Solution Based Methods for the Fabrication of Carbon Nanotube Modified Atomic Force Microscopy Probes
Abstract
:1. Introduction
2. Results
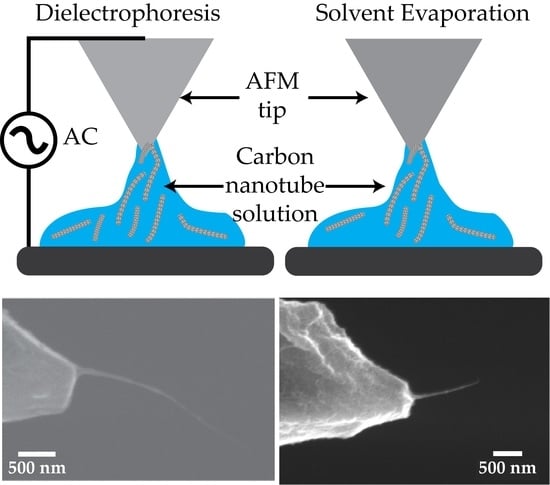
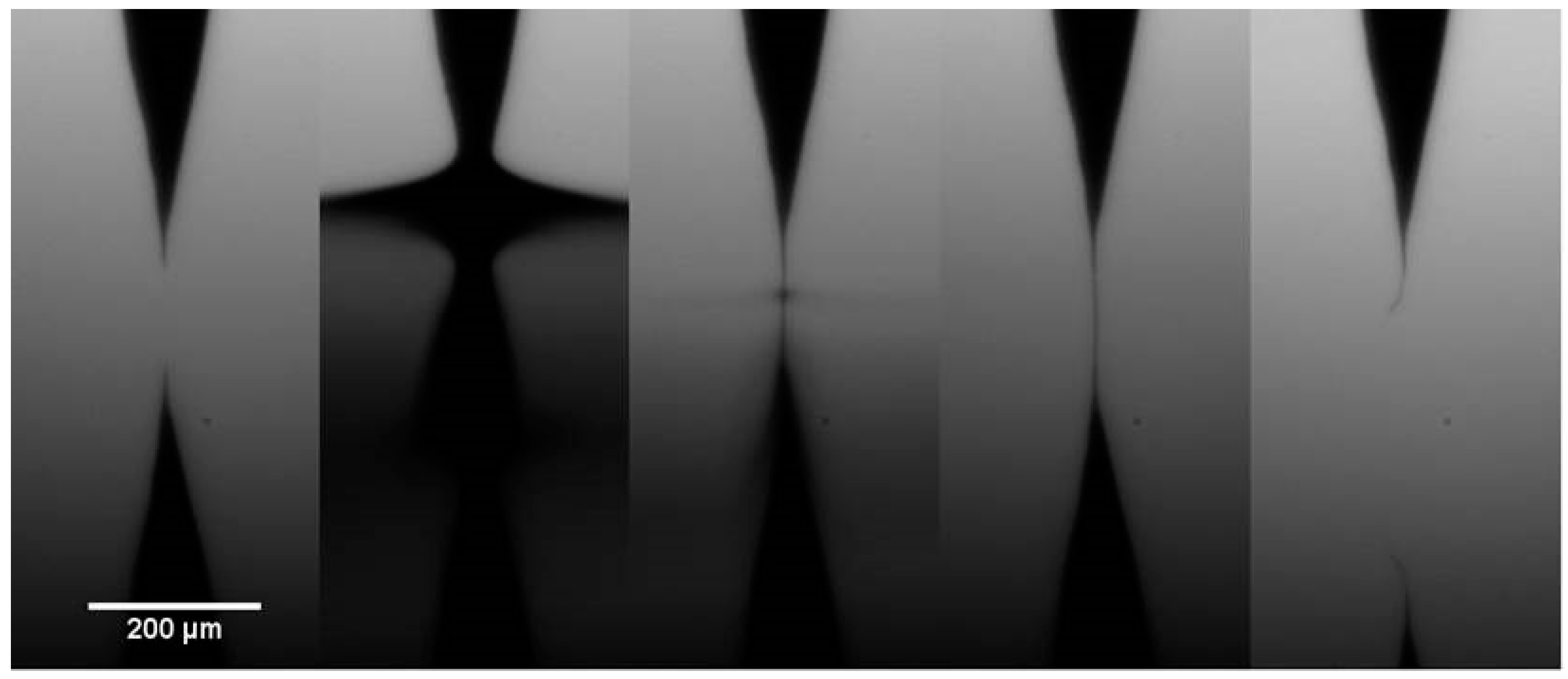
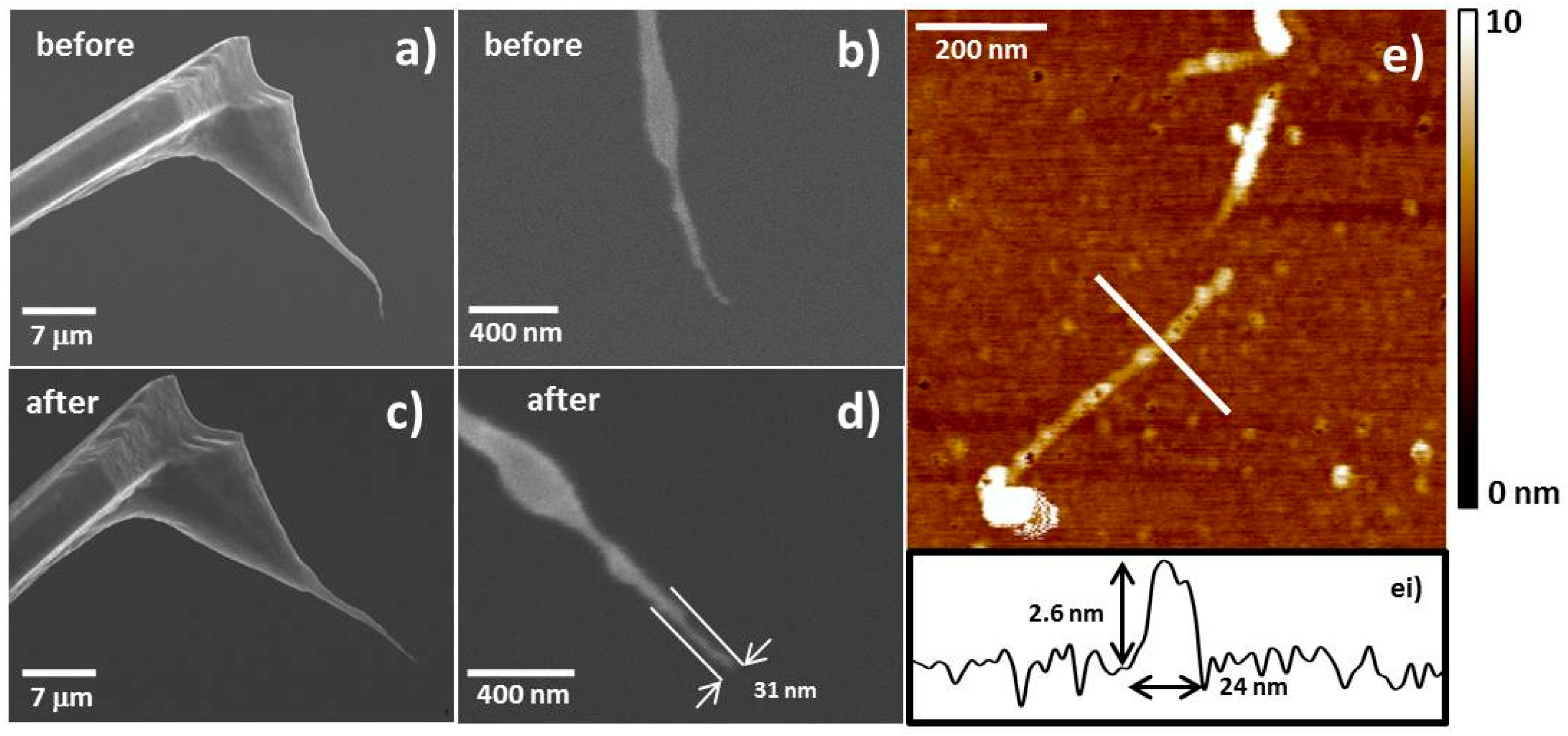
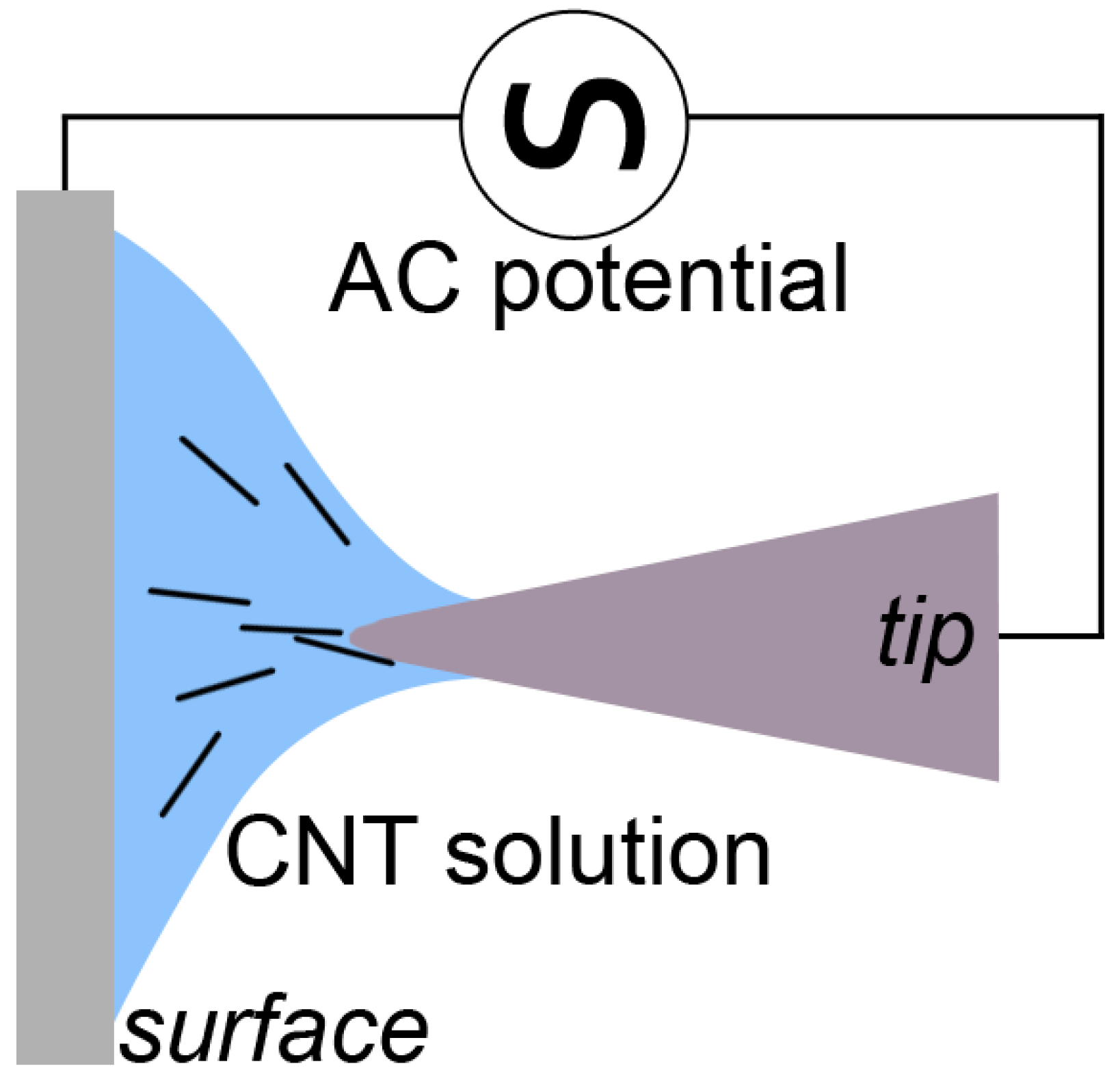
2.1. Production of CNT Fibre Probes Using Dielectrophoresis
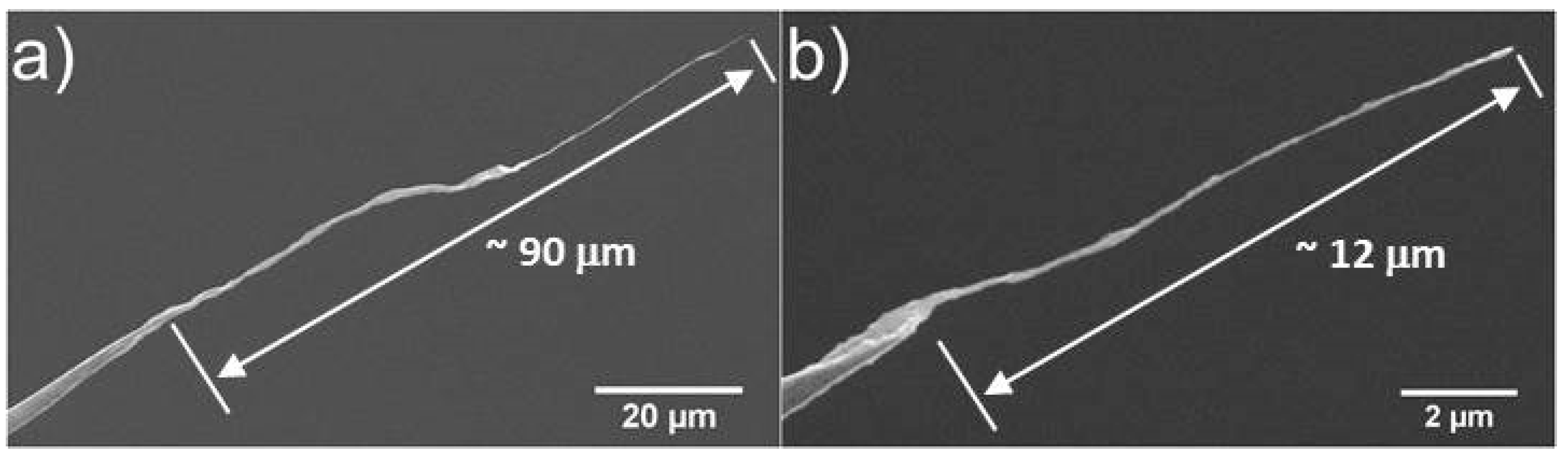
2.2. Production of CNT Fibre Probes Using Solvent Evaporation
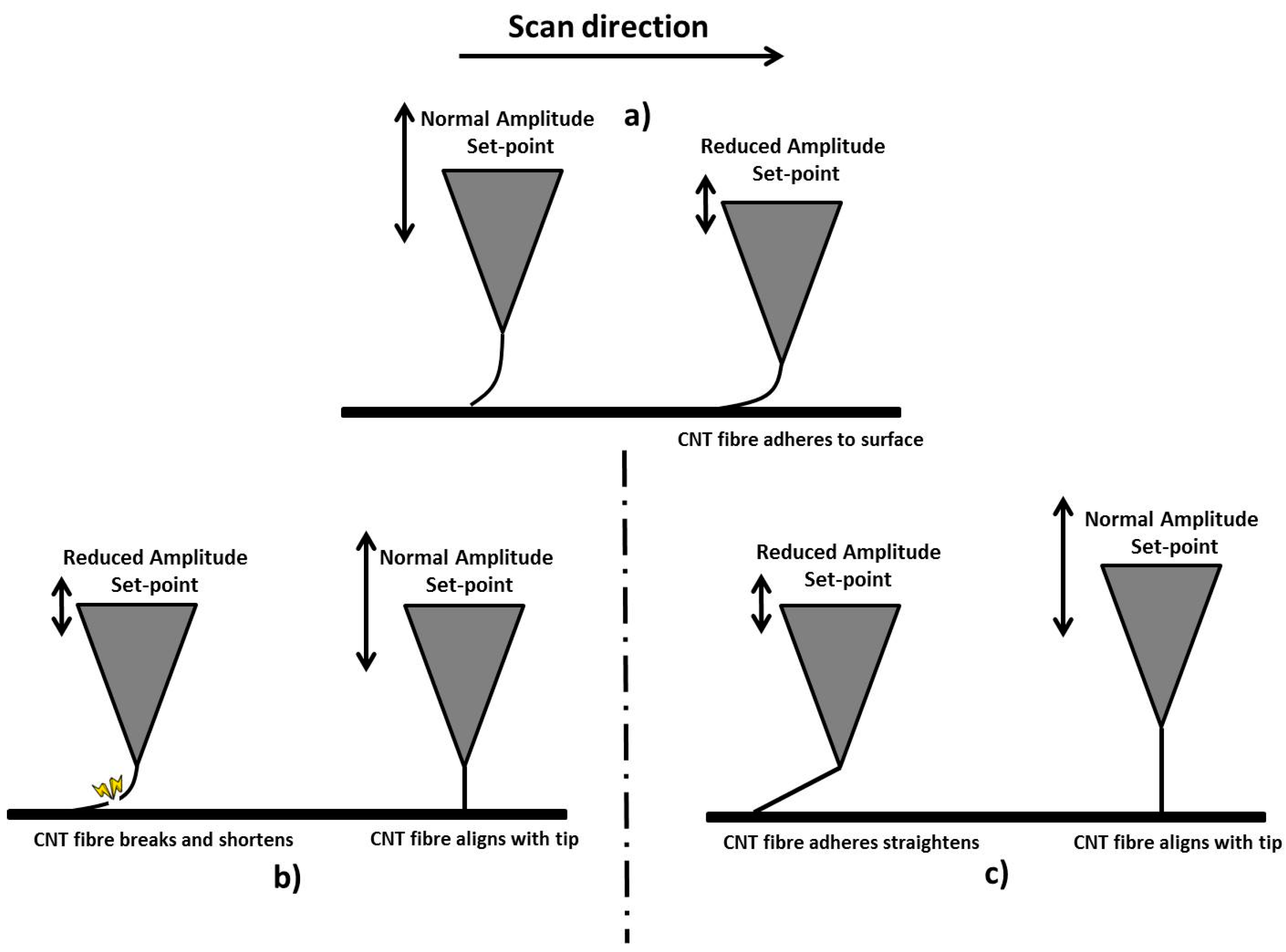
2.3. Fibre Processing and Stabilisation
3. Discussion
4. Materials and Methods
4.1. SWCNT Solutions
4.2. Production of CNT Fibre Probes Using Dielectrophoresis
4.3. Solvent Evaporation
4.4. AFM Analysis and CNT Fibre Processing
4.5. SEM Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, N.R.; Macpherson, J.V. Carbon nanotube tips for atomic force microscopy. Nat. Nanotechnol. 2009, 4, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Slattery, A.D.; Blanch, A.J.; Quinton, J.S.; Gibson, C.T. Efficient attachment of carbon nanotubes to conventional and high-frequency AFM probes enhanced by electron beam processes. Nanotechnology 2013, 24, 235705. [Google Scholar] [CrossRef] [PubMed]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate thickness measurement of graphene. Nanotechnology 2016, 27, 125704. [Google Scholar] [CrossRef] [PubMed]
- Slattery, A.D.; Shearer, C.J.; Gibson, C.T.; Shapter, J.G.; Lewis, D.A.; Stapleton, A.J. Carbon nanotube modified probes for stable and high sensitivity conductive atomic force microscopy. Nanotechnology 2016, 27, 475708. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J.H.; Cheung, C.L.; Lieber, C.M. Direct Growth of Single-Walled Carbon Nanotube Scanning Probe Microscopy Tips. J. Am. Chem. Soc. 1999, 121, 9750–9751. [Google Scholar] [CrossRef]
- Shu-Cheng, C.; Yuan-Chih, C.; Chia-Seng, C. The fabrication of carbon nanotube probes utilizing ultra-high vacuum transmission electron microscopy. Nanotechnology 2009, 20, 285307. [Google Scholar] [CrossRef]
- Dai, H.; Hafner, J.H.; Rinzler, A.G.; Colbert, D.T.; Smalley, R.E. Nanotubes as nanoprobes in scanning probe microscopy. Nature 1996, 384, 147–150. [Google Scholar] [CrossRef]
- Cheung, C.L.; Hafner, J.H.; Odom, T.W.; Kim, K.; Lieber, C.M. Growth and fabrication with single-walled carbon nanotube probe microscopy tips. Appl. Phys. Lett. 2000, 76, 3136–3138. [Google Scholar] [CrossRef] [Green Version]
- Gibson, C.T.; Carnally, S.; Roberts, C.J. Attachment of carbon nanotubes to atomic force microscope probes. Ultramicroscopy 2007, 107, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J.H.; Cheung, C.-L.; Oosterkamp, T.H.; Lieber, C.M. High-Yield Assembly of Individual Single-Walled Carbon Nanotube Tips for Scanning Probe Microscopies. J. Phys. Chem. B 2001, 105, 743–746. [Google Scholar] [CrossRef]
- Wade, L.A.; Shapiro, I.R.; Ma, Z.; Quake, S.R.; Collier, C.P. Correlating AFM Probe Morphology to Image Resolution for Single-Wall Carbon Nanotube Tips. Nano Lett. 2004, 4, 725–731. [Google Scholar] [CrossRef]
- Tang, J.; Yang, G.; Zhang, Q.; Parhat, A.; Maynor, B.; Liu, J.; Qin, L.C.; Zhou, O. Rapid and reproducible fabrication of carbon nanotube AFM probes by dielectrophoresis. Nano Lett. 2005, 5, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Nishijima, H.; Nakayama, Y.; Tokumasu, F.; Takeyasu, K. Carbon nanotube tips for a scanning probe microscope: Their fabrication and properties. J. Phys. D 1999, 32, 1044–1048. [Google Scholar] [CrossRef]
- Kim, J.E.; Han, C.S. Use of dielectrophoresis in the fabrication of an atomic force microscope tip with a carbon nanotube: A numerical analysis. Nanotechnology 2005, 16, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, S.H.; Kwak, Y.K.; Lee, E.S.; Han, C.S. The effect of the shape of a tip’s apex on the fabrication of an AFM tip with an attached single carbon nanotube. Sens. Actuator A-Phys. 2005, 125, 41–49. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, W.S.; Choi, B.S.; Choi, S.W.; Kim, J.H. Fabrication of carbon nanotube AFM probes using the Langmuir-Blodgett technique. Ultramicroscopy 2008, 108, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Shin, Y.H.; Yoon, Y.H. Fabrication and characterization of carbon nanotube tip modified by focused ion beam. In Proceedings of the 2nd IEEE International Conference of Nano/Micro Engineered and Molecular Systems, Bangkok, Thailand, 16–19 January 2007; pp. 1197–1200. [Google Scholar] [CrossRef]
- Shin, Y.H.; Song, J.W.; Lee, E.S.; Han, C.S. Imaging characterization of carbon nanotube tips modified using a focused ion beam. Appl. Surf. Sci. 2007, 253, 6872–6877. [Google Scholar] [CrossRef]
- Raghuveer, M.S.; Ganesan, P.G.; D’Arcy-Gall, J.; Ramanath, G.; Marshall, M.; Petrov, I. Nanomachining carbon nanotubes with ion beams. Appl. Phys. Lett. 2004, 84, 4484–4486. [Google Scholar] [CrossRef]
- Hulman, M.; Tajmar, M. The dielectrophoretic attachment of nanotube fibres on tungsten needles. Nanotechnology 2007, 18, 145504. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, J.; Yang, G.; Qiu, Q.; Qin, L.C.; Zhou, O. Efficient fabrication of carbon nanotube point electron sources by dielectrophoresis. Adv. Mater. 2004, 16, 1219–1222. [Google Scholar] [CrossRef]
- Pethig, R. Review Article-Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Khoshmanesh, K.; Mitchell, A.; Kalantar-zadeh, K. Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems. Anal. Bioanal. Chem. 2010, 396, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Ondarcuhu, T.; Joachim, C. Drawing a single nanofibre over hundreds of microns. EPL 1998, 42, 215–220. [Google Scholar] [CrossRef]
- Kozlov, M.E.; Capps, R.C.; Sampson, W.M.; Ebron, V.H.; Ferraris, J.P.; Baughman, R.H. Spinning solid and hollow polymer-free carbon nanotube fibers. Adv. Mater. 2005, 17, 614–617. [Google Scholar] [CrossRef]
- Ericson, L.M.; Fan, H.; Peng, H.Q.; Davis, V.A.; Zhou, W.; Sulpizio, J.; Wang, Y.H.; Booker, R.; Vavro, J.; Guthy, C.; et al. Macroscopic, neat, single-walled carbon nanotube fibers. Science 2004, 305, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Behabtu, N.; Green, M.J.; Pasquali, M. Carbon nanotube-based neat fibers. Nano Today 2008, 3, 24–34. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; ter Waarbeek, R.F.; de Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.J.; Koziol, K.K.K.; Kinloch, I.A.; Windle, A.H. Macroscopic fibers of well-aligned carbon nanotubes by wet spinning. Small 2008, 4, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Li, Q.W.; Kinloch, I.A.; Windle, A.H. Ordering in a Droplet of an Aqueous Suspension of Single-Wall Carbon Nanotubes on a Solid Substrate. Langmuir 2010, 26, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Aime, J.P.; Marsaudon, S.; Levy, R.; Bonnot, A.M.; Nguyen, C.; Mariolle, D.; Bertin, F.; Chabli, A. Drying nano particles solution on an oscillating tip at an air liquid interface: What we can learn, what we can do. Nanoscale Res. Lett. 2007, 2, 309–318. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Atkinson, K.R.; Hawkins, S.C.; Huynh, C.; Skourtis, C.; Dai, J.; Zhang, M.; Fang, S.L.; Zakhidov, A.A.; Lee, S.B.; Aliev, A.E.; et al. Multifunctional carbon nanotube yarns and transparent sheets: Fabrication, properties, and applications. Physica B-Condens. Matter 2007, 394, 339–343. [Google Scholar] [CrossRef]
- Gibson, C.T.; Watson, G.S.; Myhra, S. Scanning force microscopy—Calibrative procedures for ‘best practice’. Scanning 1997, 19, 564–581. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.Y. Carbon nanotubes: A promising standard for quantitative evaluation of AFM tip apex geometry. Ultramicroscopy 2007, 107, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Hertel, T.; Walkup, R.E.; Avouris, P. Deformation of carbon nanotubes by surface van der Waals forces. Phys. Rev. B 1998, 58, 13870–13873. [Google Scholar] [CrossRef]
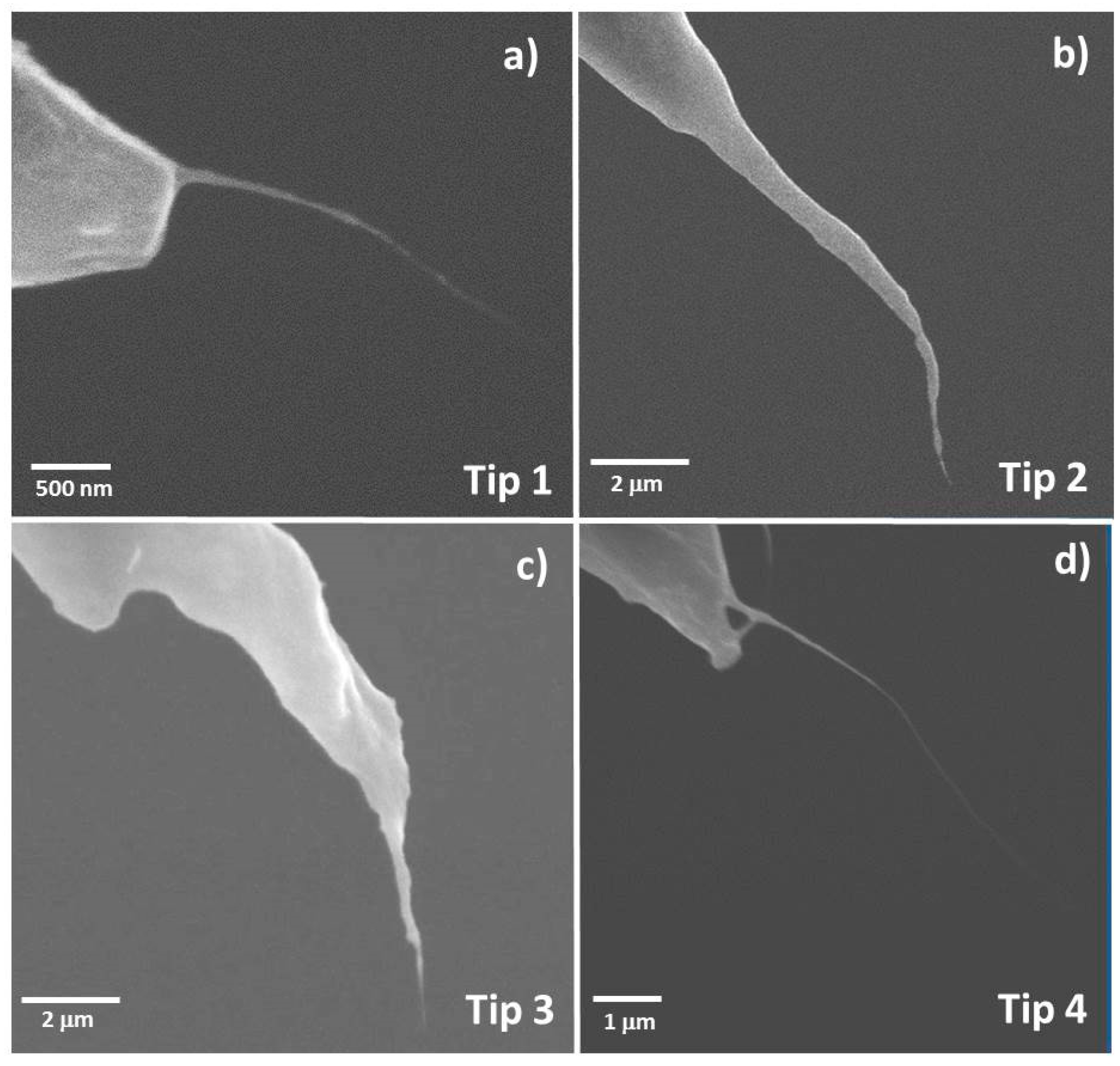
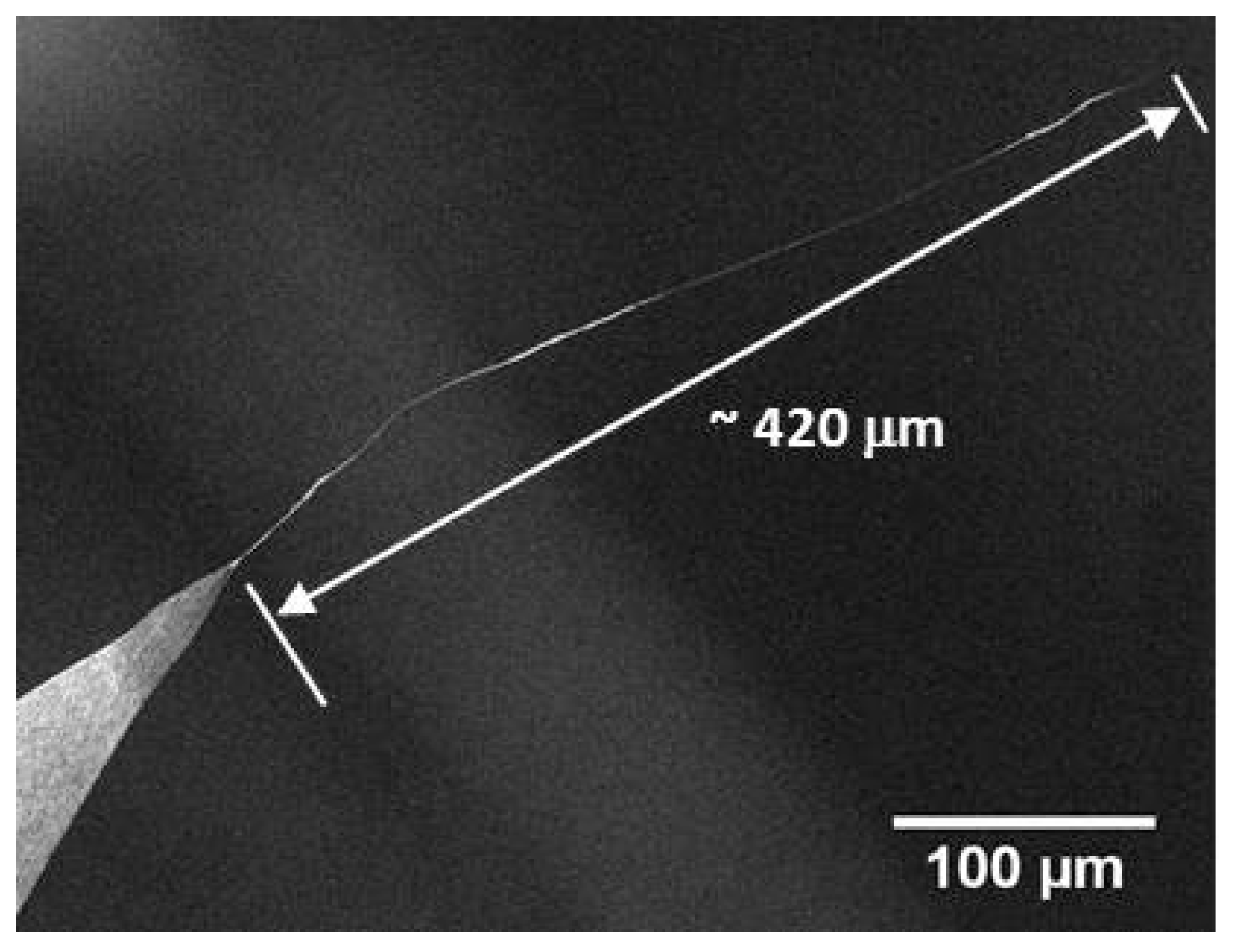
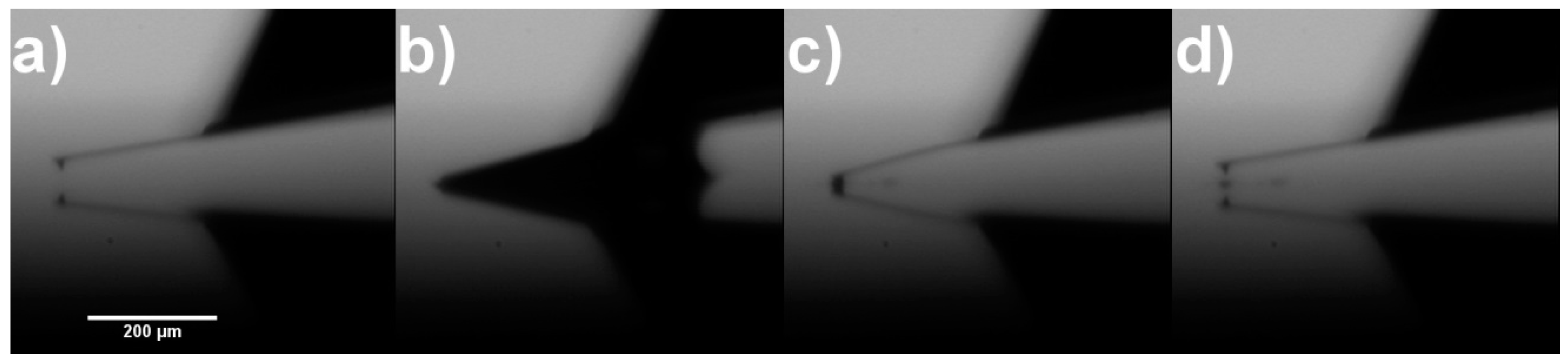
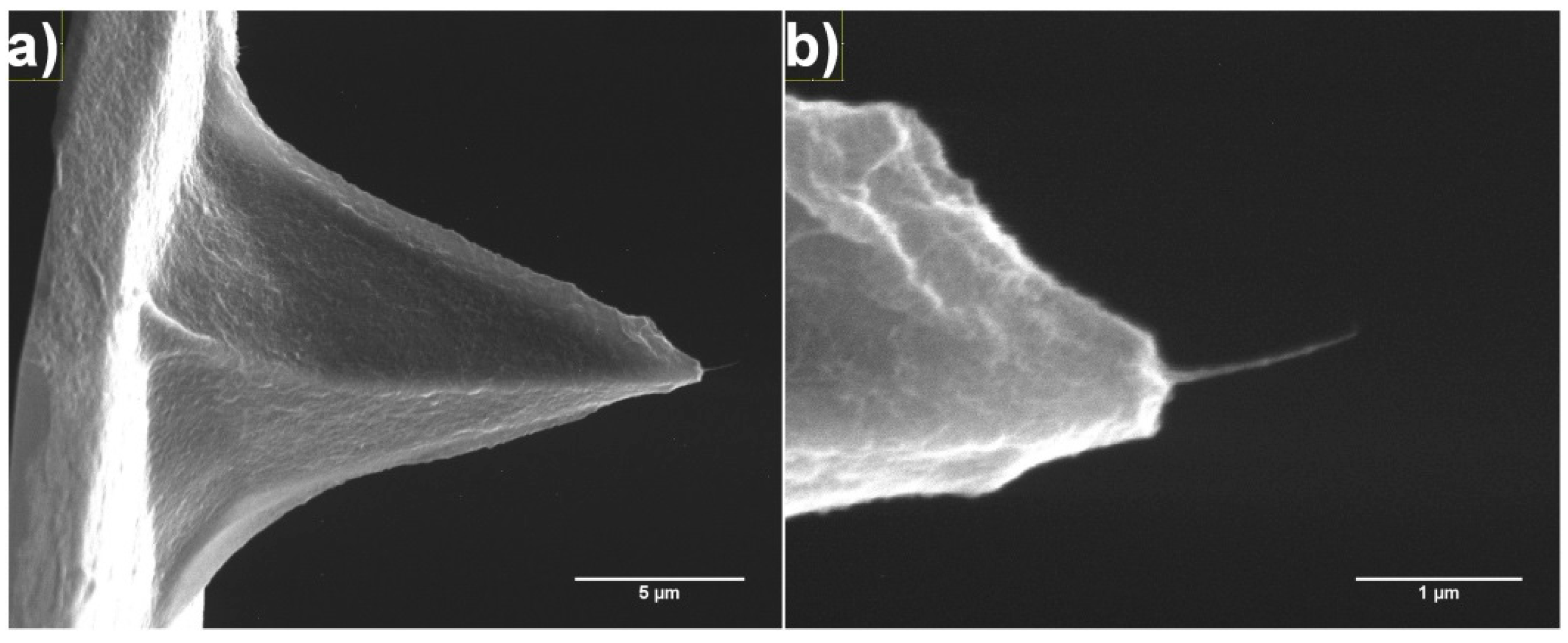
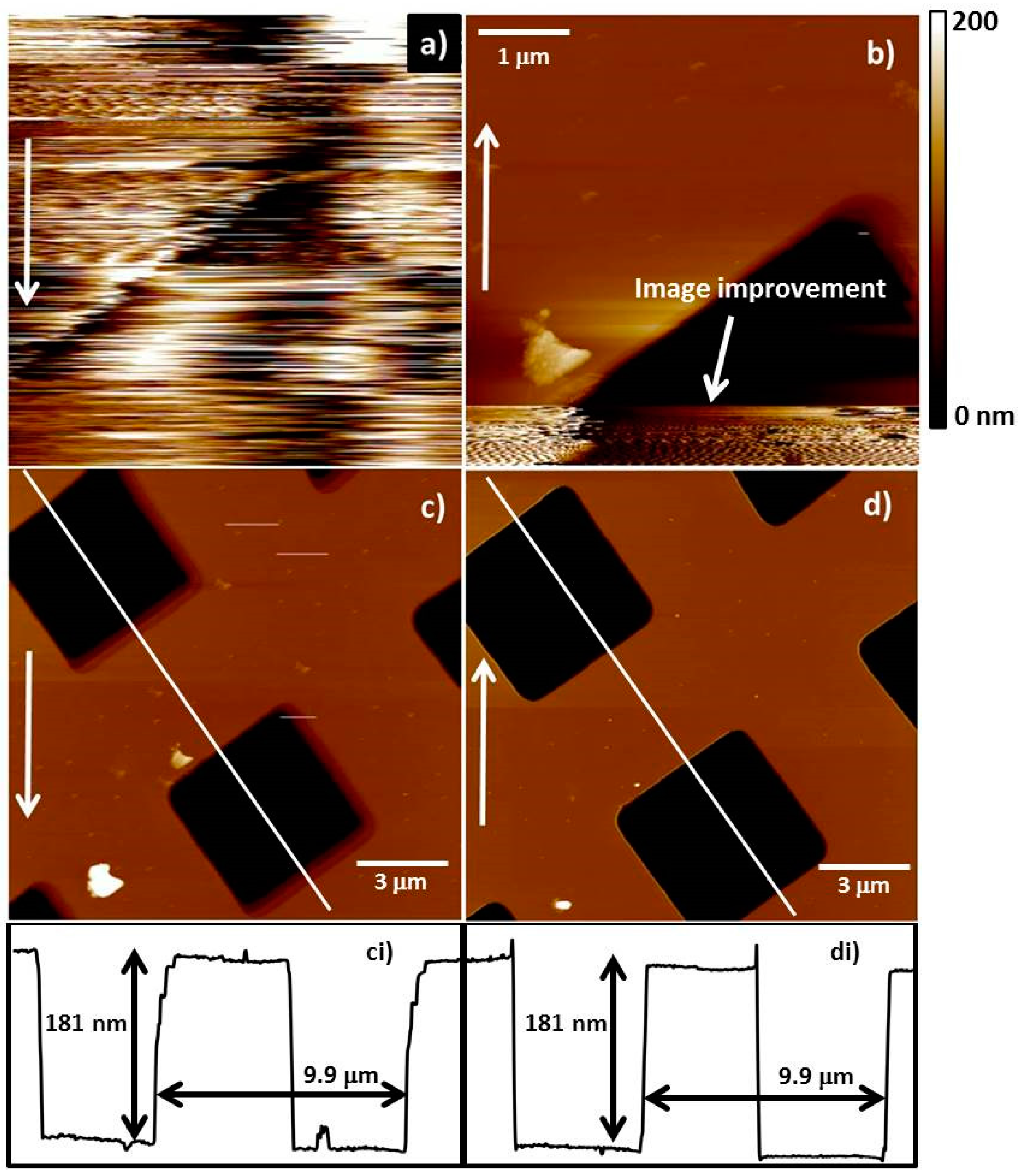
| AFM Tip | Diameter of Attached CNT Fibre Measured Using SEM (nm) | Diameter of Attached CNT Fibre Determined Using Equation (1) (nm) |
|---|---|---|
| Tip 1 | 19 | 63 ± 9.2 |
| Tip 2 | 31 | 78 ± 27 |
| Tip 3 | 40 | 94 ± 20 |
| Without CNT | - | 112 ± 40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slattery, A.D.; Shearer, C.J.; Shapter, J.G.; Quinton, J.S.; Gibson, C.T. Solution Based Methods for the Fabrication of Carbon Nanotube Modified Atomic Force Microscopy Probes. Nanomaterials 2017, 7, 346. https://doi.org/10.3390/nano7110346
Slattery AD, Shearer CJ, Shapter JG, Quinton JS, Gibson CT. Solution Based Methods for the Fabrication of Carbon Nanotube Modified Atomic Force Microscopy Probes. Nanomaterials. 2017; 7(11):346. https://doi.org/10.3390/nano7110346
Chicago/Turabian StyleSlattery, Ashley D., Cameron J. Shearer, Joseph G. Shapter, Jamie S. Quinton, and Christopher T. Gibson. 2017. "Solution Based Methods for the Fabrication of Carbon Nanotube Modified Atomic Force Microscopy Probes" Nanomaterials 7, no. 11: 346. https://doi.org/10.3390/nano7110346