One-Pot Facile Methodology to Synthesize Chitosan-ZnO-Graphene Oxide Hybrid Composites for Better Dye Adsorption and Antibacterial Activity
Abstract
:1. Introduction
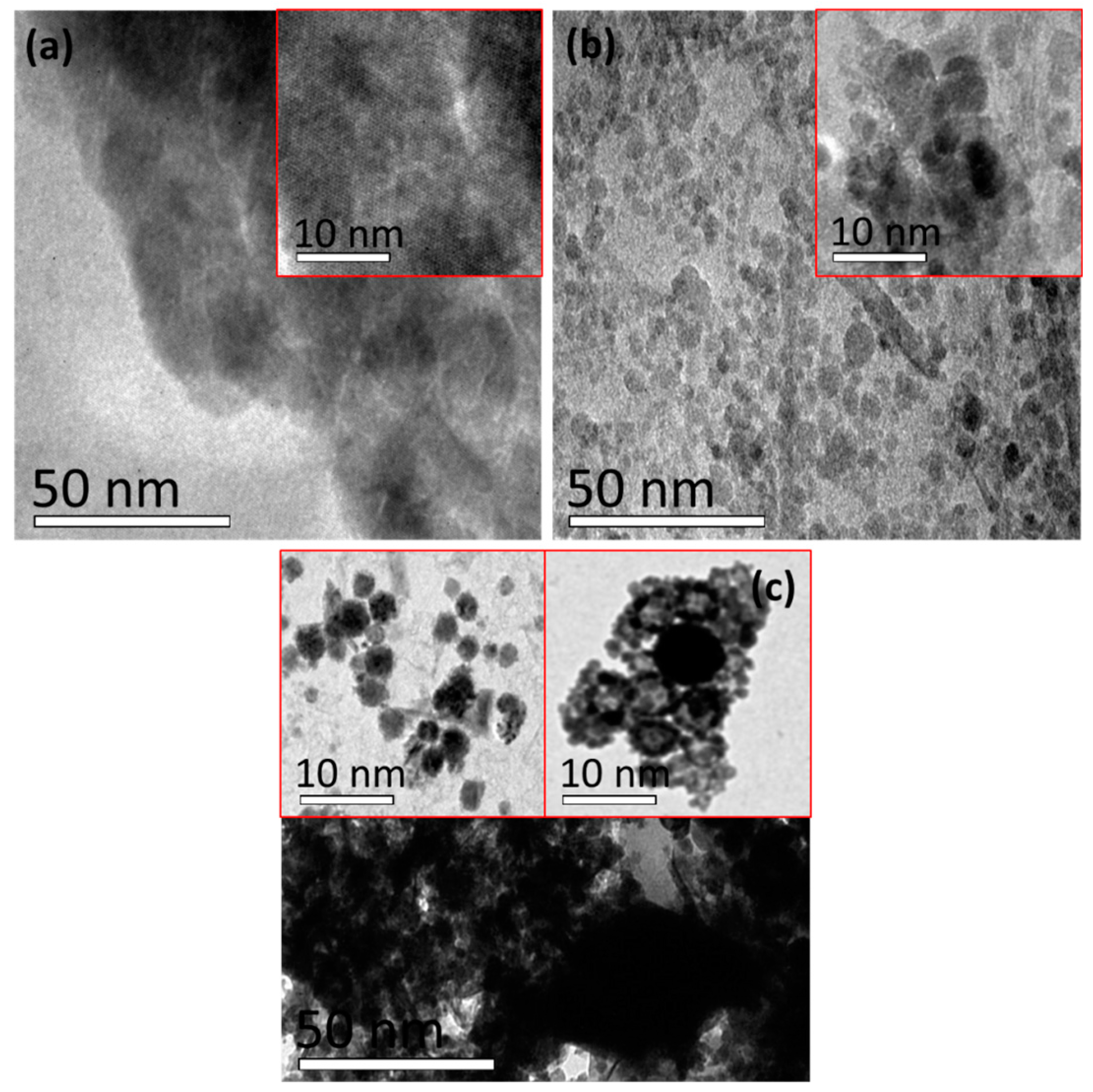
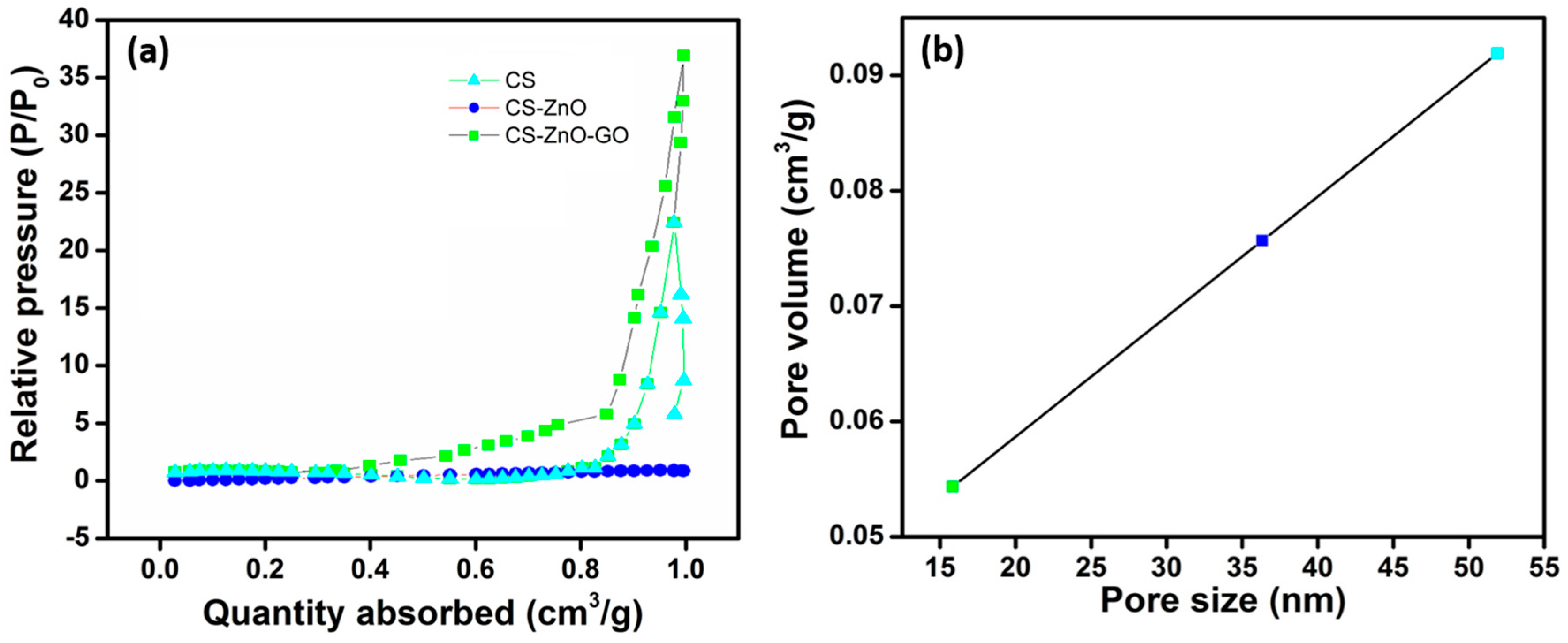
2. Results
3. Materials and Methods
3.1. Materials
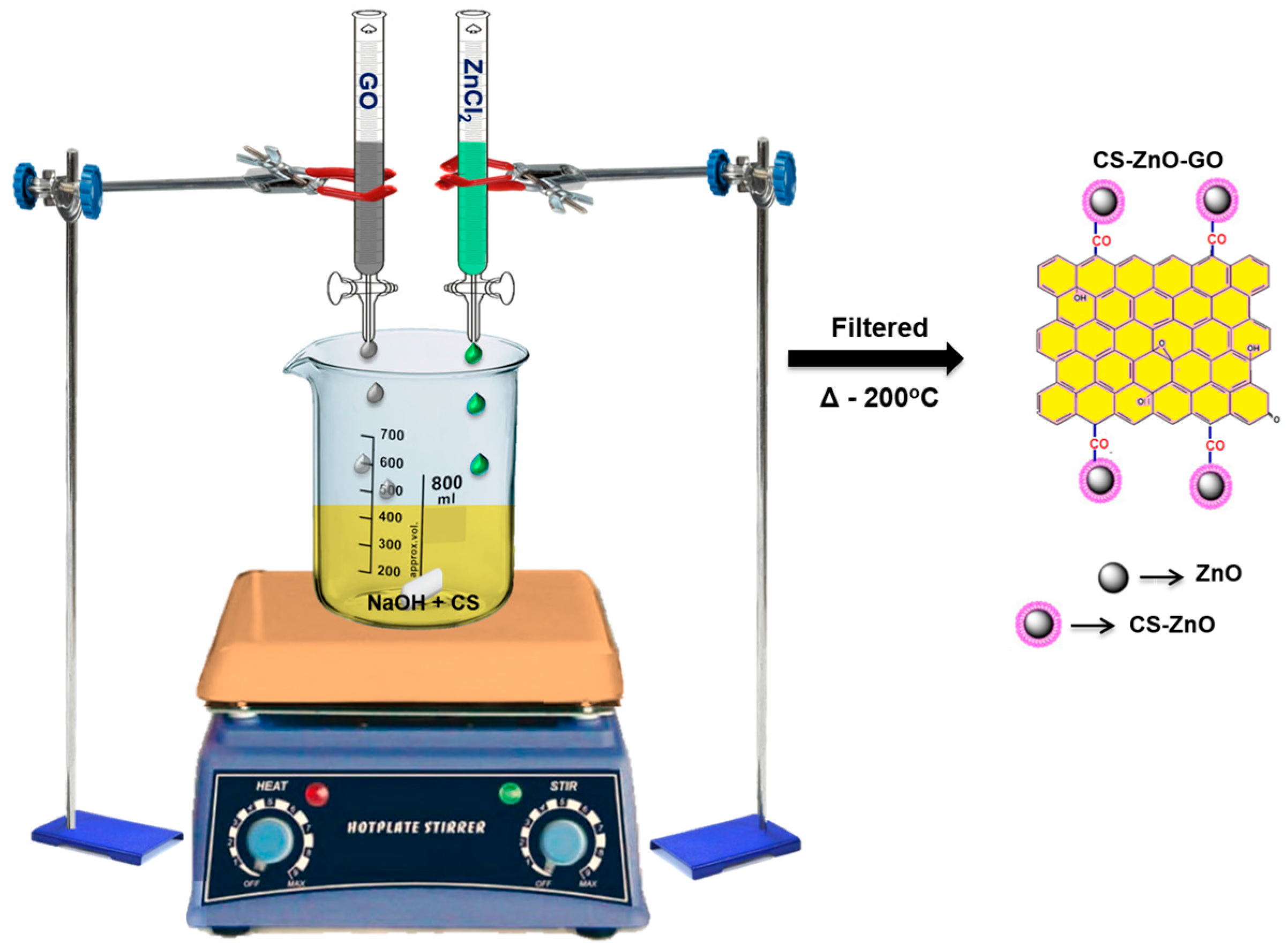
3.2. Synthesis of Hybrid Composites
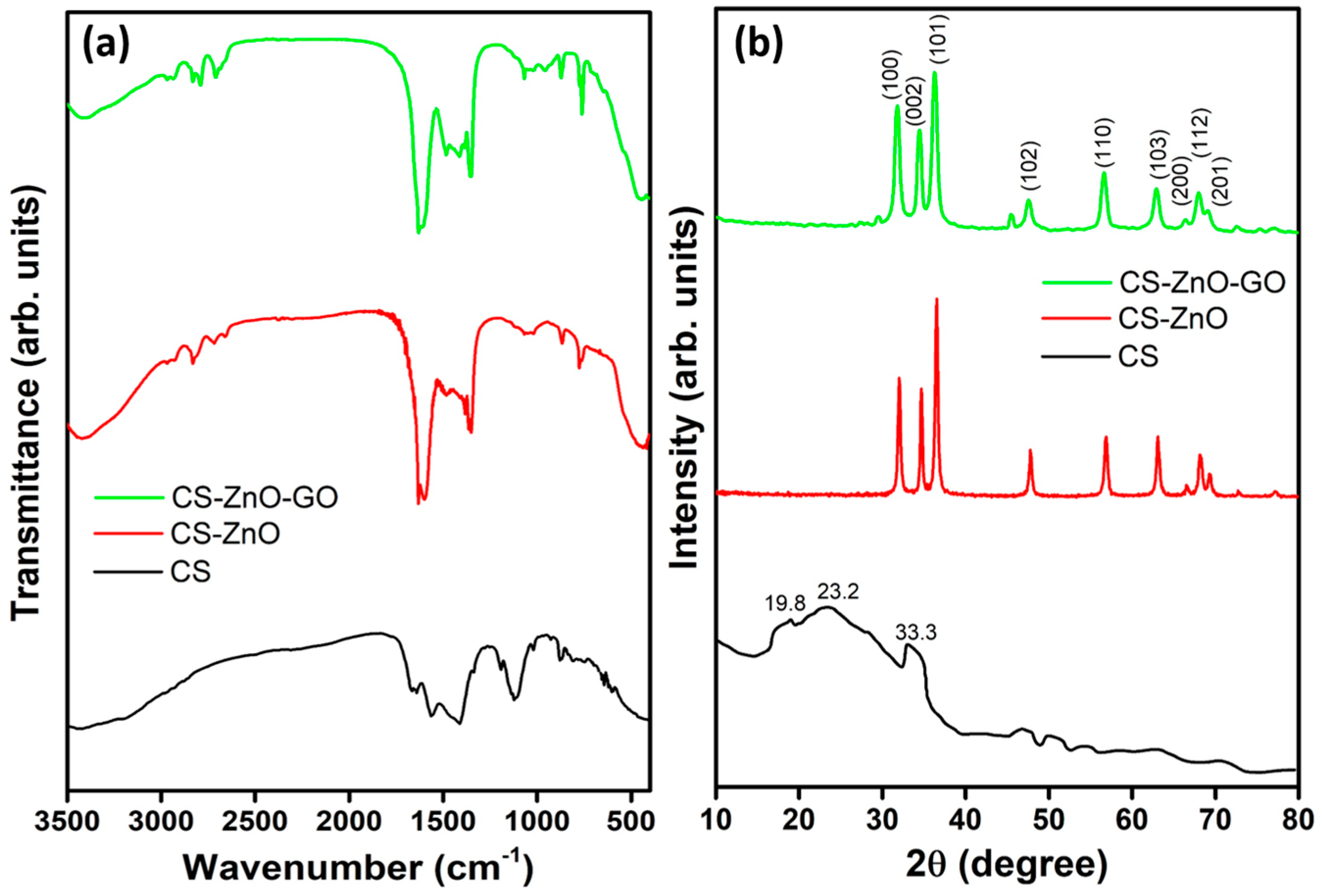
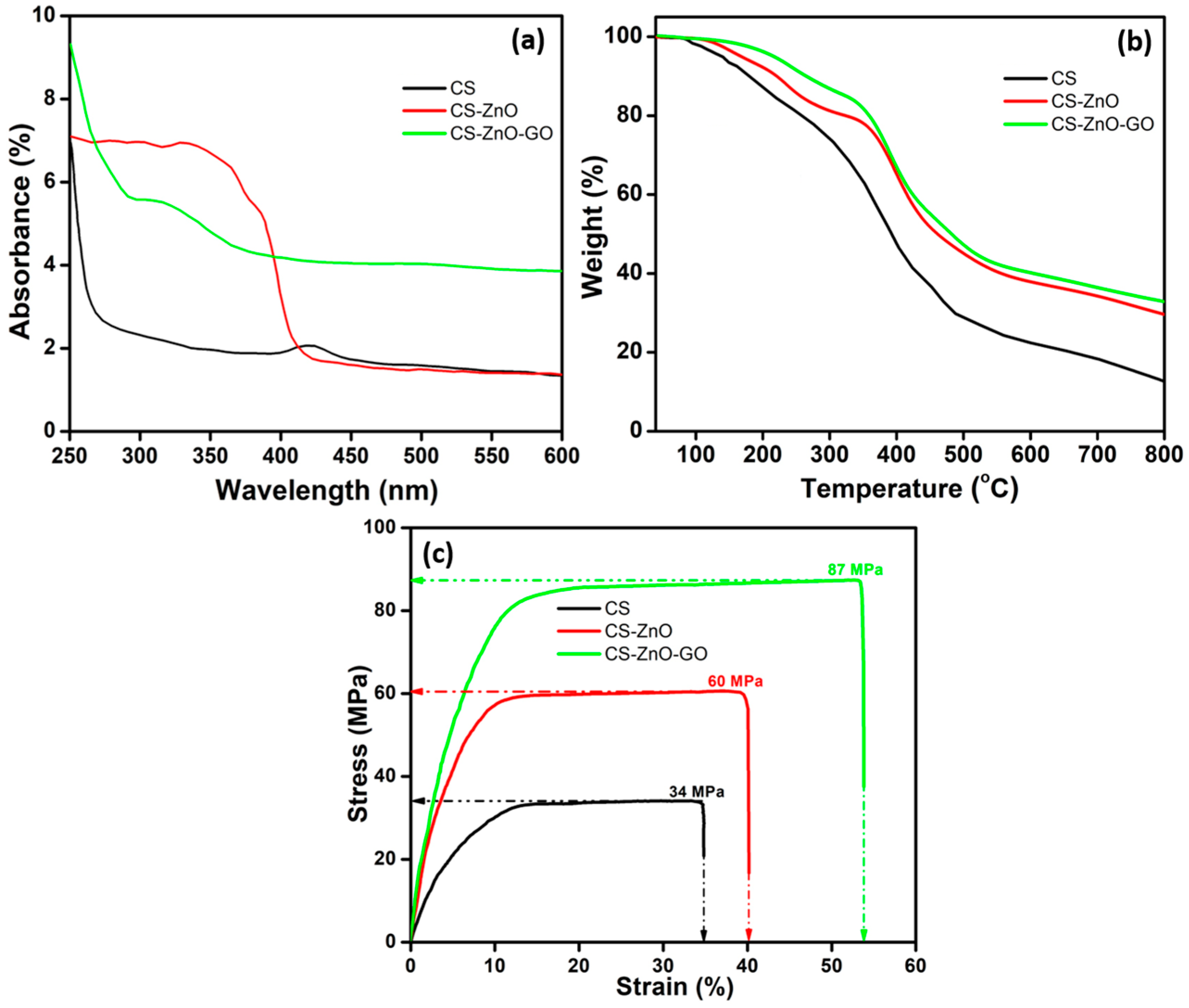
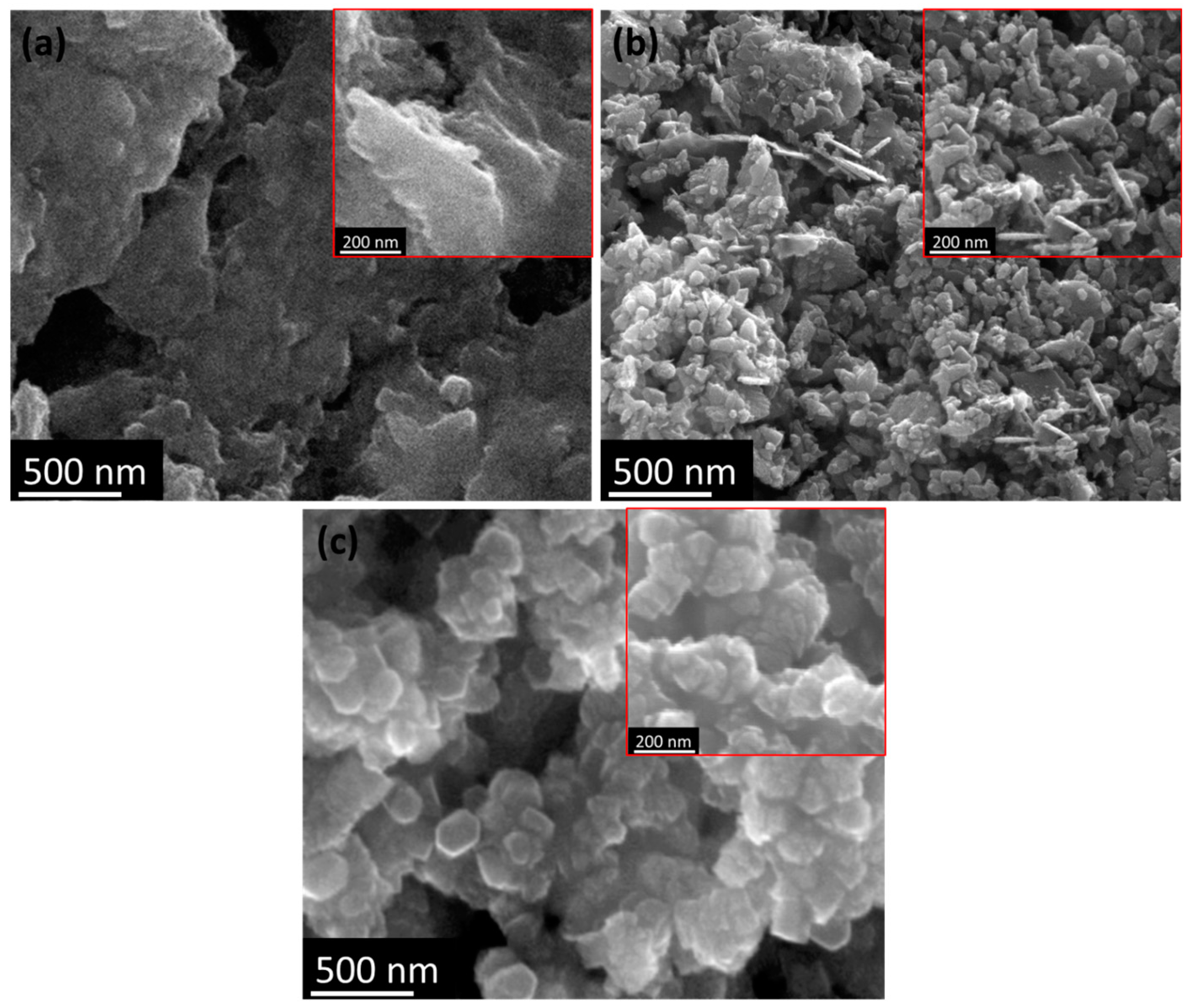
3.3. Characterization
3.4. Dye Absorption and Antibacterial Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kahrilas, G.A.; Haggren, W.; Read, R.L.; Wally, L.M.; Fredrick, S.J.; Hiskey, M.; Prieto, A.L.; Owens, J.E. Investigation of Antibacterial Activity by Silver Nanoparticles Prepared by Microwave-Assisted Green Syntheses with Soluble Starch, Dextrose, and Arabinose. ACS Sustain. Chem. Eng. 2014, 2, 590–598. [Google Scholar] [CrossRef]
- Ramstedt, M.; Cheng, N.; Azzaroni, O.; Mossialos, D.; Mathieu, H.J.; Huck, W.T.S. Synthesis and Characterization of Poly(3-sulfopropylmethacrylate) Brushes for Potential Antibacterial Applications. Langmuir 2007, 23, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Krouse, J.; Kotov, N.A.; Eghtedari, M.; Vargas, G.; Motamedi, M. Rapid Aqueous Photo-Polymerization Route to polymer and Polymer-Composite Hydrogel 3d Inverted Colloidal Crystal Scaffolds. J. Biomed. Mater. Res. Part A 2007, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver Nanoparticles: Green synthesis and Their Antimicrobial Activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Hyland, R.M.; Beck, P.; Mulvey, G.L.; Kitov, P.I.; Armstrong, G.D. N-acetyllactosamine Conjugated to Gold Nanoparticles Inhibits Enteropathogenic Escherichia Coli Colonization of the Epithelium in Human Intestinal Biopsy Specimens. Infect. Immun. 2006, 74, 5419–5421. [Google Scholar] [CrossRef] [PubMed]
- Letfullin, R.; Joenathan, C.; George, T.; Zharov, V. Cancer cell Killing by Laser-Induced Thermal Explosion of Nanoparticles. Nanomedicine 2006, 1, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P. An overview of textile pollution and its remedy. Indian J. Environ. Prot. 1994, 14, 443–446. [Google Scholar]
- Chen, K.-C.; Wu, J.-Y.; Huang, C.-C.; Liang, Y.-M.; Hwang, S.-C.J. Decolorization of Azo Dye Using Pva-Immobilized Microorganisms. J. Biotechnol. 2003, 101, 241–252. [Google Scholar] [CrossRef]
- Gong, R.; Ding, Y.; Li, M.; Yang, C.; Liu, H.; Sun, Y. Utilization of Powdered Peanut Hull as Biosorbent for Removal of Anionic Dyes from Aqueous Solution. Dyes Pigments 2005, 64, 187–192. [Google Scholar] [CrossRef]
- Aksu, Z. Application of Biosorption for the Removal of Organic Pollutants: A review. Proc. Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Barquist, K.; Larsen, S.C. Chromate Adsorption on Bifunctional, Magnetic Zeolite Composites. Microporous Mesoporous Mater. 2010, 130, 197–202. [Google Scholar] [CrossRef]
- Denizli, A.; Say, R.; Pişkin, E. Removal of Aluminium by Alizarin Yellow-Attached Magnetic Poly (2-Hydroxyethyl Methacrylate) Beads. React. Funct. Polym. 2003, 55, 99–107. [Google Scholar] [CrossRef]
- Safarik, I.; Safarikova, M.; Buricova, V. Sorption of Water Soluble Organic Dyes on Magnetic Poly (Oxy-2, 6-Dimethyl-1, 4-Phenylene). Collect. Czechoslov. Chem. Commun. 1995, 60, 1448–1456. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharyya, K.G. Adsorption of Methylene Blue on Kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Ghosh, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioremediation of Chromium Complex Dyes and Treatment of Sludge Generated during the Process. Int. Biodeterior. Biodegrad. 2017, 119, 448–460. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Juang, R.-S. Adsorption of Tannic Acid, Humic Acid, and Dyes from Water Using the Composite of Chitosan and Activated Clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Pinto, R.J.; Fernandes, S.C.; Freire, C.S.; Sadocco, P.; Causio, J.; Neto, C.P.; Trindade, T. Antibacterial Activity of Optically Transparent Nanocomposite Films Based on Chitosan or Its Derivatives and Silver Nanoparticles. Carbohydr. Res. 2012, 348, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Luo, J.; Yang, J.; Wang, W.; Kennedy, J.F. A Novel Biopolymer/Rectorite Nanocomposite with Antimicrobial Activity. Carbohydr. Polym. 2009, 77, 449–456. [Google Scholar] [CrossRef]
- Sharma, S.; Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Fabrication of Antibacterial Silver Nanoparticle—Sodium Alginate–Chitosan Composite Films. RSC Adv. 2012, 2, 5837–5843. [Google Scholar] [CrossRef]
- Crini, G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.S.; Beppu, M.M. Interaction of Natural and Crosslinked Chitosan Membranes With hg (ii) Ions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 196–207. [Google Scholar] [CrossRef]
- Juang, R.-S.; Ju, C.-Y. Equilibrium Sorption of Copper (ii)-Ethylenediaminetetraacetic Acid Chelates onto Cross-Linked, Polyaminated Chitosan Beads. Ind. Eng. Chem. Res. 1997, 36, 5403–5409. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Xu, Y.-J. Basic Principles for Observing the Photosensitizer Role of Graphene in the Graphene–Semiconductor Composite Photocatalyst from a Case Study on Graphene–Zno. J. Phys. Chem. C 2013, 117, 21724–21734. [Google Scholar] [CrossRef]
- Kavitha, T.; Gopalan, A.I.; Lee, K.-P.; Park, S.-Y. Glucose Sensing, Photocatalytic and Antibacterial Properties of Graphene–Zno Nanoparticle Hybrids. Carbon 2012, 50, 2994–3000. [Google Scholar] [CrossRef]
- Khan, Y.; Durrani, S.; Mehmood, M.; Ahmad, J.; Khan, M.R.; Firdous, S. Low Temperature Synthesis of Fluorescent Zno Nanoparticles. Appl. Surf. Sci. 2010, 257, 1756–1761. [Google Scholar] [CrossRef]
- Shi, R.; Yang, P.; Dong, X.; Ma, Q.; Zhang, A. Growth of Flower-Like Zno on Zno Nanorod Arrays Created on Zinc Substrate through Low-Temperature Hydrothermal Synthesis. Appl. Surf. Sci. 2013, 264, 162–170. [Google Scholar] [CrossRef]
- Dutta, S.; Ganguly, B.N. Characterization of Zno Nanoparticles Grown in Presence of Folic Acid Template. J. Nanobiotechnol. 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of zno nanoparticles (zno nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior Antibacterial Activity of Zinc Oxide/Graphene Oxide Composites Originating from High Zinc Concentration Localized around Bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Mohan Kumar, K.; Mandal, B.K.; Appala Naidu, E.; Sinha, M.; Siva Kumar, K.; Sreedhara Reddy, P. Synthesis and Characterisation of Flower Shaped Zinc Oxide Nanostructures and Its Antimicrobial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Anat, L.; Yeshayahu, N.; Aharon, G.; Rachel, L. Antifungal Activity of Zno Nanoparticles—The Role of Ros Mediated Cell Injury. Nanotechnology 2011, 22, 105101. [Google Scholar]
- You, J.; Zhang, Y.; Hu, Z. Bacteria and Bacteriophage Inactivation by Silver and Zinc Oxide Nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Seil, J.T.; Taylor, E.N.; Webster, T.J. Reduced Activity of Staphylococcus Epidermidis in the Presence of Sonicated Piezoelectric Zinc Oxide Nanoparticles. In Proceedings of the 2009 IEEE 35th Annual Northeast Bioengineering Conference, Cambridge, MA, USA, 3–5 April 2009; pp. 1–2. [Google Scholar]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Hu, Z.; Oskam, G.; Searson, P.C. Influence of Solvent on the Growth of Zno Nanoparticles. J. Colloid Interface Sci. 2003, 263, 454–460. [Google Scholar] [CrossRef]
- Ramesha, G.; Kumara, A.V.; Muralidhara, H.; Sampath, S. Graphene and Graphene Oxide as Effective Adsorbents toward Anionic and Cationic Dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In Vitro Toxicity Evaluation of Graphene Oxide on a549 Cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping Bacteria by Graphene Nanosheets for Isolation from Environment, Reactivation by Sonication, and Inactivation by Near-Infrared Irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, M.; Lee, M.-H.; Krishnamoorthy, K.; Yun, K. Synthesis, Characterization and Electrochemical Properties of Functionalized Graphene Oxide. Carbon 2012, 50, 4228–4238. [Google Scholar] [CrossRef]
- Zhang, N.; Qiu, H.; Si, Y.; Wang, W.; Gao, J. Fabrication of Highly Porous Biodegradable Monoliths Strengthened by Graphene Oxide and Their Adsorption of Metal Ions. Carbon 2011, 49, 827–837. [Google Scholar] [CrossRef]
- Moharram, M.A.; Ereiba, K.M.; hotaby, W.E.; Bakr, A. Synthesis and Characterization of Graphene Oxide/Crosslinked Chitosan Nanaocomposite for Lead Removal Form Aqueous Solution. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 17. [Google Scholar]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.-M. Well-Dispersed Chitosan/Graphene Oxide Nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, A.R.; Tripathy, S.; Chandra, S.; Roy, S.; Sahu, S.K. A Zno Decorated Chitosan–Graphene Oxide Nanocomposite Shows Significantly Enhanced Antimicrobial Activity with Ros Generation. RSC Adv. 2015, 5, 49420–49428. [Google Scholar] [CrossRef]
- Halder, A.; Zhang, M.; Chi, Q. Electrocatalytic Applications of Graphene–Metal oxide Nanohybrid Materials. In Advanced Catalytic Materials—Photocatalysis and Other Current Trends; Norena, L.E., Wang, J.-A., Eds.; InTech: Rijeka, Croatia, 2016; Chapter 14. [Google Scholar]
- Hu, H.; Xin, J.H.; Hu, H.; Chan, A.; He, L. Glutaraldehyde–Chitosan and Poly (Vinyl Alcohol) Blends, and Fluorescence of Their Nano-Silica Composite Films. Carbohydr. Polym. 2013, 91, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Sundar, K.; Harikarthick, V.; Karthika, V.S.; Ravindran, A. Preparation of Chitosan-Graphene Oxide Nanocomposite and Evaluation of Its Antimicrobial Activity. J. Bionanosci. 2014, 8, 207–212. [Google Scholar] [CrossRef]
- Konwar, A.; Kalita, S.; Kotoky, J.; Chowdhury, D. Chitosan–Iron Oxide Coated Graphene Oxide Nanocomposite Hydrogel: A robust And Soft Antimicrobial Biofilm. ACS Appl. Mater. Interfaces 2016, 8, 20625–20634. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.C.; Ismail, R.; Basri, M.; Nang, H.L.L.; Tejo, B.A.; Abu Hassan, H.; May, C.Y. Testing of Glyceryl Monoesters for Their Anti-Microbial Susceptibility and Their Influence in Emulsions. J. Oil Palm. Res. 2010, 22, 846–855. [Google Scholar]
- Das, M.R.; Sarma, R.K.; Saikia, R.; Kale, V.S.; Shelke, M.V.; Sengupta, P. Synthesis of Silver Nanoparticles in an Aqueous Suspension of Graphene Oxide Sheets and Its Antimicrobial Activity. Colloids Surf. B Biointerfaces 2011, 83, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Dhanasekaran, V.; Mahalingam, T. Structural and Magnetic Properties of High Magnetic Moment Electroplated Conife Thin Films. Ionics 2011, 17, 835–842. [Google Scholar] [CrossRef]
- Sanmugam, A.; Vikraman, D.; Venkatesan, S.; Park, H.J. Optical and Structural Properties of Solvent Free Synthesized Starch/Chitosan-Zno Nanocomposites. J. Nanomater. 2017, 2017, 7536364. [Google Scholar] [CrossRef]
- Wang, S.-M.; Huang, Q.-Z.; Wang, Q.-S. Study on the Synergetic Degradation of Chitosan with Ultraviolet Light and Hydrogen Peroxide. Carbohydr. Res. 2005, 340, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Tajdidzadeh, M.; Azmi, B.Z.; Yunus, W.M.M.; Talib, Z.A.; Sadrolhosseini, A.R.; Karimzadeh, K.; Gene, S.A.; Dorraj, M. Synthesis of Silver Nanoparticles Dispersed in Various Aqueous Media Using Laser Ablation. Sci. World J. 2014, 2014, 324921. [Google Scholar] [CrossRef] [PubMed]
- Anandhavelu, S.; Thambidurai, S. Preparation of Chitosan–Zinc Oxide Complex during Chitin Deacetylation. Carbohydr. Polym. 2011, 83, 1565–1569. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel Synthesis of Thorn-Like Zno Nanoparticles Endorsing Mechanical Stirring Effect and Their Antimicrobial Activities: Potential Role as Nano-Antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-Level Dispersion of Graphene into Poly (Vinyl Alcohol) and Effective Reinforcement of Their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Wang, S.-F.; Shen, L.; Zhang, W.-D.; Tong, Y.-J. Preparation and Mechanical Properties of Chitosan/Carbon Nanotubes Composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Dong, H.; Li, C.M.; Lucia, L.A. The Enhanced Mechanical Properties of a Covalently Bound Chitosan-Multiwalled Carbon Nanotube Nanocomposite. J. Appl. Polym. Sci. 2009, 113, 466–472. [Google Scholar] [CrossRef]
- Gorga, R.E.; Cohen, R.E. Toughness Enhancements in Poly (Methyl Methacrylate) by Addition of Oriented Multiwall Carbon Nanotubes. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 2690–2702. [Google Scholar] [CrossRef]
- Blond, D.; Barron, V.; Ruether, M.; Ryan, K.P.; Nicolosi, V.; Blau, W.J.; Coleman, J.N. Enhancement of Modulus, Strength, and Toughness in Poly (Methyl Methacrylate)-Based Composites by the Incorporation of Poly (Methyl Methacrylate)-Functionalized Nanotubes. Adv. Funct. Mater. 2006, 16, 1608–1614. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Sreekumar, T.; Kumar, S.; Moore, V.C.; Hauge, R.H.; Smalley, R.E. Poly (Vinyl Alcohol)/Swnt Composite Film. Nano Lett. 2003, 3, 1285–1288. [Google Scholar] [CrossRef]
- Liu, L.; Barber, A.H.; Nuriel, S.; Wagner, H.D. Mechanical Properties of Functionalized Single-Walled Carbon-Nanotube/Poly (Vinyl Alcohol) Nanocomposites. Adv. Funct. Mater. 2005, 15, 975–980. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Gun’ko, Y.K. Mechanical Reinforcement of Polymers Using Carbon Nanotubes. Adv. Mater. 2006, 18, 689–706. [Google Scholar] [CrossRef]
- Neumann, M.G.; Gessner, F.; Schmitt, C.C.; Sartori, R. Influence of the Layer Charge and Clay Particle Size on the Interactions between the Cationic Dye Methylene Blue and Clays in an Aqueous Suspension. J. Colloid Interface Sci. 2002, 255, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S. Synthesis of Silver Nanoparticles Using Fresh Bark of Pongamia Pinnata and Characterization of its Antibacterial Activity against Gram Positive and Gram Negative Pathogens. Resour.-Effic. Technol. 2016, 2, 30–35. [Google Scholar] [CrossRef]
- Huang, K.C.; Mukhopadhyay, R.; Wen, B.; Gitai, Z.; Wingreen, N.S. Cell Shape and Cell-Wall Organization in Gram-Negative Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 19282–19287. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yun, K. Graphene Oxide-moDified Zno Particles: Synthesis, Characterization, and Antibacterial Properties. Int. J. Nanomed. 2015, 10, 79–92. [Google Scholar]
- Patel, M.B.; Harikrishnan, U.; Valand, N.N.; Modi, N.R.; Menon, S.K. Novel Cationic Quinazolin-4 (3h)-One Conjugated Fullerene Nanoparticles as Antimycobacterial and Antimicrobial Agents. Archiv Der Pharm. 2013, 346, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, D.-Q.; Lin, Y.-J.; Wei, M.; Evans, D.G.; Duan, X. Controllable Preparation of Nano-Mgo and Investigation of Its Bactericidal Properties. J. Inorg. Biochem. 2005, 99, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Bhargava, P.; Bahadur, D. Fluorescent Zno for Imaging and Induction of DNA Fragmentation and Ros-Mediated Apoptosis in Cancer Cells. J. Mater. Chem. B 2015, 3, 1968–1978. [Google Scholar] [CrossRef]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-Assisted Synthesis of Nanocrystalline Mgo and Its Use as a Bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Anandhavelu, S.; Dhanasekaran, V.; Sethuraman, V.; Park, H.J. Chitin and Chitosan Based Hybrid Nanocomposites for Super Capacitor Applications. J. Nanosci. Nanotechnol. 2017, 17, 1321–1328. [Google Scholar] [CrossRef]

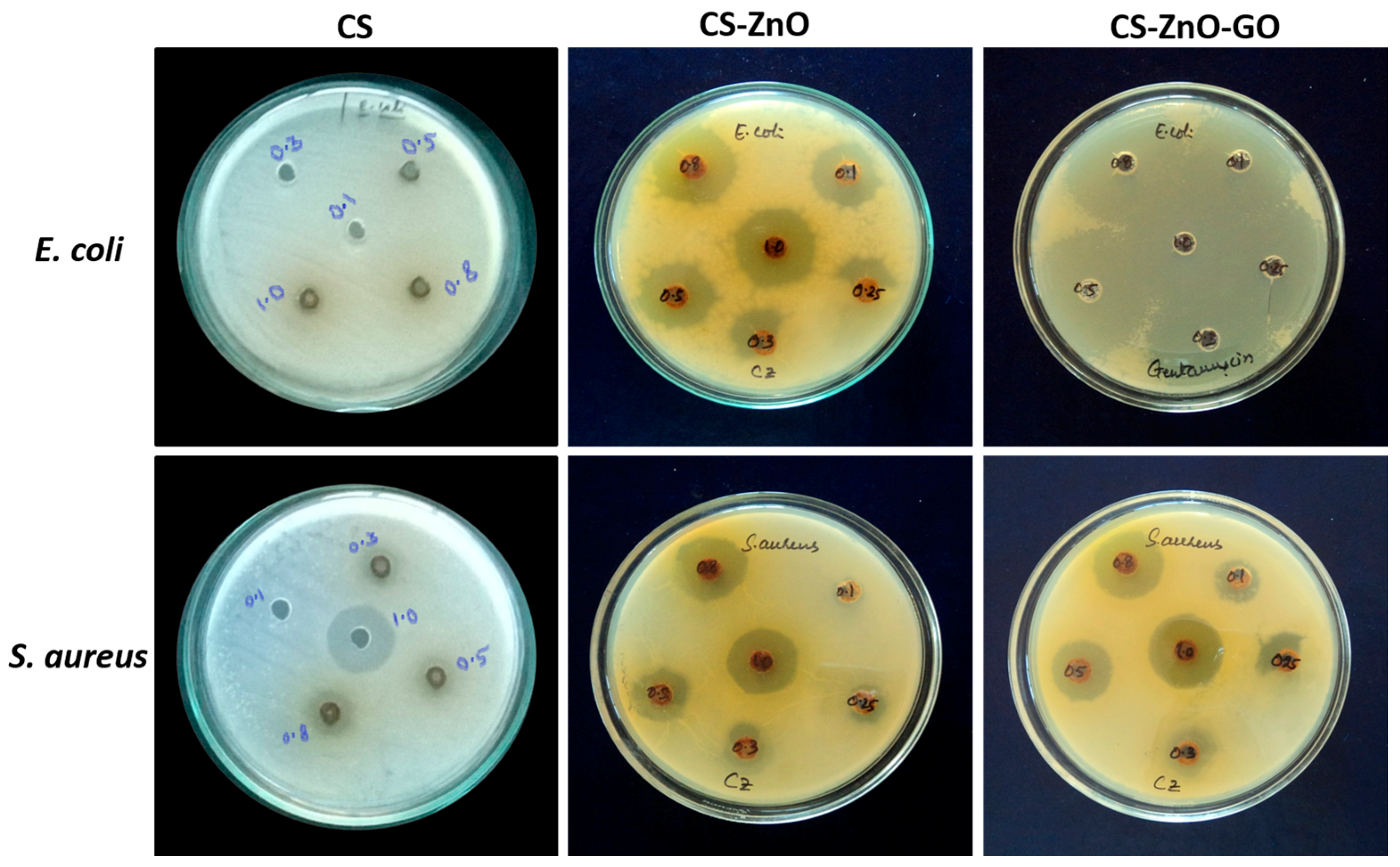
| Bacteria | MIC of CS (µg/mL) | MIC of CS–ZnO (µg/mL) | MIC of CS–ZnO–GO (µg/mL) |
|---|---|---|---|
| E. coli | 0.5 | 0.1 | 0.1 |
| S. aureus | 0.3 | 0.1 | 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmugam, A.; Vikraman, D.; Park, H.J.; Kim, H.-S. One-Pot Facile Methodology to Synthesize Chitosan-ZnO-Graphene Oxide Hybrid Composites for Better Dye Adsorption and Antibacterial Activity. Nanomaterials 2017, 7, 363. https://doi.org/10.3390/nano7110363
Sanmugam A, Vikraman D, Park HJ, Kim H-S. One-Pot Facile Methodology to Synthesize Chitosan-ZnO-Graphene Oxide Hybrid Composites for Better Dye Adsorption and Antibacterial Activity. Nanomaterials. 2017; 7(11):363. https://doi.org/10.3390/nano7110363
Chicago/Turabian StyleSanmugam, Anandhavelu, Dhanasekaran Vikraman, Hui Joon Park, and Hyun-Seok Kim. 2017. "One-Pot Facile Methodology to Synthesize Chitosan-ZnO-Graphene Oxide Hybrid Composites for Better Dye Adsorption and Antibacterial Activity" Nanomaterials 7, no. 11: 363. https://doi.org/10.3390/nano7110363






