Phonon Confinement Induced Non-Concomitant Near-Infrared Emission along a Single ZnO Nanowire: Spatial Evolution Study of Phononic and Photonic Properties
Abstract
:1. Introduction
2. Results
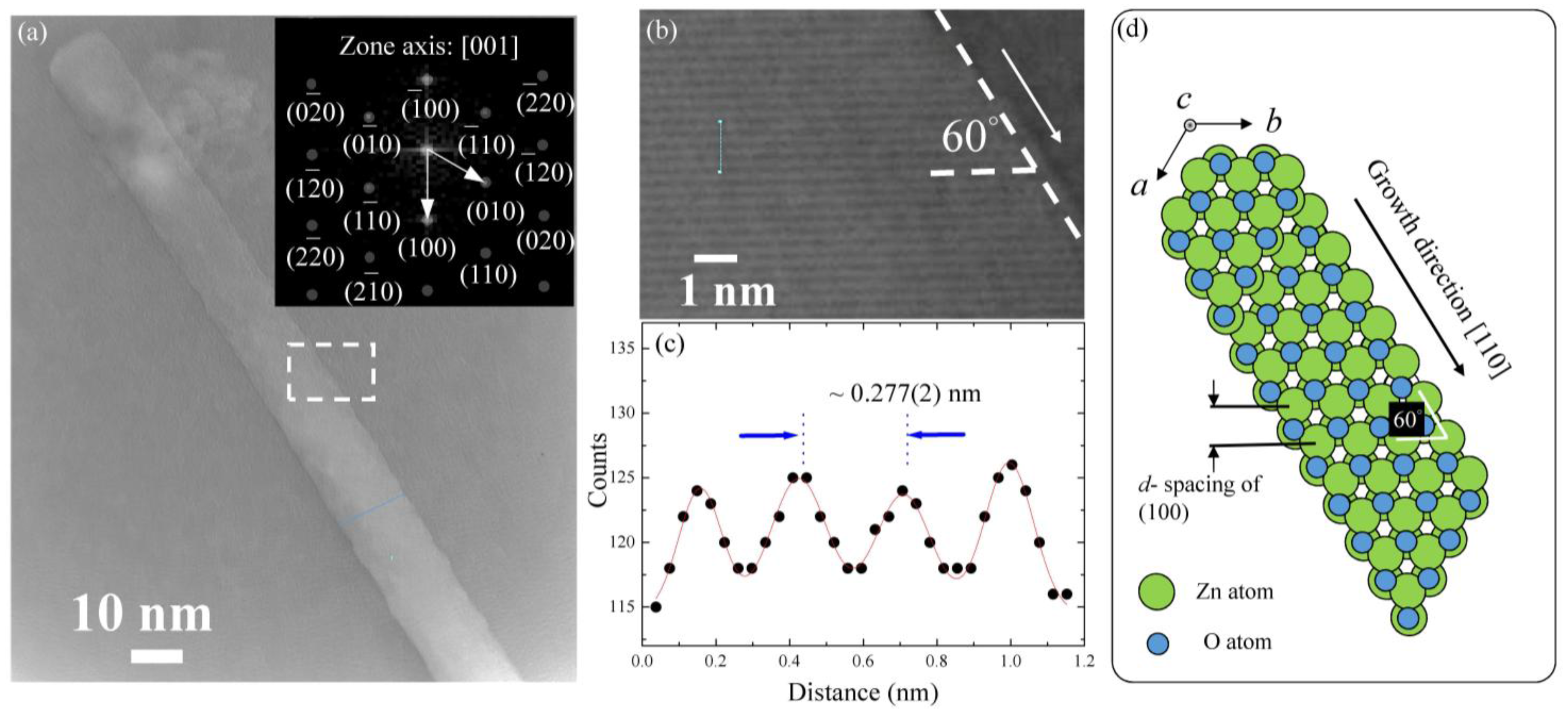
2.1. Sample Characterization and TEM Analysis
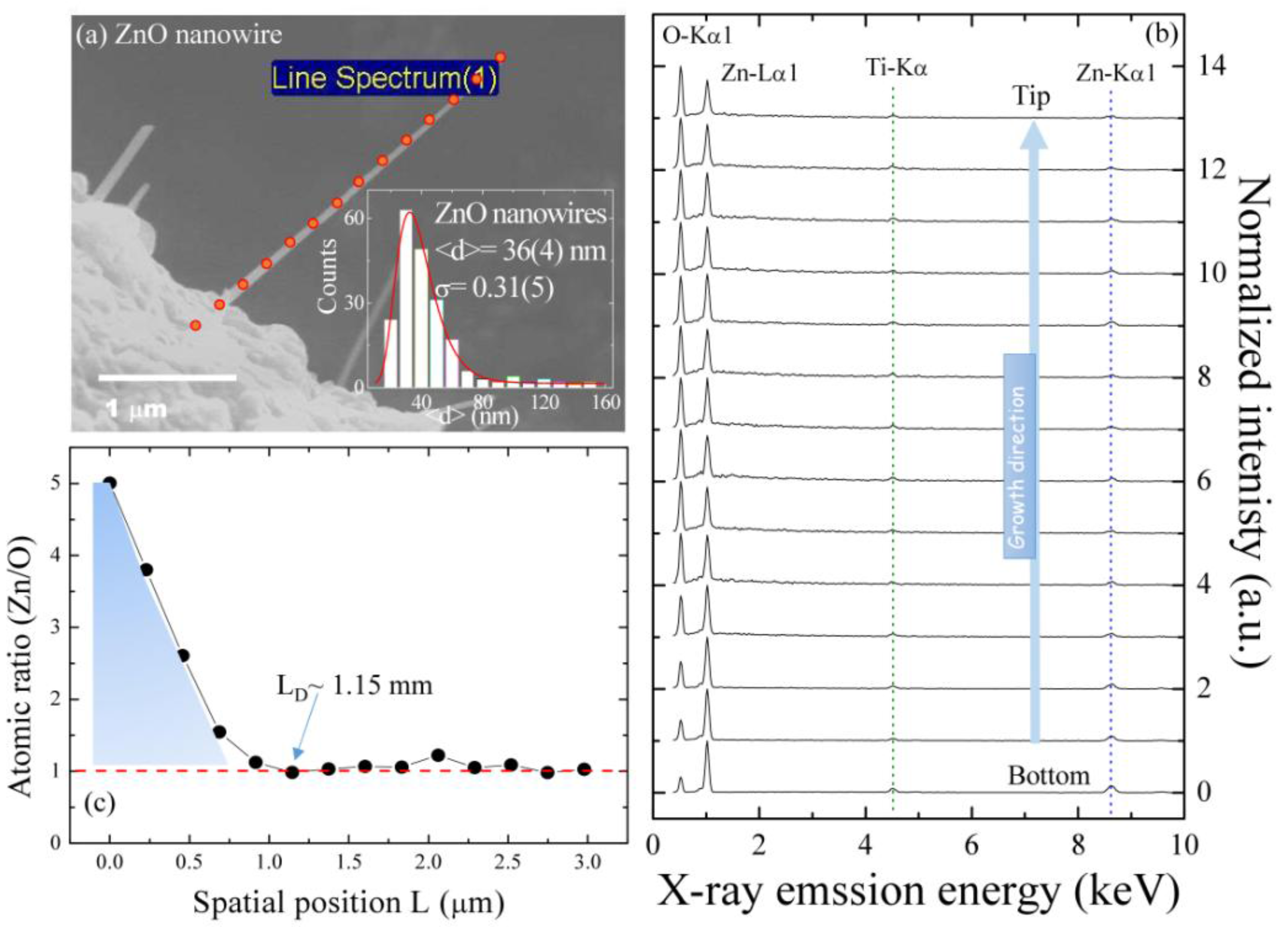
2.2. High Spatial Resolution of Energy Dispersive Spectroscopy Mapping
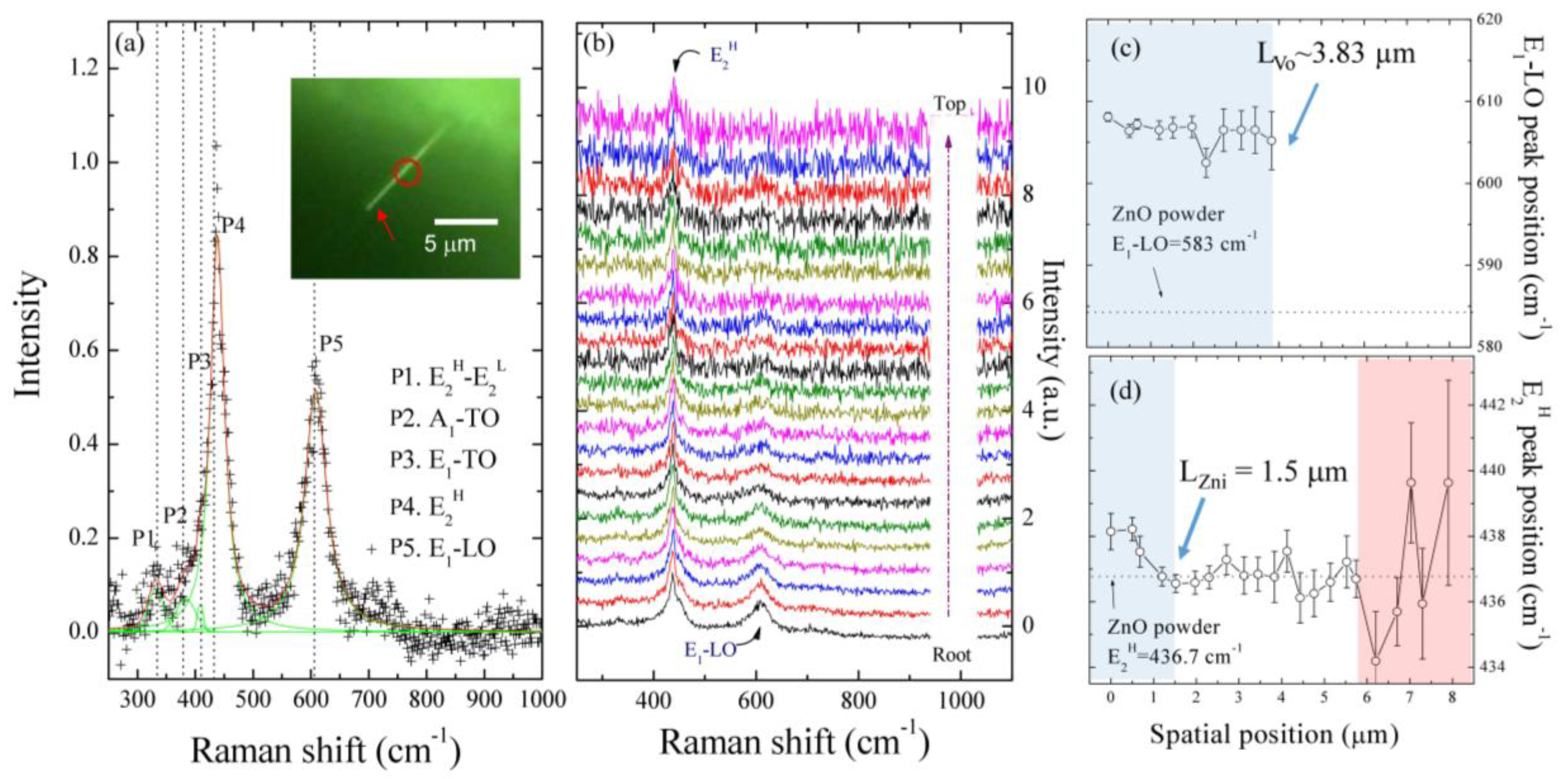
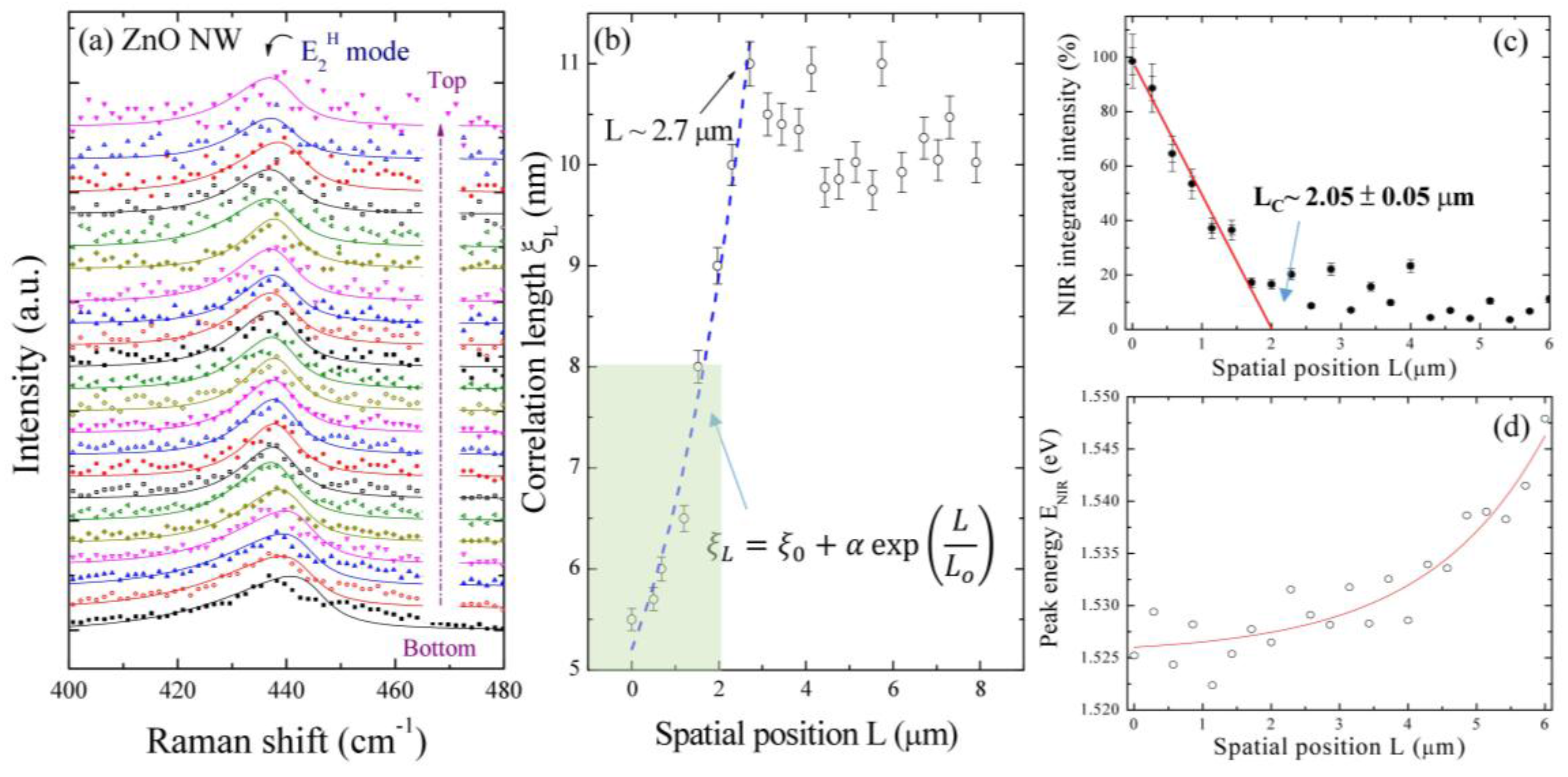
2.3. Spatial Scanning Confocal Raman Measurements along a Single ZnO NW
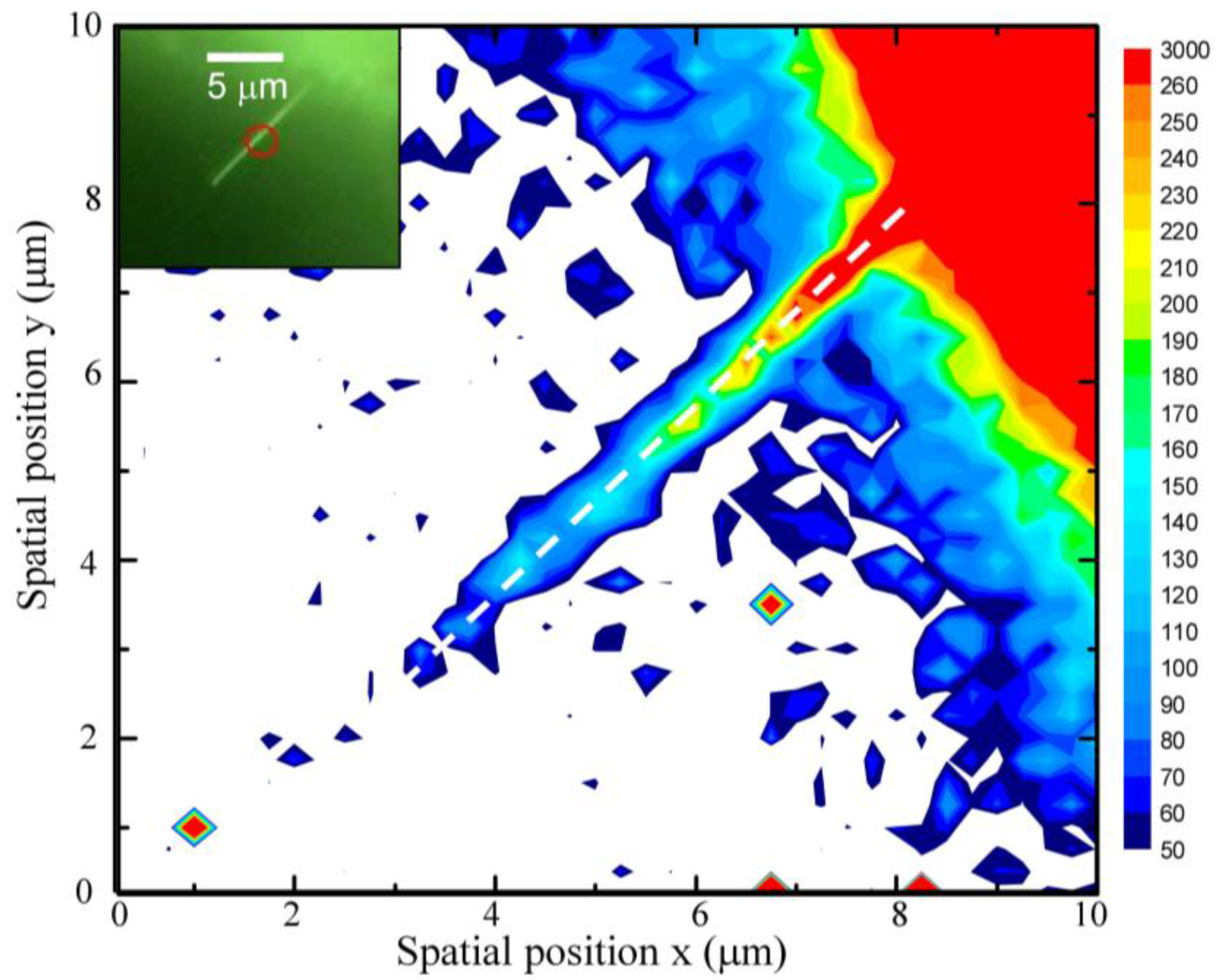
2.4. NIR Emission Mapping along a ZnO NW
2.5. Linear Chain Modeling and the Phonon Peak of E2H Mode Mapping
3. Discussion
4. Method
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials, and Device Technology, 1st ed.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Shih, H.Y.; Chen, Y.T.; Huang, N.H.; Wei, C.M.; Chen, Y.F. Size-dependent photo elastic effect in ZnO nanorods. Appl. Phys. Lett. 2009, 94, 021908. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-Temperature Ultraviolet Nanowire Nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, F.; Villani, M.; Catellani, A.; Calzolari, A.; Cicero, G.; Calestani, D.; Calestani, G.; Zappettini, A.; Dierre, B.; Sekiguchi, T.; et al. Zn vacancy induced green luminescence on non-polar surfaces in ZnO nanostructures. Sci. Rep. 2014, 4, 5188. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.S.; Gandhi, A.C.; Pandit, S.D.; Pant, J.; Chan, T.-S.; Cheng, C.-L.; Ma, Y.-R.; Wu, S.Y. Oxygen induced strained ZnO nanoparticles: An investigation of Raman scattering and visible photoluminescence. J. Mater. Chem. C 2014, 2, 7264–7274. [Google Scholar] [CrossRef]
- Shih, P.-H.; Cheng, C.-L.; Ma, Y.-R.; Wu, S.Y. Short range correlation length study in a single ZnO nanowire and its impact on phonon confinement. Appl. Phys. Lett. 2016, 108, 113101. [Google Scholar] [CrossRef]
- Geng, C.; Jiang, Y.; Meng, X.; Zapien, J.A.; Lee, C.S.; Lifshitz, Y.; Lee, S.T. Well-aligned ZnO nanowire arrays fabricated on silicon substrates. Adv. Funct. Mater. 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, S.M.; Wang, H.W.; Du, Z.L. Raman scattering and photoluminescence of Fe-doped ZnO nanocantilever arrays. Chin. Sci. Bull. 2008, 53, 1639–1643. [Google Scholar]
- Ashkenov, N.; Mbenkum, B.N.; Bundesmann, C.; Riede, V.; Lorenz, M.; Spemann, D.; Kaidashev, E.M.; Kasic, A.; Schubert, M.; Grundmann, M.; et al. Infrared dielectric functions and phonon modes of high-quality ZnO films. J. Appl. Phys. 2003, 93, 126–133. [Google Scholar] [CrossRef]
- Scott, J.F. UV Resonant Raman Scattering in ZnO. Phys. Rev. B 1970, 2, 1209. [Google Scholar] [CrossRef]
- Bǿrseth, T.M.; Svensson, B.G.; Kuznetsov, A.Y.; Klason, P.; Zhao, Q.X.; Willander, M. Identification of oxygen and zinc vacancy optical signals in ZnO. Appl. Phys. Lett. 2006, 89, 262112. [Google Scholar] [CrossRef]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1995, 68, 403–405. [Google Scholar] [CrossRef]
- Wu, X.L.; Siu, G.G.; Fu, C.L.; Ong, H.C. Photoluminescence and cathode luminescence studies of stoichiometric and oxygen-deficient ZnO films. Appl. Phys. Lett. 2001, 78, 2285–2287. [Google Scholar] [CrossRef]
- Lin, B.; Fu, Z.; Anhui, H.; Tsai, C.L. Green luminescent center in undoped zinc oxide films deposited on silicon substrates. Appl. Phys. Lett. 2001, 79, 943–945. [Google Scholar] [CrossRef]
- Shalish, I.; Temkin, H.; Narayanamurti, V. Size-dependent surface luminescence in ZnO nanowires. Phys. Rev. B 2004, 69, 245401. [Google Scholar] [CrossRef]
- Hui, K.C.; Ong, H.C.; Lee, P.F.; Dai, J.Y. Role of recombination pathway competition in spatially resolved cathodoluminescence spectroscopy. Appl. Phys. Lett. 2005, 86, 152116. [Google Scholar] [CrossRef]
- Richters, J.-P.; Voss, T.; Wischmeier, L.; Rückmann, I.; Gutowski, J. Influence of polymer coating on the low-temperature photoluminescence properties of ZnO nanowires. Appl. Phys. Lett. 2008, 92, 011103. [Google Scholar] [CrossRef]
- Lauer, R.B. The IR photoluminescence emission band in zinc oxide. J. Phys. Chem. Solids 1973, 34, 249–253. [Google Scholar] [CrossRef]
- Lehmann, W. Zinc Oxide and Zinc-Cadmium Oxide Phosphors. J. Electrochem. Soc. 1968, 115, 538–540. [Google Scholar] [CrossRef]
- Gorelkinskii, Y.V.; Watkins, G.D. Defects produced in ZnO by 2.5-MeV electron irradiation at 4.2 K: Study by optical detection of electron paramagnetic resonance. Phys. Rev. B 2004, 69, 115212. [Google Scholar] [CrossRef]
- Dong, Y.; Tuomisto, F.; Svensson, B.G.; Kuznetsov, A.Y.; Brillson, L.J. Vacancy defect and defect cluster energetics in ion-implanted ZnO. Phys. Rev. B 2010, 81, 081201. [Google Scholar] [CrossRef]
- Shih, P.-H.; Hung, H.-J.; Ma, Y.-R.; Wu, S.Y. Tuning the dimensionality of ZnO nanowires through thermal treatment: An investigation of growth mechanism. Nanoscale Res. Lett. 2012, 7, 354. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.; Wu, S.Y. Growth Mechanism Studies of ZnO Nanowires: Experimental Observations and Short-Circuit Diffusion Analysis. Nanomaterials 2017, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-L.; Ma, Y.-R.; Chou, M.H.; Huang, C.Y.; Yeh, V.; Wu, S.Y. Direct observation of short-circuit diffusion during the formation of a single cupric oxide nanowire. Nanotechnology 2007, 18, 245604. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Gupta, S.K.; Desai, R.; Jha, P.K.; Sahoo, S.; Kirin, D. Titanium dioxide synthesized using titanium chloride: Size effect study using Raman spectroscopy and photoluminescence. J. Raman Spectrosc. 2010, 41, 350–355. [Google Scholar] [CrossRef]
- Yang, L.-L.; Yang, J.-H.; Wang, D.-D.; Zhang, Y.-J.; Wang, Y.-X.; Liu, H.-L.; Fan, H.-G.; Lang, J.-H. Photoluminescence and Raman analysis of ZnO nanowires deposited on Si(100) via vapor-liquid-solid process. Physica E 2008, 40, 920–923. [Google Scholar] [CrossRef]
- Zeng, J.N.; Low, J.K.; Ren, Z.M.; Liew, T.; Lu, Y.F. Effect of deposition conditions on optical and electrical properties of ZnO films prepared by pulsed laser deposition. Appl. Surf. Sci. 2012, 197–198, 362–367. [Google Scholar] [CrossRef]
- Bratos, S.; Marechal, E. Raman Study of Liquids. I. Theory of the Raman Spectra of Diatomic Molecules in Inert Solutions. Phys. Rev. A 1971, 4, 1078. [Google Scholar] [CrossRef]
- Bartoli, F.J.; Litovitz, T.A. Analysis of Orientational Broadening of Raman Line Shapes. J. Chem. Phys. 1972, 56, 404–412. [Google Scholar] [CrossRef]
- Song, J.; Kulinich, S.A.; Yan, J.; Zhigang, L.; He, J.; Kan, C.; Zeng, H. Field Emitters: Epitaxial ZnO Nanowire-on-Nanoplate Structures as Efficient and Transferable Field Emitters. Adv. Mater. 2013, 25, 5678. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, A.; Ji, X.; Zhu, Y.; Gui, X.; Huang, F.; Tang, Z. Growth of vertically aligned ZnO nanowire arrays on ZnO single crystals. Mater. Lett. 2015, 154, 40–43. [Google Scholar] [CrossRef]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline CeO2 thin films. Solid State Ionics 2002, 149, 99–105. [Google Scholar]
- Yoshikawa, M.; Mori, Y.; Obata, H.; Maegawa, M.; Katagiri, G.; Ishida, H.; Ishitani, A. Raman scattering from nanometer sized diamond. Appl. Phys. Lett. 1995, 67, 694–696. [Google Scholar] [CrossRef]
- Xu, C.Y.; Zhang, P.X.; Yan, L. Blue shift of Raman peak from coated TiO2 nanoparticles. J. Raman Spectrosc. 2001, 32, 862–865. [Google Scholar] [CrossRef]
- Fukata, N.; Oshima, T.; Murakami, K.; Kizuka, T.; Tsurui, T.; Ito, S. Phonon confinement effect of silicon nanowires synthesized by laser ablation. Appl. Phys. Lett. 2005, 86, 213112. [Google Scholar] [CrossRef] [Green Version]
- Selim, F.A.; Weber, M.H.; Solodovnikov, D.; Lynn, K.G. Nature of Native Defects in ZnO. Phys. Rev. Lett. 2007, 99, 085502. [Google Scholar] [CrossRef] [PubMed]
- Tu, N.; Tuan, N.T.; Nguyen, V.; Cuong, N.D.; Kien, N.D.T.; Huy, P.T.; Nguyen, V.; Nguyen, D.H. Near-infrared emission from ZnO nanorods grown by thermal evaporation. J. Lumin. 2014, 156, 199–204. [Google Scholar]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Zhang, Y.; Kim, E.J.; Hahn, S.H.; Seong, S.G. Near-infrared luminescence from ZnO. Appl. Phys. Lett. 2012, 100, 101906. [Google Scholar] [CrossRef]
- Biroju, R.K.; Giri, P.K. Strong visible and near infrared photoluminescence from ZnO nanorods/nanowires grown on single layer graphene studied using sub-band gap excitation. J. Appl. Phys. 2005, 122, 044301. [Google Scholar] [CrossRef]
- Al-Hilli, S.; Willander, M. The pH Response and Sensing Mechanism of n-Type ZnO/Electrolyte Interfaces. Sensors 2009, 9, 7445–7480. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Faraci, G.; Gibilisco, S.; Russo, P.; Pennisi, A.R.; La Rosa, S. Modified Raman confinement model for Si nanocrystals. Phys. Rev. B 2006, 73, 033307. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhong, H.M.; Li, Z.F.; Lu, W. Raman study for E2 phonon of ZnO in Zn1−xMnxO nanoparticles. J. Appl. Phys. 2005, 97, 086105. [Google Scholar] [CrossRef]
- Wang, R.-P.; Zhou, G.-W.; Liu, Y.-L.; Pan, S.-H.; Zhang, H.-Z.; Yu, D.-P.; Zhang, Z. Raman spectral study of silicon nanowires: High-order scattering and phonon confinement effects. Phys. Rev. B 2000, 61, 16827. [Google Scholar] [CrossRef]
- Zhu, K.-R.; Zhang, M.-S.; Chen, Q.; Yin, Z. Size and phonon-confinement effects on low-frequency Raman mode of anatase TiO2 nanocrystal. Phys. Lett. A 2005, 340, 220–227. [Google Scholar] [CrossRef]
- Begum, N.; Bhatti, A.S.; Jabeen, F.; Rubini, S.; Martell, F. Phonon Confinement Effect in III-V Nanowires. In Nanowires; Prete, P., Ed.; InTechOpen: Rijeka, Croatia, 2010. [Google Scholar]
- Bersani, D.; Lottici, P.P.; Ding, X.-Z. Phonon confinement effects in the Raman scattering by TiO2 nanocrystals. Appl. Phys. Lett. 1998, 72, 73–75. [Google Scholar] [CrossRef]
- Rajalakshmi, M.; Arora, A.K.; Bendre, B.S.; Mahamuni, S. Optical phonon confinement in zinc oxide nanoparticles. J. Appl. Phys. 2000, 87, 2445–2448. [Google Scholar] [CrossRef]
- Yang, M.; Huang, D.; Hao, P.; Zhang, F.; Hou, X.; Wang, X. Study of the Raman peak shift and the linewidth of light-emitting porous silicon. J. Appl. Phys. 1994, 75, 651–653. [Google Scholar] [CrossRef]
- Akhilesh, K.; Arora, M.; Rajalakshmi, T.R. Ravindran, Phonon Confinement in Nanostructured Materials. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2004; Volume 8. [Google Scholar]
- Arora, A.K.; Rajalakshmi, M.; Ravindran, T.R.; Sivasubramanian, V. Raman spectroscopy of optical phonon confinement in nanostructured materials. J. Raman Spectrosc. 2007, 38, 604–617. [Google Scholar] [CrossRef]
- Adu, K.W.; Xiong, Q.; Gutierrez, H.R.; Chen, G.; Eklund, P.C. Raman scattering as a probe of phonon confinement and surface optical modes in semiconducting nanowires. Appl. Phys. A 2006, 85, 287–297. [Google Scholar] [CrossRef]
- Venugopal, R.; Lin, P.-I.; Liu, C.-C.; Chen, Y.T. Surface-Enhanced Raman Scattering and Polarized Photoluminescence from Catalytically Grown CdSe Nanobelts and Sheets. J. Am. Chem. Soc. 2005, 127, 11262–11268. [Google Scholar] [CrossRef] [PubMed]
- Barriga-Castro, E.D.; García, J.; Mendoza-Resendez, R.; Pridab, V.M.; Luna, C. Pseudo-monocrystalline properties of cylindrical nanowires confinedly grown by electrodeposition in nanoporous alumina templates. RSC Adv. 2017, 7, 13817–13826. [Google Scholar] [CrossRef]

| Spatial Position (μm) | E2H Mode (cm−1) | E1-LO Mode (cm−1) | ||
|---|---|---|---|---|
| Position | Width | Position | Width | |
| 0.00 | 438.1 ± 0.56 | 29.3 | 608.1 ± 0.54 | 45.4 |
| 0.50 | 438.2 ± 0.36 | 26.3 | 606.4 ± 0.80 | 44.7 |
| 0.68 | 437.5 ± 0.47 | 23.9 | 607.2 ± 0.62 | 40.4 |
| 1.20 | 436.8 ± 0.29 | 24.0 | 606.5 ± 1.11 | 51.8 |
| 1.52 | 436.6 ± 0.27 | 20.9 | 606.8 ± 1.27 | 46.7 |
| 1.97 | 436.6 ± 0.35 | 24.6 | 606.9 ± 1.31 | 44.9 |
| 2.30 | 436.7 ± 0.35 | 22.9 | 602.5 ± 1.76 | 63.1 |
| 2.71 | 437.3 ± 0.45 | 19.8 | 606.5 ± 2.59 | 55.1 |
| 3.12 | 436.8 ± 0.57 | 30.0 | 606.5 ± 2.36 | 50.6 |
| 3.45 | 436.8 ± 0.51 | 22.0 | 606.5 ± 2.83 | 44.7 |
| 3.83 | 436.8 ± 0.78 | 29.3 | 605.2 ± 3.54 | 53.7 |
| 4.13 | 437.5 ± 0.64 | 21.6 | - | - |
| 4.43 | 436.1 ± 0.77 | 21.4 | - | - |
| 4.76 | 436.3 ± 0.71 | 21.4 | - | - |
| 5.14 | 436.6 ± 0.59 | 15.8 | - | - |
| 5.53 | 437.2 ± 0.79 | 22.6 | - | - |
| 5.74 | 436.7 ± 0.57 | 13.4 | - | - |
| 6.20 | 434.2 ± 1.51 | 36.5 | - | - |
| 6.71 | 435.7 ± 1.04 | 20.8 | - | - |
| 7.03 | 439.6 ± 1.83 | 42.2 | - | - |
| 7.30 | 435.9 ± 1.69 | 24.6 | - | - |
| 7.90 | 439.6 ± 3.13 | 43.4 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, P.-H.; Li, T.-Y.; Yeh, Y.-C.; Wu, S.Y. Phonon Confinement Induced Non-Concomitant Near-Infrared Emission along a Single ZnO Nanowire: Spatial Evolution Study of Phononic and Photonic Properties. Nanomaterials 2017, 7, 353. https://doi.org/10.3390/nano7110353
Shih P-H, Li T-Y, Yeh Y-C, Wu SY. Phonon Confinement Induced Non-Concomitant Near-Infrared Emission along a Single ZnO Nanowire: Spatial Evolution Study of Phononic and Photonic Properties. Nanomaterials. 2017; 7(11):353. https://doi.org/10.3390/nano7110353
Chicago/Turabian StyleShih, Po-Hsun, Tai-Yue Li, Yu-Chen Yeh, and Sheng Yun Wu. 2017. "Phonon Confinement Induced Non-Concomitant Near-Infrared Emission along a Single ZnO Nanowire: Spatial Evolution Study of Phononic and Photonic Properties" Nanomaterials 7, no. 11: 353. https://doi.org/10.3390/nano7110353





