Influence of VO2 Nanoparticle Morphology on the Colorimetric Assay of H2O2 and Glucose
Abstract
:1. Introduction
2. Results and Discussions
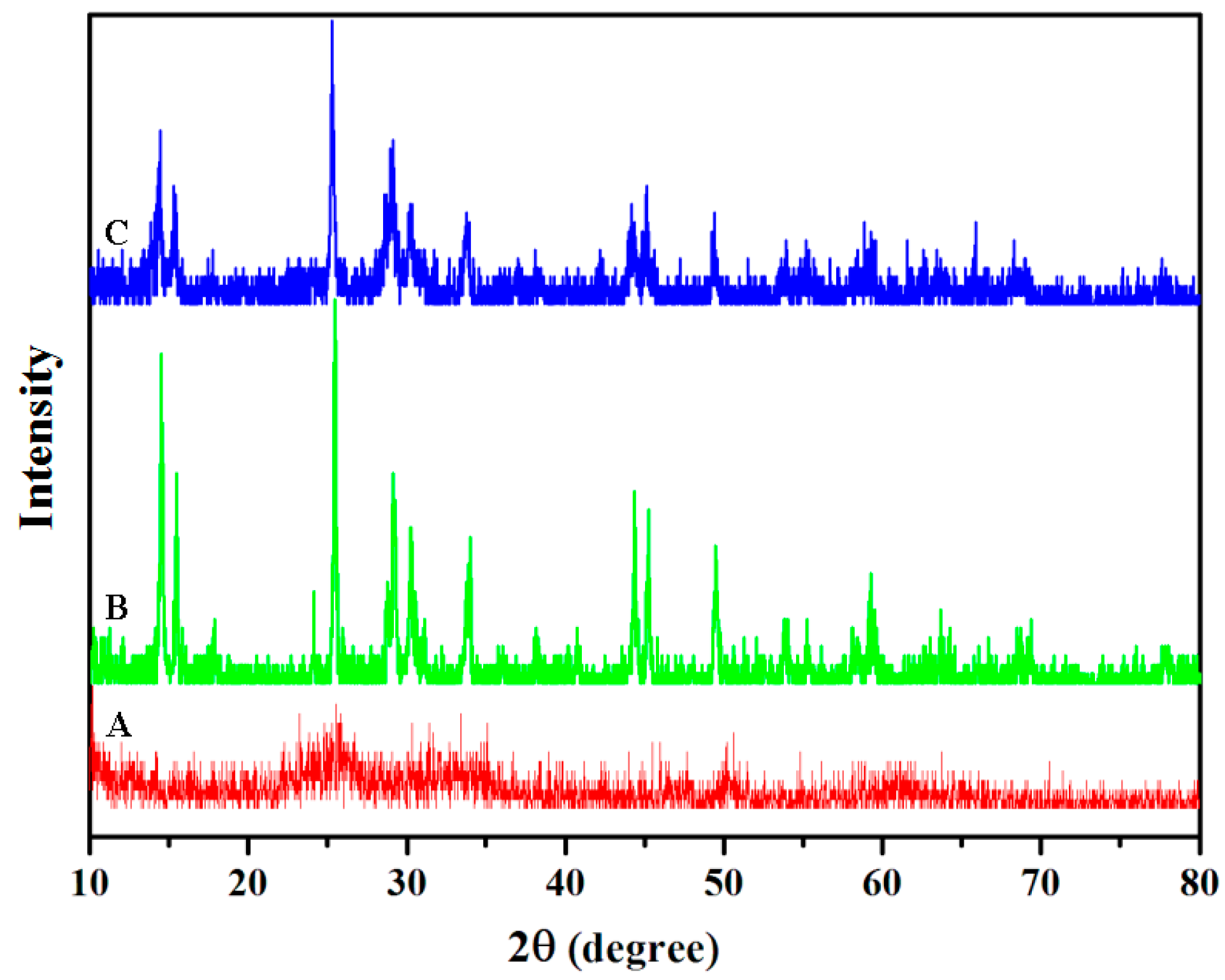
2.1. Characterization of VO2 Nanoparticles
2.2. Principle
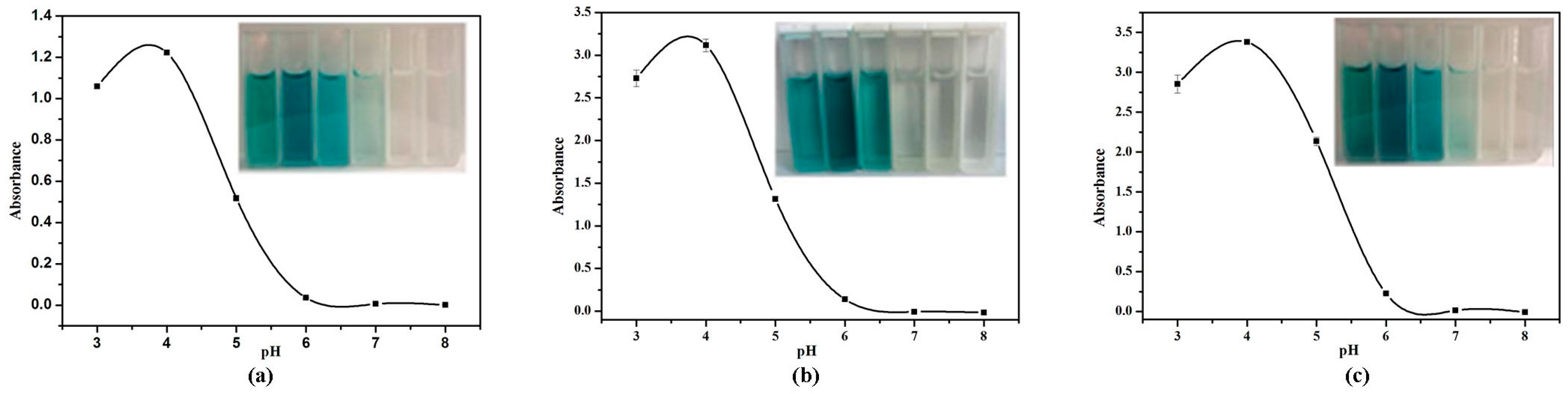
2.3. Effect of pH
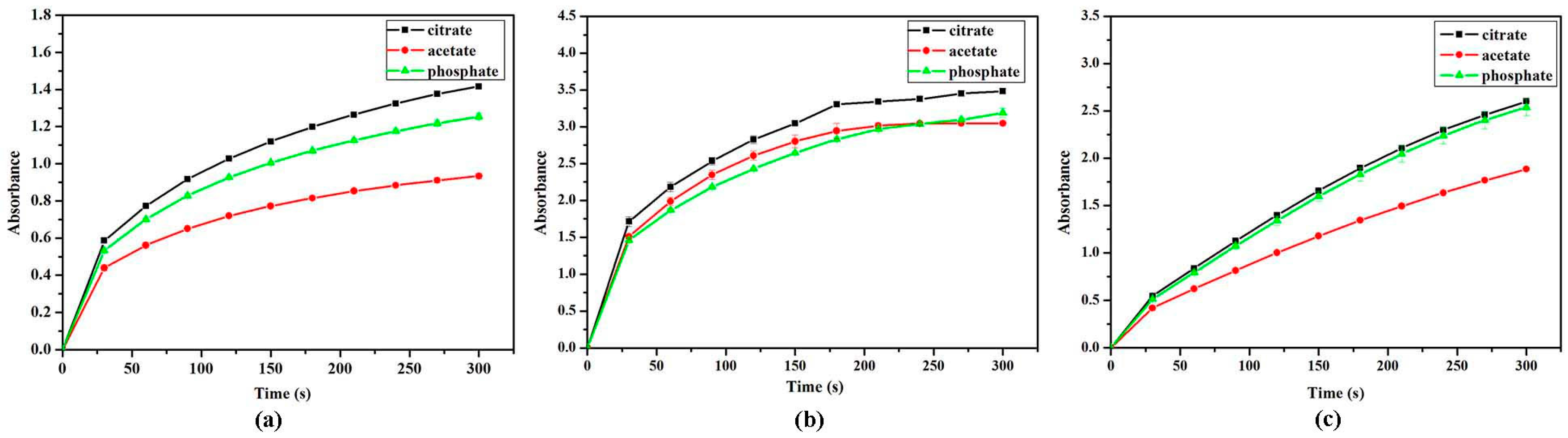
2.4. Effect of Buffers
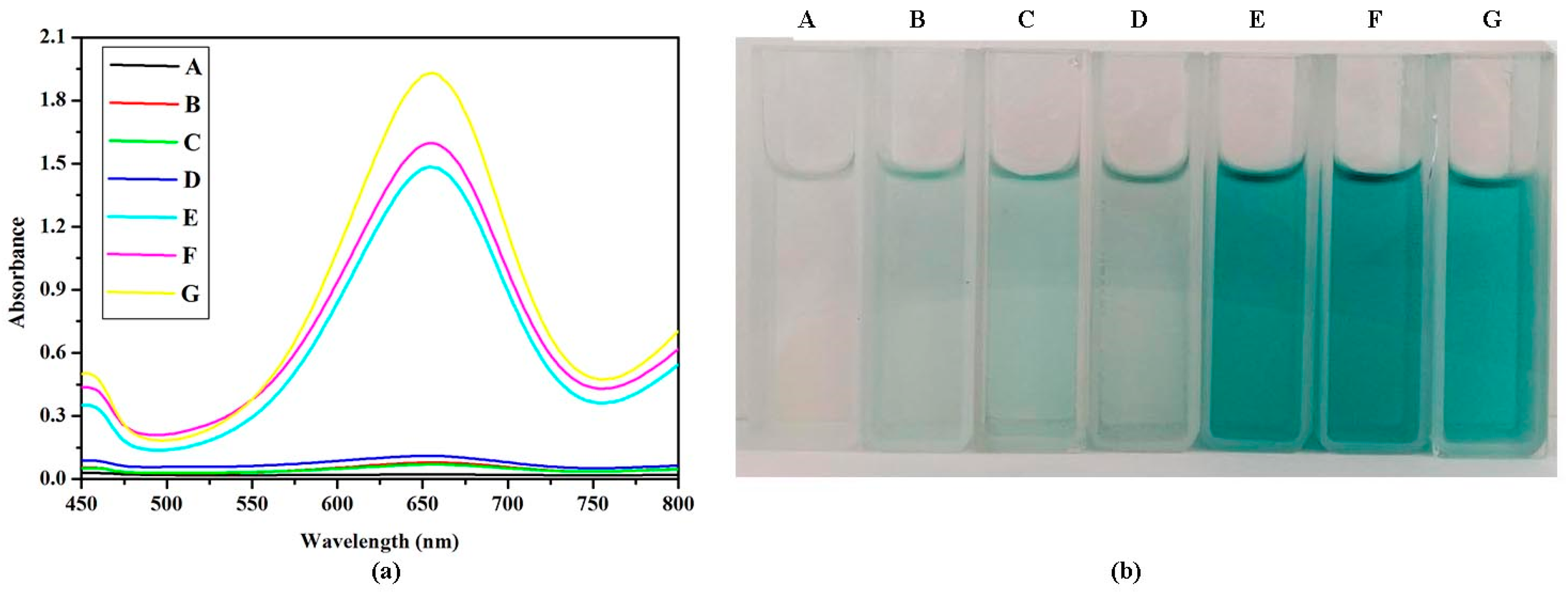
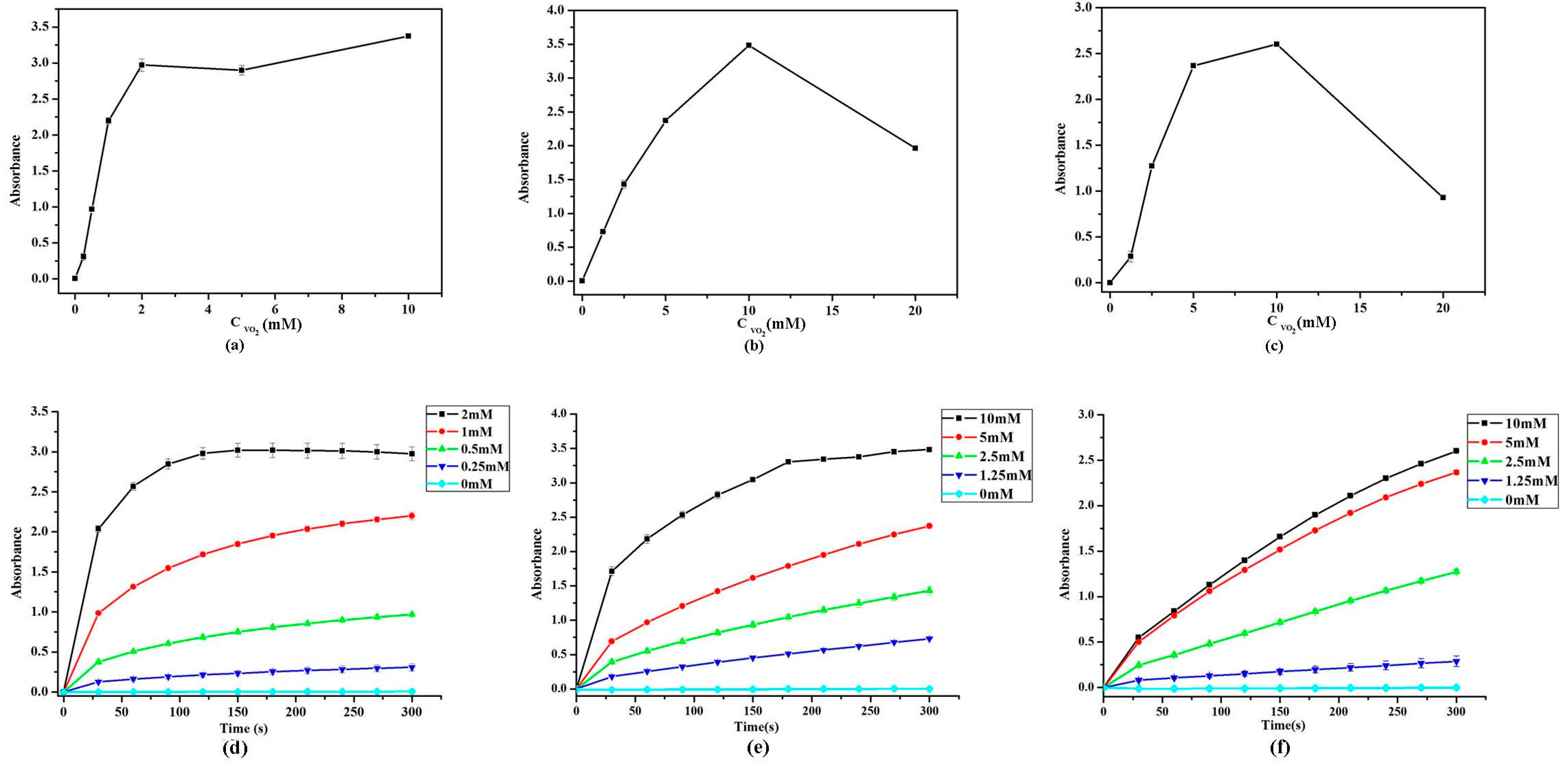
2.5. Effect of VO2 Nanoparticle Morphologies and Concentrations
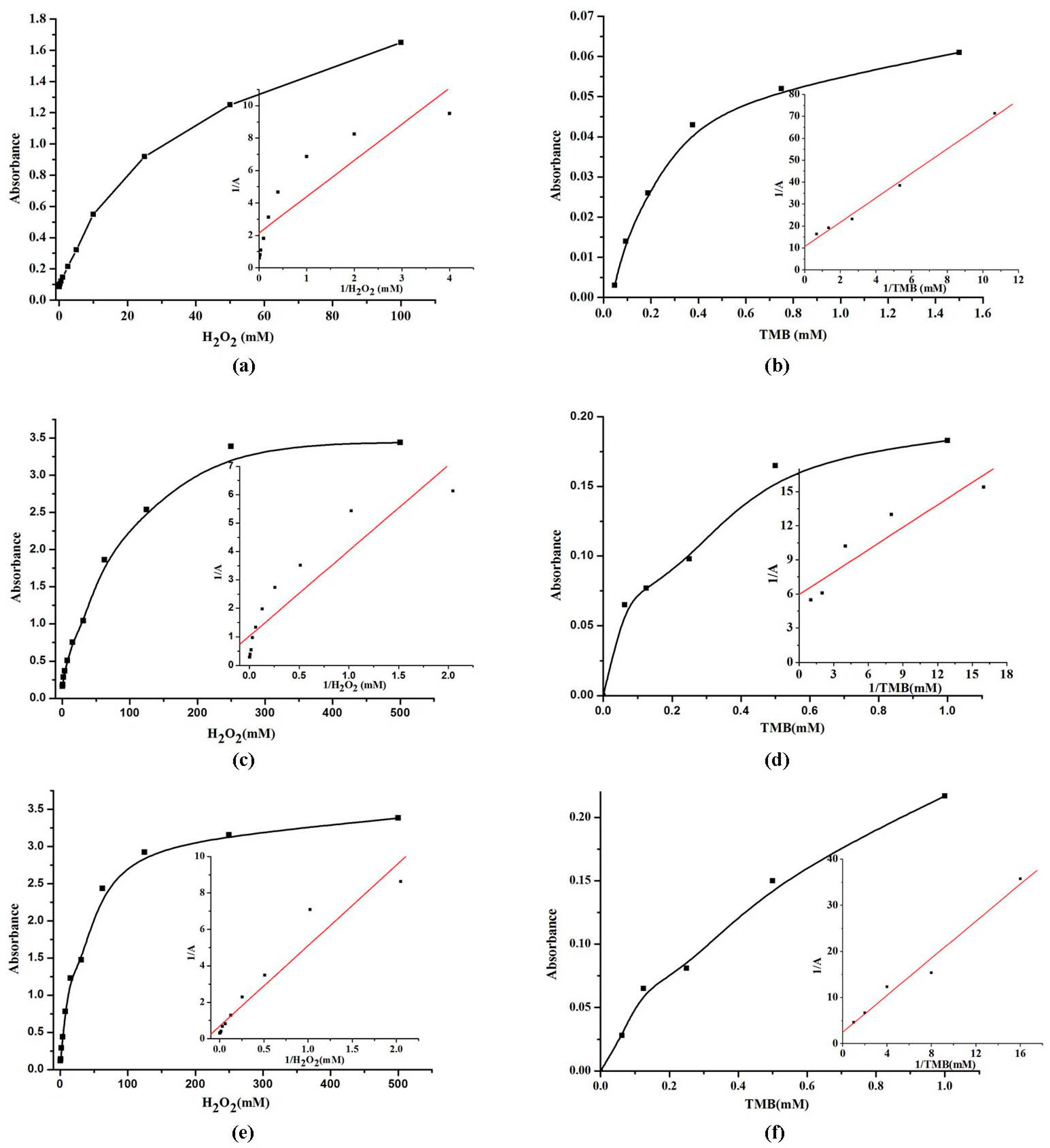
2.6. Steady-State Kinetic Assay
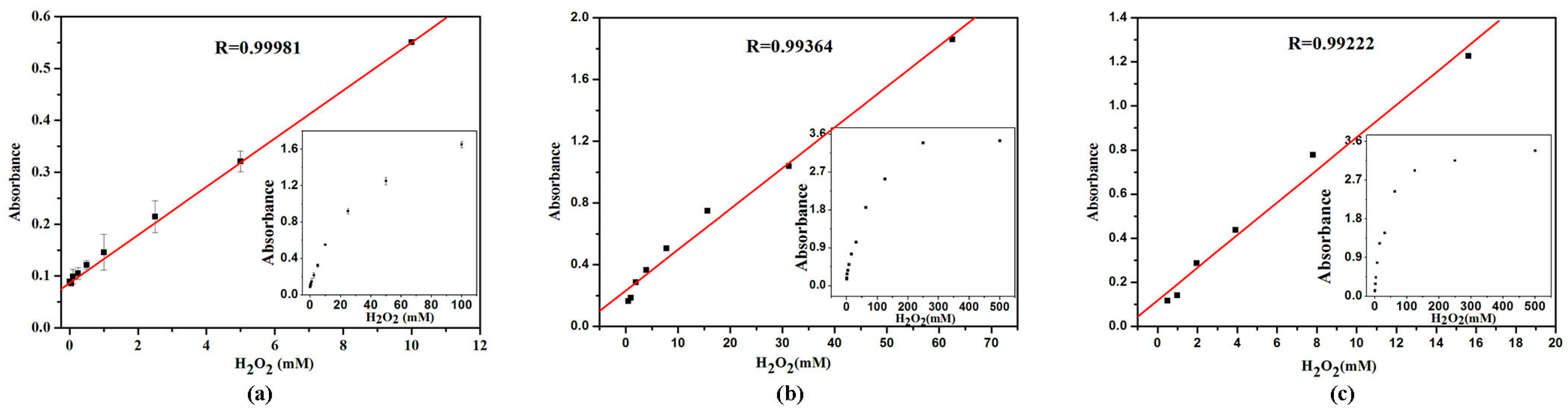
2.7. Calibration Curve for H2O2 and Glucose Detection
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of VO2 Nanoparticles
3.3. Physical Characterization
3.4. H2O2 Detection Using VO2 Nanoparticles as Peroxidase Mimetics
3.5. Glucose Detection Using VO2 Nanoparticles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, Y.Y.; Ran, X.; Lin, Y.H.; Ren, J.S.; Qu, X.G. Self-assembly of an organic-inorganic hybrid nanoflower as an efficient biomimetic catalyst for self-activated tandem reactions. Chem. Commun. 2015, 51, 4386–4389. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.H.; Wang, X.L.; Wen, G.Q.; Jiang, Z.L. A sensitive and selective Victoria blue 4R SERS molecular probe for sodium lauryl sulfate in AuNP/AgCl sol substrate. Sens. Actuators B 2017, 244, 275–281. [Google Scholar] [CrossRef]
- Gao, L.Z.; Zhuang, J.; Nie, L.; Zhang, J.B.; Zhang, Y.; Gu, N.; Wang, T.H.; Feng, J.; Yang, D.L.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Srikanth Vallabani, N.V.; Karakoti, A.S.; Singh, S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: One step detection of blood glucose at physiological pH. Colloids Surfaces B 2017, 153, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics and Their Applications in H2O2 and Glucose Detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Gong, S.W.; Zhang, Y.; Yang, T.; Wang, C.Y.; Gu, N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. Mater. Chem. 2010, 20, 5110–5116. [Google Scholar] [CrossRef]
- Shi, W.B.; Zhang, X.D.; He, S.H.; Huang, Y.M. CoFe2O4 magnetic nanoparticles as a peroxidase mimic mediated chemiluminescence for hydrogen peroxide and glucose. Chem. Commun. 2011, 47, 10785–10787. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Q.; Huang, Y.Z.; Cole, A.J.; Yang, V.C. The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials 2009, 30, 4716–4722. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Lu, F.; Xing, R.M.; Zhu, J.J. Structural Effects of Fe3O4 Nanocrystals on Peroxidase-Like Activity. Chem. Eur. J. 2011, 17, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, M.I.; Cho, D.Y.; Park, H.G. Label-Free Colorimetric Detection of Nucleic Acids Based on Target-Induced Shielding Against the Peroxidase-Mimicking Activity of Magnetic Nanoparticles. Small 2011, 7, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, L.H.; Wang, M.Q.; Wang, D.L.; Tang, H.Q. Sono-enhanced degradation of dye pollutants with the use of H2O2 activated by Fe3O4 magnetic nanoparticles as peroxidase mimetic. Ultrason. Sonochem. 2010, 17, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 14, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-Like Activity of Polymer-Coated Cerium Oxide Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.; Seal, S.S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed]
- He, W.W.; Wu, X.C.; Liu, J.B.; Hu, X.N.; Zhang, K.; Hou, S.; Zhou, W.Y.; Xie, S.S. Design of AgM Bimetallic Alloy Nanostructures (M = Au, Pd, Pt) with Tunable Morphology and Peroxidase-Like Activity. Chem. Mater. 2010, 22, 2988–2994. [Google Scholar] [CrossRef]
- Jv, Y.; Li, B.X.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yin, J.J.; Ning, B.; Wu, X.C.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.Y.; Zhao, Y.L.; Nie, G.J. Direct evidence for catalase and peroxidase activities of ferritine—platinum nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- He, W.W.; Liu, Y.; Yuan, J.S.; Yin, J.J.; Wu, X.C.; Hu, X.N.; Zhang, K.; Liu, J.B.; Chen, C.Y.; Ji, Y.L.; et al. Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials 2011, 32, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Hu, X.N.; Hou, S.; Wen, T.; Liu, W.Q.; Zhu, X.; Wu, X.C. Screening of inhibitors for oxidase mimics of Au@Pt nanorods by catalytic oxidation of OPD. Chem. Commun. 2011, 47, 10981–10983. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Wu, Q.; Shan, Z.; Huang, Q.M. BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosens. Bioelectron. 2011, 26, 3614–3619. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, Z.H.; Cao, H.Y.; Huang, Y.M. Peroxidase-like activity of chitosan stabilized silver nanoparticles for visual and colorimetric detection of glucose. Analyst 2012, 137, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Chen, Z.P.; Choo, J.; Chen, L.X. Naked-eye sensitive ELISA-like assay based on gold-enhanced peroxidase-like immunogold activity. Anal. Bioanal. Chem. 2016, 408, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Liu, A.L.; Wang, L.M.; Li, G.W.; Lin, X.H. Peroxidase-like activity of cupric oxide nanoparticle. Chem. Cat. Chem. 2011, 3, 1151–1154. [Google Scholar] [CrossRef]
- Song, Y.J.; Qu, K.G.; Zhao, C.; Ren, J.S.; Qu, X.G. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Wang, X.H.; Zhao, C.; Qu, K.G.; Ren, J.S.; Qu, X.G. Label-Free Colorimetric Detection of Single Nucleotide Polymorphism by Using Single-Walled Carbon Nanotube Intrinsic Peroxidase-Like Activity. Chem. Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.B.; Wang, Q.L.; Long, Y.J.; Cheng, Z.L.; Chen, S.H.; Zheng, H.Z.; Huang, Y.M. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Chen, Y.; Feng, L.Y.; Ren, J.S.; Qu, X.G. Selective and quantitative cancer cell detection using target-directed functionalized graphene and its synergetic peroxidase-like activity. Chem. Commun. 2011, 47, 4436–4438. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Qu, K.G.; Xu, B.L.; Ren, J.S.; Qu, X.G. Multicolor Luminescent Carbon Nanoparticles: Synthesis, Supramolecular Assembly with Porphyrin, Intrinsic Peroxidase-Like Catalytic Activity and Applications. Nano Res. 2011, 4, 908–920. [Google Scholar] [CrossRef]
- Andre, R.; Natalio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schroder, H.C.; Muller, W.E.G.; Tremel, W. V2O5 Nanowires with an Intrinsic Peroxidase-Like Activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- Sun, J.H.; Li, C.Y.; Qi, Y.F.; Guo, S.L.; Liang, X. Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose. Sensors 2016, 16, 584. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Hong, J.P.; Wang, T.; Xu, X.L.; Yuan, Y.H.; Li, J.L. Oxidative Degradation of Organic Dyes Over Supported Perovskite Oxide LaFeO3/SBA-15 Under Ambient Conditions. Springer 2013, 143, 887–894. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhou, Y.; Qi, H.; Pan, Q.W.; Zhang, Z.; Shi, N.N.; Lu, M.; Stein, A.C.; Li, Y.; Ramanathan, S.; et al. Correlated Perovskites as a New Platform for Super-Broadband-Tunable Photonics. Adv. Mater. 2016, 28, 9117–9125. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.D.; Zhang, L.; Lei, J.Y.; Yang, L.; Zhang, Z.; Lu, X.F.; Wang, C. Monocrystalline VO2 (B) nanobelts: Large-scale synthesis, intrinsic peroxidase-like activity and application in biosensing. J. Mater. Chem. A 2014, 2, 2910–2914. [Google Scholar] [CrossRef]
- Baudrin, E.; Sudant, G.; Larcher, D.; Dunn, B.; Tarascon, J.M. Preparation of Nanotextured VO2[B] from Vanadium Oxide Aerogels. Chem. Mater. 2006, 18, 4369–4374. [Google Scholar] [CrossRef]
- Pan, J.; Zhong, L.; Li, M.; Luo, Y.Y.; Li, G.H. Microwave-Assisted Solvothermal Synthesis of VO2 Hollow Spheres and Their Conversion into V2O5 Hollow Spheres with Improved Lithium Storage Capability. Chem. Eur. J. 2016, 22, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.F.; Cai, M.; Balogh, M.P.; Tessema, M.M.; Lu, Y.F. Symmetric Growth of Pt Ultrathin Nanowires from Dumbbell Nuclei for Use as Oxygen Reduction Catalysts. Nano Res. 2012, 5, 145–151. [Google Scholar] [CrossRef]
- Han, X.G.; Jin, M.S.; Xie, S.F.; Kuang, Q.; Jiang, Z.Y.; Jiang, Y.Q.; Xie, Z.X.; Zheng, L.S. Synthesis of Tin Dioxide Octahedral Nanoparticles with Exposed High-Energy {221} Facets and Enhanced Gas-Sensing Properties. Angew. Chem. Int. Ed. 2009, 48, 9180–9183. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal Synthesis and Photoreactivity of Anatase TiO2 Nanosheets with Dominant {001} Facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.B.; Thomas, P.J.; O’Brien, P. Pyramidal Lead Sulfide Crystallites with High Energy {113} Facets. J. Am. Chem. Soc. 2008, 130, 10892–10894. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.R.; Habas, S.; Yang, P.D. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Zhou, Y.; Park, J.W.; Shi, J.; Chhowalla, M.; Park, H.; Weitz, D.A.; Ramanathan, S. Control of Emergent Properties at a Correlated Oxide Interface with Graphene. Nano Lett. 2015, 15, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.J.; Meng, J.S.; Han, C.H.; Zhao, K.N.; Yan, M.Y.; Mai, L.Q. VO2 Nanowires Assembled into Hollow Microspheres for High-Rate and Long-Life Lithium Batteries. Nano Lett. 2014, 14, 2873–2878. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Wei, Q.L.; An, Q.Y.; Tian, X.C.; Zhao, Y.L.; Xu, X.; Xu, L.; Chang, L.; Zhang, Q.J. Nanoscroll Buffered Hybrid Nanostructural VO2 (B) Cathodes for High-Rate and Long-Life Lithium Storage. Adv. Mater. 2013, 25, 2969–2973. [Google Scholar] [CrossRef] [PubMed]
- Quites, F.J.; Pastore, H.O. Hydrothermal synthesis of nanocrystalline VO2 from poly (diallyldimethylammonium) chloride and V2O5. Mater. Res. Bull. 2010, 45, 892–896. [Google Scholar] [CrossRef]
- Uchaker, E.; Gu, M.; Zhou, N.; Li, Y.W.; Wang, C.M.; Cao, G.Z. Enhanced Intercalation Dynamics and Stability of Engineered Micro/Nano-Structured Electrode Materials: Vanadium Oxide Mesocrystals. Small 2013, 9, 3880–3886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, K.N.; Xu, W.W.; Meng, J.S.; He, L.; An, Q.Y.; Xu, X.; Luo, Y.Z.; Zhao, T.W.; Mai, L.Q. Mesoporous VO2 nanowires with excellent cycling stability and enhanced rate capability for lithium batteries. RSC Adv. 2014, 4, 33332–33337. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, C.R.; Rajamathi, M.; Gautam, U.K.; Wang, X.; Golberg, D.; Bando, Y. N-Doped Graphene—VO2 (B) Nanosheet-Built 3D Flower Hybrid for Lithium Ion Battery. ACS Appl. Mater. Interfaces 2013, 5, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.I.; Shim, J.; Li, T.; Lee, J.; Park, H.G. Fabrication of Nanoporous Nanocomposites Entrapping Fe3O4 Magnetic Nanoparticles and Oxidases for Colorimetric Biosensing. Chem. Eur. J. 2011, 17, 10700–10707. [Google Scholar] [CrossRef] [PubMed]
- Edgar, P.; Yona, K. A Simple Colorimetric Method for the Measurement of Hydrogen Peroxide Produced by Cells in Culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar]
- Chen, X.; Zhou, X.D.; Hu, J. Pt-DNA complexes as peroxidase mimetics and their applications in colorimetric detection of H2O2 and glucose. Anal. Methods 2012, 4, 2183–2187. [Google Scholar] [CrossRef]


| Nanozymes | Substrate | KM (mM) | Vmax (M·S−1) |
|---|---|---|---|
| VO2 nanofibers | TMB | 0.518 | 9.3 × 10−5 |
| VO2 nanofibers | H2O2 | 1.043 | 4.66 × 10−4 |
| VO2 nanosheets | TMB | 0.111 | 1.68 × 10−4 |
| VO2 nanosheets | H2O2 | 2.924 | 9.73 × 10−4 |
| VO2 nanorods | TMB | 0.801 | 3.99 × 10−4 |
| VO2 nanorods | H2O2 | 6.469 | 1.46 × 10−3 |
| V2O5 nanozymes | TMB | 0.738 | 1.85 × 10−5 |
| V2O5 nanozymes | H2O2 | 0.232 | 1.29 × 10−5 |
| Fe3O4 MNPS | TMB | 0.434 | 10.00 × 10−8 |
| Fe3O4 MNPS | H2O2 | 154 | 9.78 × 10−8 |
| HRP | TMB | 0.434 | 1.24 × 10−8 |
| HRP | H2O2 | 3.70 | 2.46 × 10−8 |
| Nanozymes | Linear Range | Limit of Detection | Reference |
|---|---|---|---|
| Fe3O4 MNPS | 1–100 μΜ | 0.5 μΜ | [48] |
| HRP | 1–60 μΜ | 1 μΜ | [49] |
| Pt-DNA complexes | 0.979–17.6 mΜ | 0.392 mΜ | [50] |
| V2O5 nanozymes | 1–500 μΜ | 1 μΜ | [30] |
| VO2 nanofibers | 0.025–10 mM | 0.018 mM | This work |
| VO2 nanosheets | 0.488–62.5 mM | 0.266 mΜ | This work |
| VO2 nanorods | 0.488–15.6 mM | 0.41 mΜ | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, R.; Sun, J.; Qi, Y.; Zhang, B.; Guo, S.; Zhao, M. Influence of VO2 Nanoparticle Morphology on the Colorimetric Assay of H2O2 and Glucose. Nanomaterials 2017, 7, 347. https://doi.org/10.3390/nano7110347
Tian R, Sun J, Qi Y, Zhang B, Guo S, Zhao M. Influence of VO2 Nanoparticle Morphology on the Colorimetric Assay of H2O2 and Glucose. Nanomaterials. 2017; 7(11):347. https://doi.org/10.3390/nano7110347
Chicago/Turabian StyleTian, Rui, Jiaheng Sun, Yanfei Qi, Boyu Zhang, Shuanli Guo, and Mingming Zhao. 2017. "Influence of VO2 Nanoparticle Morphology on the Colorimetric Assay of H2O2 and Glucose" Nanomaterials 7, no. 11: 347. https://doi.org/10.3390/nano7110347