Endocytosis of Corn Oil-Caseinate Emulsions In Vitro: Impacts of Droplet Sizes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Varying Droplet Size Emulsions
2.3. Droplet Diameter Analysis
2.4. Droplet Size Stability
2.5. Transmission Electron Microscopy (TEM) Analysis
2.6. Cytotoxicity of Lipid-Based Emulsions
2.7. Cellular Uptake
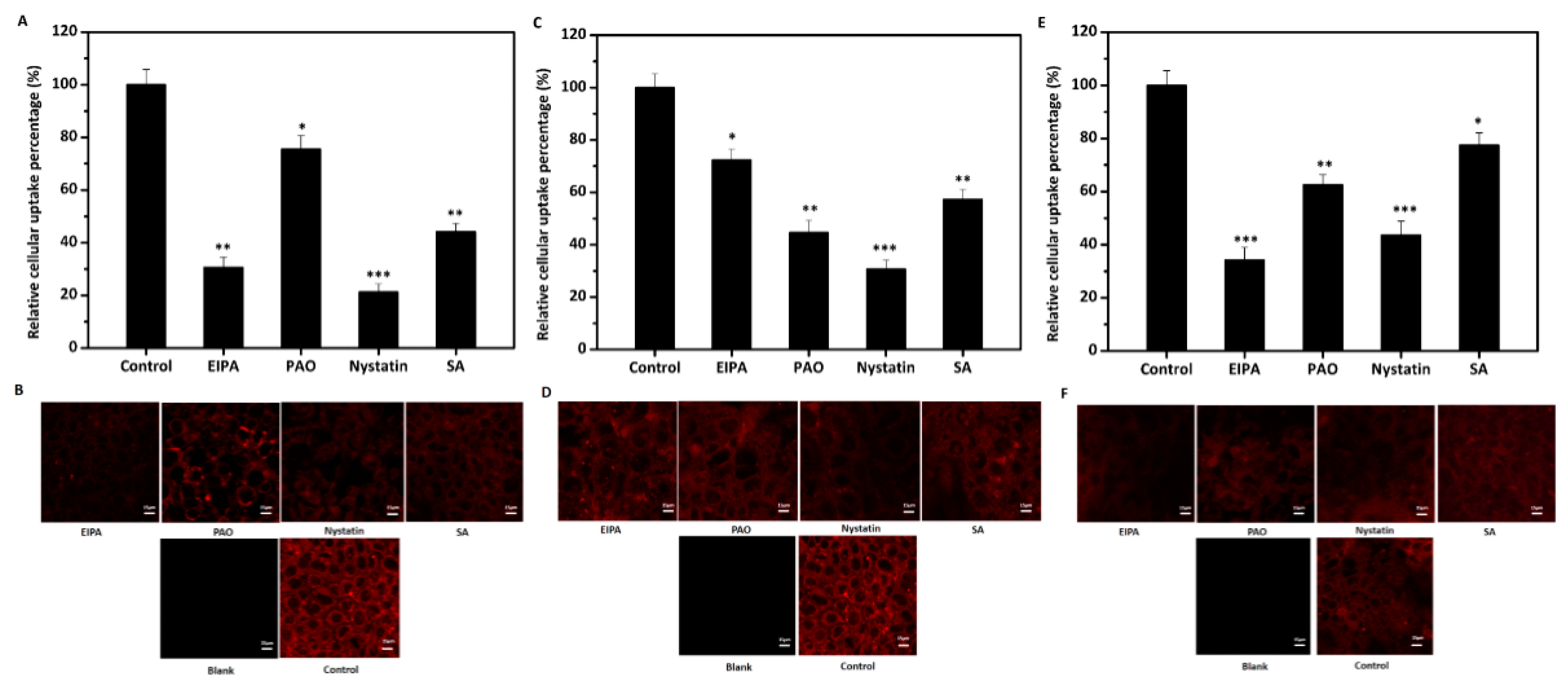
2.8. Effects of Inhibitors on Cellular Uptake of Lipid-Based Nanoemulsions
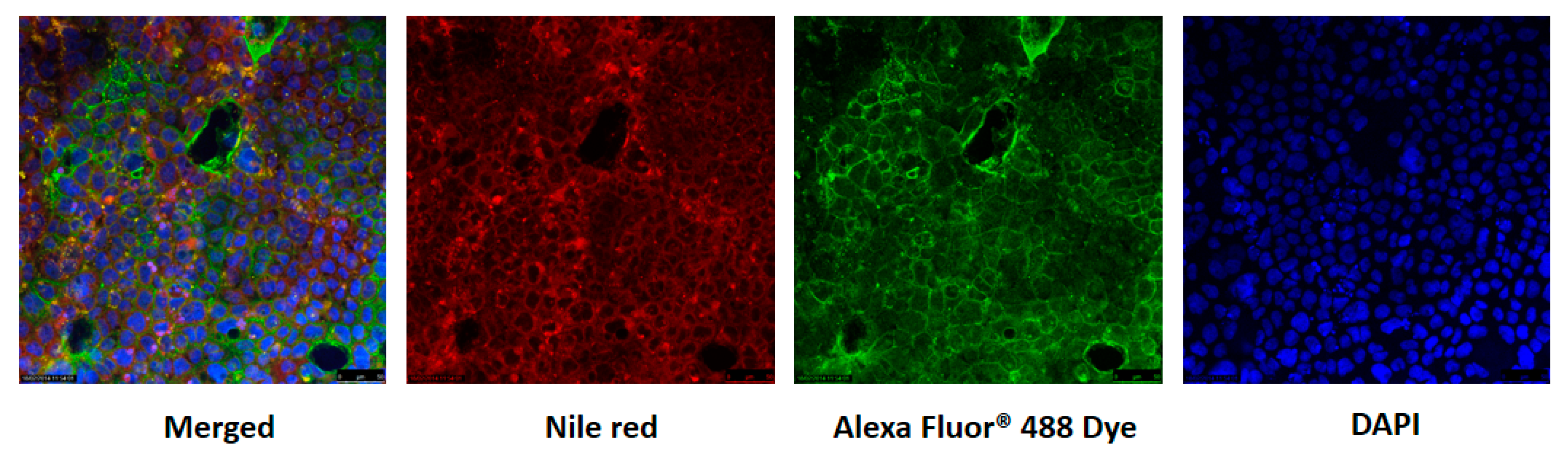
2.9. Confocal Laser Scanning Microscopy (CLSM)
2.10. Quantification Study
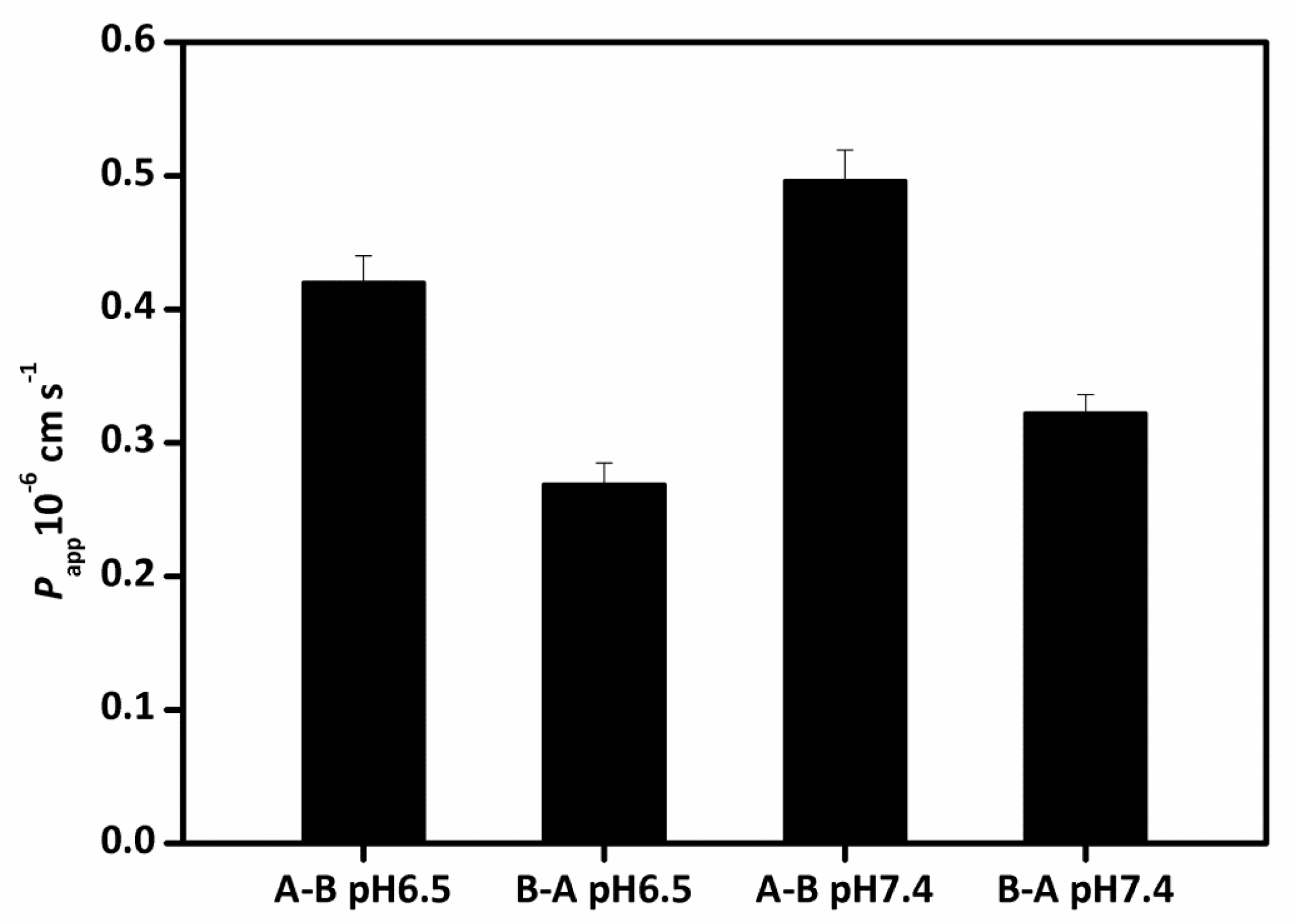
2.11. Transport Study
2.12. Statistical Analysis
3. Results and Discussion
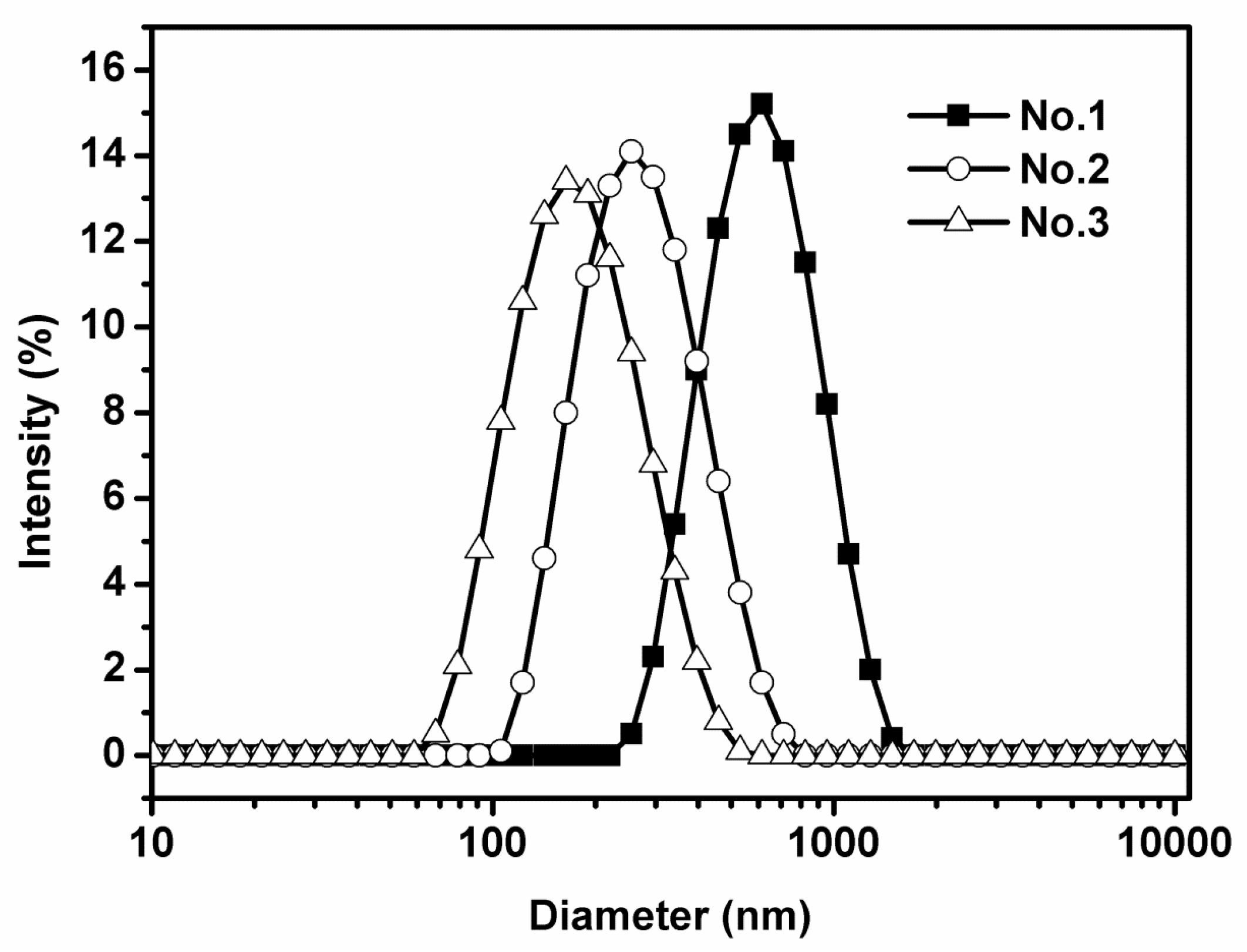
3.1. Characteristics of Emulsions
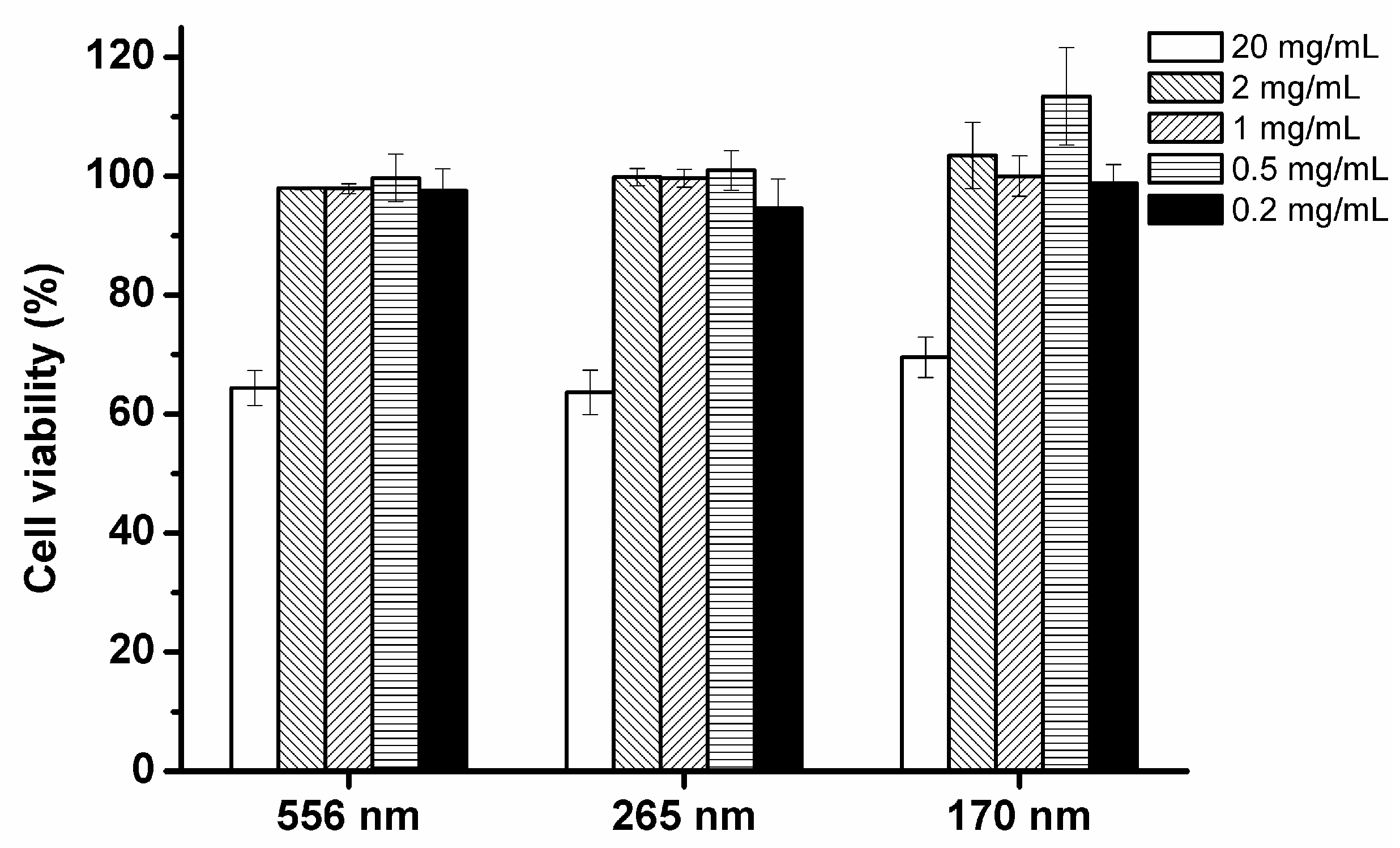
3.2. Cytotoxicity of Lipid-Based Emulsions
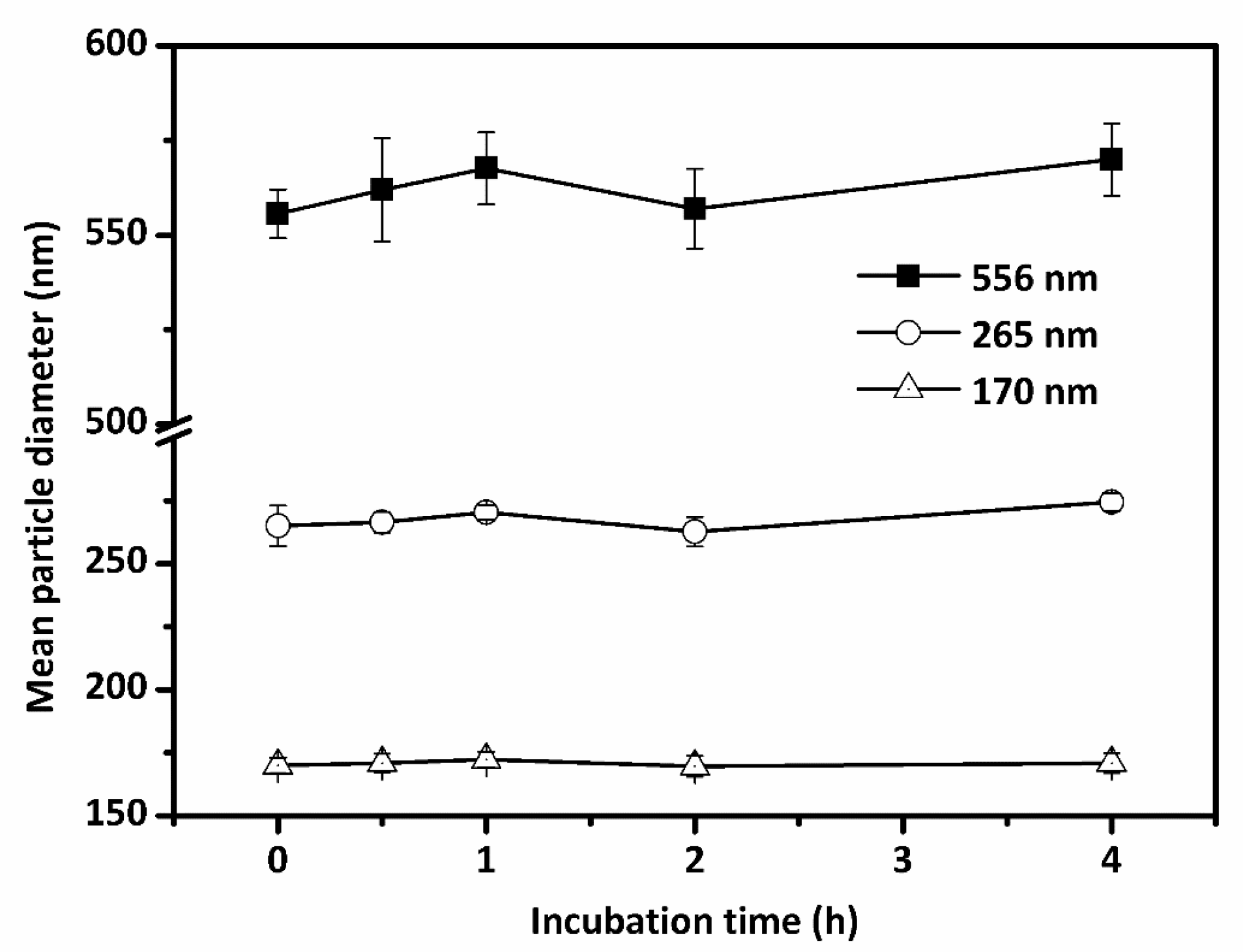
3.3. Mean Droplet Diameter Stability of Lipid-Based Emulsions
3.4. Effects of Droplet Size on Cellular Uptake of Lipid-Based Emulsions
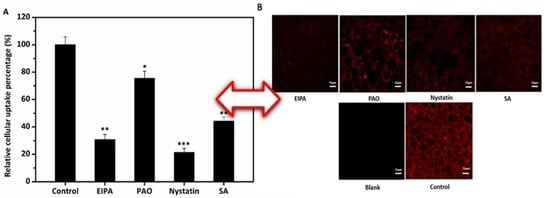
3.5. Effects of Four Inhibitors on the Cellular Uptake of Lipid-Based Emulsions
3.6. Effects of Temperature on the Cellular Uptake of Nanoemulsions
3.7. Transport Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SC | sodium caseinate |
| CLSM | Confocal laser scanning microscopy |
| BC | beat-carotene |
| o/w | Oil-in-water |
| ROS | reactive oxygen species |
| EIPA | 5-(N-Ethyl-N-isopropyl)amiloride |
| PAO | phenylarsine oxide |
| FBS | fetal bovine serum |
| TER | transepithelial electrical resistance |
| Dz | mean droplet sizes |
| PDI | polydispersity index |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| TEM | Transmission electron microscopy |
| DMEM | the dulbecco’s modified eagle medium |
References
- Augustin, M.A.; Sanguansri, L. Challenges and Solutions to Incorporation of Nutraceuticals in Foods. Annu. Rev. Food Sci. Technol. 2015, 6, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Fan, Y.; Zhang, Y.; Zhao, L. Characterization of catechin-α-lactalbumin conjugates and the improvement in β-carotene retention in an oil-in-water nanoemulsion. Food Chem. 2016, 205, 73–80. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; McClements, D.J. Food-grade microemulsions, nanoemulsions and emulsions: Fabrication from sucrose monopalmitate & lemon oil. Food Hydrocoll. 2011, 25, 1413–1423. [Google Scholar]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Dickinson, E. Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf. B Biointerfaces 2010, 81, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Fan, Y.; Yokoyama, W.; Zhang, Y.; Zhao, L. Thermal Degradation and Isomerization of β-Carotene in Oil-in-Water Nanoemulsions Supplemented with Natural Antioxidants. J. Agric. Food Chem. 2016, 64, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhang, Y.; Liang, R.; Zhong, F.; Ma, J. Beta-Carotene Chemical Stability in Nanoemulsions Was Improved by Stabilized with Beta-Lactoglobulin-Catechin Conjugates through Free Radical Method. J. Agric. Food Chem. 2015, 63, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Bhushani, J.A.; Karthik, P.; Anandharamakrishnan, C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016, 56, 372–382. [Google Scholar] [CrossRef]
- Heo, W.; Kim, J.H.; Pan, J.H.; Kim, Y.J. Lecithin-Based Nano-emulsification Improves the Bioavailability of Conjugated Linoleic Acid. J. Agric. Food Chem. 2016, 64, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Li, Y.; Zhong, F.; Yokoyama, W. The physicochemical stability and in vitro bioaccessibility of beta-carotene in oil-in-water sodium caseinate emulsions. Food Hydrocoll. 2014, 35, 19–27. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Y.; Song, M.; Fang, X.; Cao, Y.; McClements, D.J.; Xiao, H. Improving intracellular uptake of 5-demethyltangeretin by food grade nanoemulsions. Food Res. Int. 2014, 62, 98–103. [Google Scholar] [CrossRef]
- Paul, D.; Mukherjee, S.; Chakraborty, R.; Mallick, S.K.; Dhar, P. Comparative real-time study of cellular uptake of a formulated conjugated linolenic acid rich nano and conventional macro emulsions and their bioactivity in ex vivo models for parenteral applications. Colloids Surf. B Biointerfaces 2015, 126, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.-H.; Xu, Y.; Chen, S.-Q.; Cheng, B.; Hu, F.-Q.; You, J.; Du, Y.-Z.; Yuan, H. Transport Mechanisms of Solid Lipid Nanoparticles across Caco-2 Cell Monolayers and their Related Cytotoxicology. ACS Appl. Mater. Interfaces 2016, 8, 5929–5940. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stoter, M.; et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Chan, W.C.W. Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Controlled Release of β-Carotene in β-Lactoglobulin-Dextran-Conjugated Nanoparticles’ in Vitro Digestion and Transport with Caco-2 Monolayers. J. Agric. Food Chem. 2014, 62, 8900–8907. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Cellular Uptake of β-Carotene from Protein Stabilized Solid Lipid Nanoparticles Prepared by Homogenization-Evaporation Method. J. Agric. Food Chem. 2014, 62, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.I.; Stanker, L.H.; Lee, K.; Jin, R.; Cheng, L.W. Translocation of botulinum neurotoxin serotype A and associated proteins across the intestinal epithelia. Cell. Microbiol. 2015, 17, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Properties of Emulsions Stabilized with Milk Proteins: Overview of Some Recent Developments. J. Dairy Sci. 1997, 80, 13. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.P. Lipid nanocarriers improve paclitaxel transport throughout human intestinal epithelial cells by using vesicle-mediated transcytosis. J. Control. Release 2009, 140, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; KimBetty, Y.S.; Rutka, J.T.; ChanWarren, C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nano 2008, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; He, L.; McClements, D.J.; Xiao, H. Uptake of Gold Nanoparticles by Intestinal Epithelial Cells: Impact of Particle Size on Their Absorption, Accumulation, and Toxicity. J. Agric. Food Chem. 2015, 63, 8044–8049. [Google Scholar] [CrossRef] [PubMed]
- Harush-Frenkel, O.; Rozentur, E.; Benita, S.; Altschuler, Y. Surface Charge of Nanoparticles Determines Their Endocytic and Transcytotic Pathway in Polarized MDCK Cells. Biomacromolecules 2008, 9, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Teng, Z.; Wang, T.T.Y.; Wang, Q. Cellular Uptake and Transport of Zein Nanoparticles: Effects of Sodium Caseinate. J. Agric. Food Chem. 2013, 61, 7621–7629. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.; Guerrero, J.M.M.; Briviba, K.; Rechkemmer, G.; Schuchmann, H.P.; Schubert, H. Cellular Uptake of Carotenoid-Loaded Oil-in-Water Emulsions in Colon Carcinoma Cells in Vitro. J. Agric. Food Chem. 2006, 54, 9366–9369. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Herant, M.; Heinrich, V.; Dembo, M. Mechanics of neutrophil phagocytosis: Behavior of the cortical tension. J. Cell Sci. 2005, 118, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Shi, X.; Gao, H. Cellular Uptake of Elastic Nanoparticles. Phys. Rev. Lett. 2011, 107, 98101. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhen, X.; Wang, X.; Wu, W.; Jiang, X. Cellular entry fashion of hollow milk protein spheres. Soft Matter 2011, 7, 11526–11534. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Nusrat, A.; Parkos, C.A. Endocytosis of Epithelial Apical Junctional Proteins by a Clathrin-mediated Pathway into a Unique Storage Compartment. Mol. Biol. Cell 2004, 15, 176–188. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lin, P.; Jia, Z.; Du, W.; Qu, W.; Yuan, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. The transport mechanisms of polymer nanoparticles in Caco-2 epithelial cells. Biomaterials 2013, 34, 6082–6098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Dai, W.; He, B.; Wang, J.; He, Z.; Zhang, X.; Zhang, Q. Monitoring the transport of polymeric micelles across MDCK cell monolayer and exploring related mechanisms. J. Control. Release 2012, 158, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.M.; Rajasekaran, D.; Ludford-Menting, M.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H. Transport of stearic acid-based solid lipid nanoparticles (SLNs) into human epithelial cells. Colloids Surf. B Biointerfaces 2016, 140, 204–212. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Investigation of the Absorption Mechanism of Solubilized Curcumin Using Caco-2 Cell Monolayers. J. Agric. Food Chem. 2011, 59, 9120–9126. [Google Scholar] [CrossRef] [PubMed]



| Endocytosis Inhibitors | Inhibitor of | Concentrations |
|---|---|---|
| EIPA | macropinocytosis pathway | 50 μM |
| PAO | clathrin-mediated endocytosis | 10 μM |
| Nystatin | caveolae/lipid raft-dependent endocytosis | 30 μM |
| Sodium azide | energy-dependent route | 1 mg/mL |
| Type | Compositions | Initial Emulsion | Emulsion after 30 Days | ||||
|---|---|---|---|---|---|---|---|
| Mean Droplet Size (nm) | PDI | Zeta-Potential | Mean Droplet Size (nm) | PDI | Zeta-Potential | ||
| 1 | 10% corn oil, 2% sodium caseinate, 88% distilled water | 556.4 ± 3.4a | 0.198 ± 0.037 | −40.5 ± 1.7a | 629.5 ± 2.7a | 0.235 ± 0.041 | −43.6 ± 1.5a |
| 2 | 10% corn oil, 2% sodium caseinate, 88% distilled water | 265.2 ± 2.5b | 0.150 ± 0.025 | −41.2 ± 1.4a | 283 ± 1.6.5b | 0.176 ± 0.017 | −41.5 ± 1.2a |
| 3 | 10% corn oil, 2% sodium caseinate, 88% distilled water | 170.3 ± 2.1c | 0.113 ± 0.021 | −42.3 ± 1.8a | 182 ± 2.4c | 0.142 ± 0.026 | −42.6 ± 1.7a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhang, Y.; Yokoyama, W.; Yi, J. Endocytosis of Corn Oil-Caseinate Emulsions In Vitro: Impacts of Droplet Sizes. Nanomaterials 2017, 7, 349. https://doi.org/10.3390/nano7110349
Fan Y, Zhang Y, Yokoyama W, Yi J. Endocytosis of Corn Oil-Caseinate Emulsions In Vitro: Impacts of Droplet Sizes. Nanomaterials. 2017; 7(11):349. https://doi.org/10.3390/nano7110349
Chicago/Turabian StyleFan, Yuting, Yuzhu Zhang, Wally Yokoyama, and Jiang Yi. 2017. "Endocytosis of Corn Oil-Caseinate Emulsions In Vitro: Impacts of Droplet Sizes" Nanomaterials 7, no. 11: 349. https://doi.org/10.3390/nano7110349