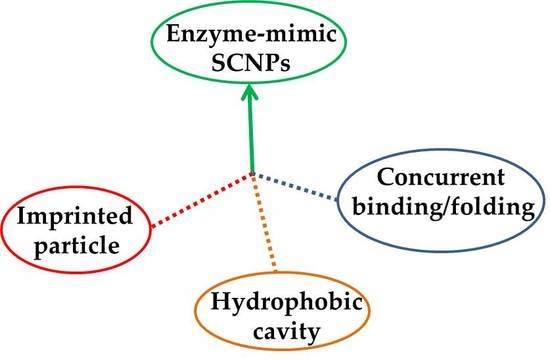
| Imprinted particle | ||||||
| Carbonate hydrolysis | H2O/MeCN | 10 | - | - | 4.4 × 10−3 | [26] |
| Hydrophobic cavity | ||||||
| Hydrogenation of ketones | H2O | 40 | 50 | 98 | 20 | [28] |
| Oxidation of secondary alcohols | H2O | r.t. | 7 × 10−2 | >99 | 600 | [48] |
| Aldol reaction | H2O | 25 | 24 | 99 | 8 | [49] |
| CuAAC | PBS | r.t. | 0.2 | >99 | 13 | [50] |
| Mono-depropargylation reaction | PBS | r.t. | 5 | >99 | 0.2 | [50] |
| Bis-depropargylation reaction | PBS | r.t. | 25 | >99 | 0.04 | [50] |
| Benzoin condensation reaction | THF | 80 | 24 | 65 | 0.3 | [51] |
| Enantioselective sulfoxidation | H2O | 25 | 1 | 99 | 198 | [52] |
| Concurrent binding/folding | ||||||
| Reduction of α-diketones | CH2Cl2 | r.t. | 0.1 | 96 | 5580 | [30] |
| Alkyne dimerization | Bulk | 60 | 8 | >98 | 25 | [14] |
| Reduction of secondary amines | THF | r.t. | 16 | >99 | 0.6 | [53] |
| Allylation of benzophenone | THF | 35 | 24 | 97 | 8 | [53] |
| Biphenyl formation | THF | 80 | 16 | >99 | 0.2 | [53] |
| Sonogashira coupling | HN(C2H5)2 | r.t. | 24 | 45 | 21 | [54] |
| CuAAC | H2O | 50 | 24 | >99 | 16,667 | [13] |
| Photoreduction of CO2 | DMF | 80 | 1.5 | - | 2529 | [55] |
| Amination of allyl alcohol | C6D6 | 100 | 24 | 99 | 16 | [56] |
| Hydroxylation of phenol | H2O | 60 | 1 | 28 | 872 | [31] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Cervilla, J.; González, E.; Pomposo, J.A. Advances in Single-Chain Nanoparticles for Catalysis Applications. Nanomaterials 2017, 7, 341. https://doi.org/10.3390/nano7100341
Rubio-Cervilla J, González E, Pomposo JA. Advances in Single-Chain Nanoparticles for Catalysis Applications. Nanomaterials. 2017; 7(10):341. https://doi.org/10.3390/nano7100341
Chicago/Turabian StyleRubio-Cervilla, Jon, Edurne González, and José A. Pomposo. 2017. "Advances in Single-Chain Nanoparticles for Catalysis Applications" Nanomaterials 7, no. 10: 341. https://doi.org/10.3390/nano7100341