Template-Assisted Formation of Nanostructured Dopamine-Modified Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
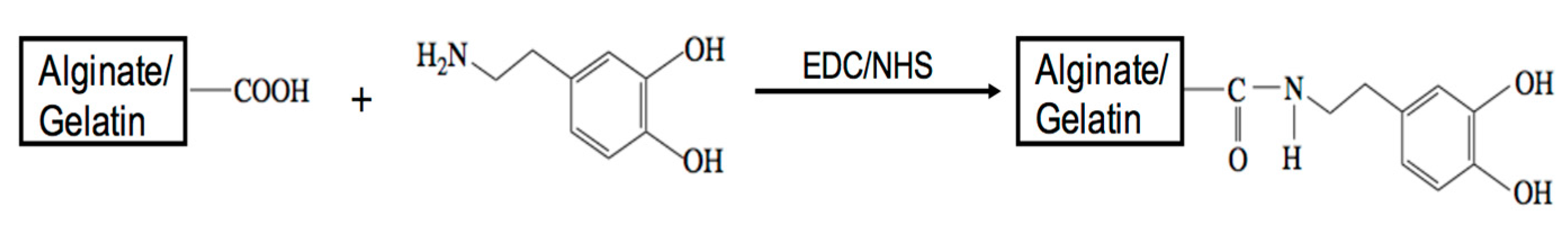
2.2. Polymer Modification
2.3. Time Course of Oxidation of Conjugates and Composites
2.4. Films Formed on Membranes and Thickness Measurement
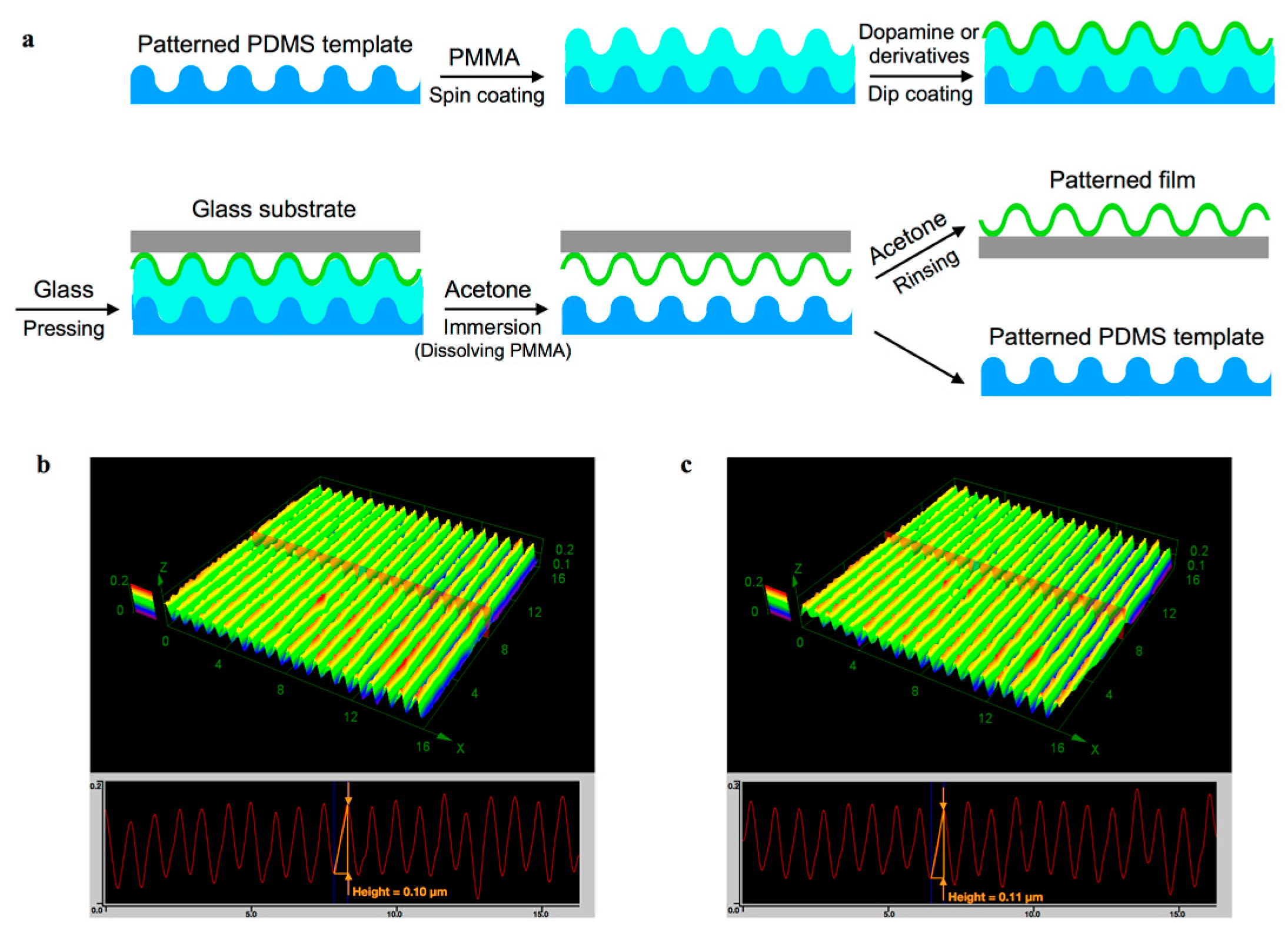
2.5. Template-Assisted Nanopattern Film Fabrication
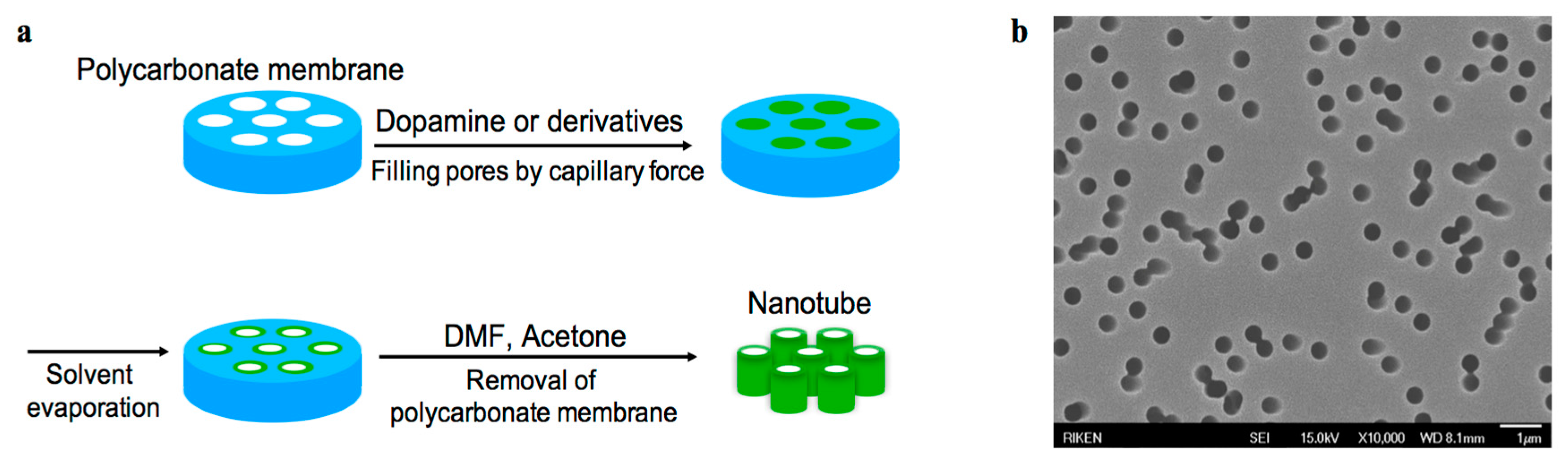
2.6. Template-Assisted Nanotube Fabrication
3. Results and Discussion
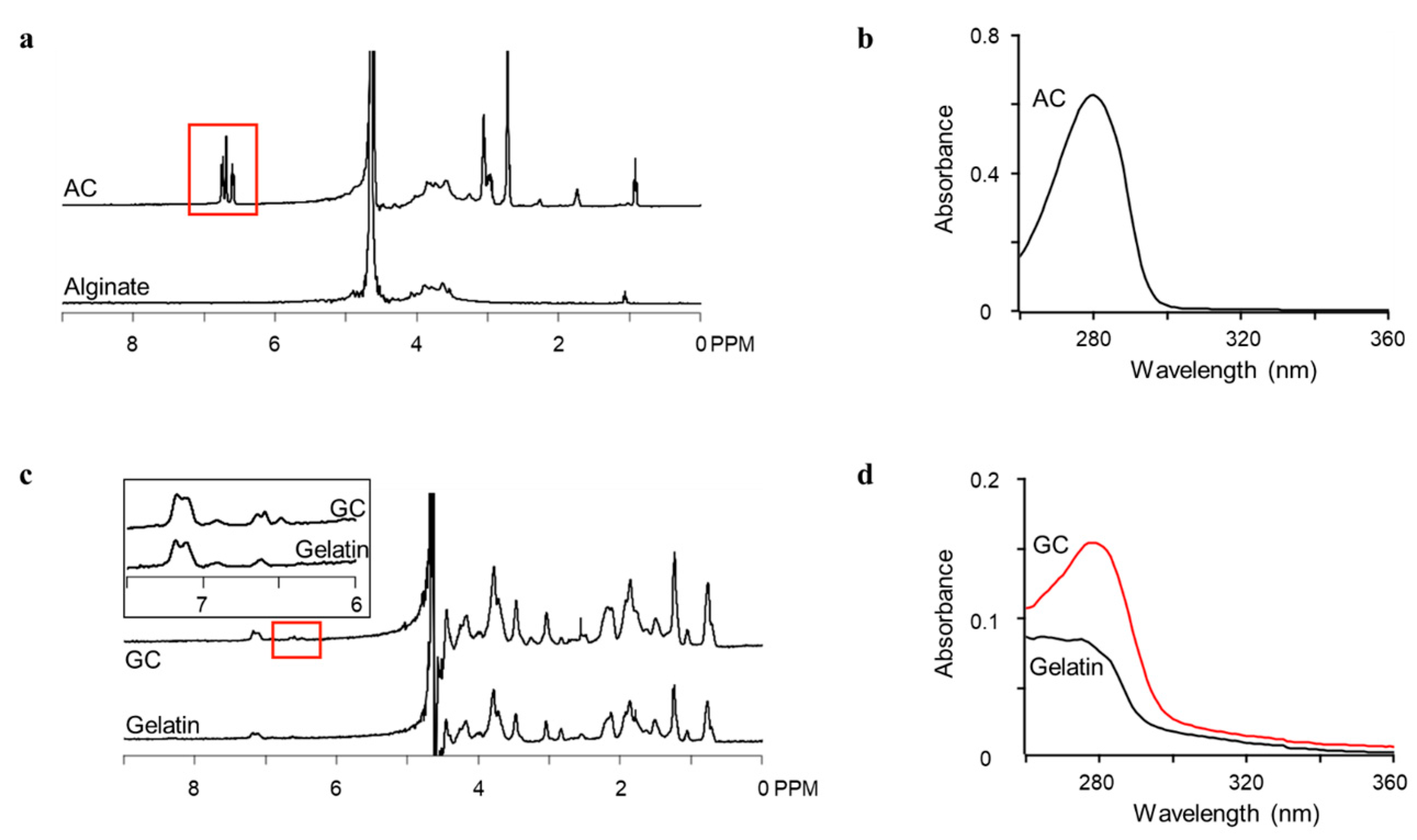
3.1. Polymer Synthesis
3.2. Oxidation and Gelation of Conjugates and Composites
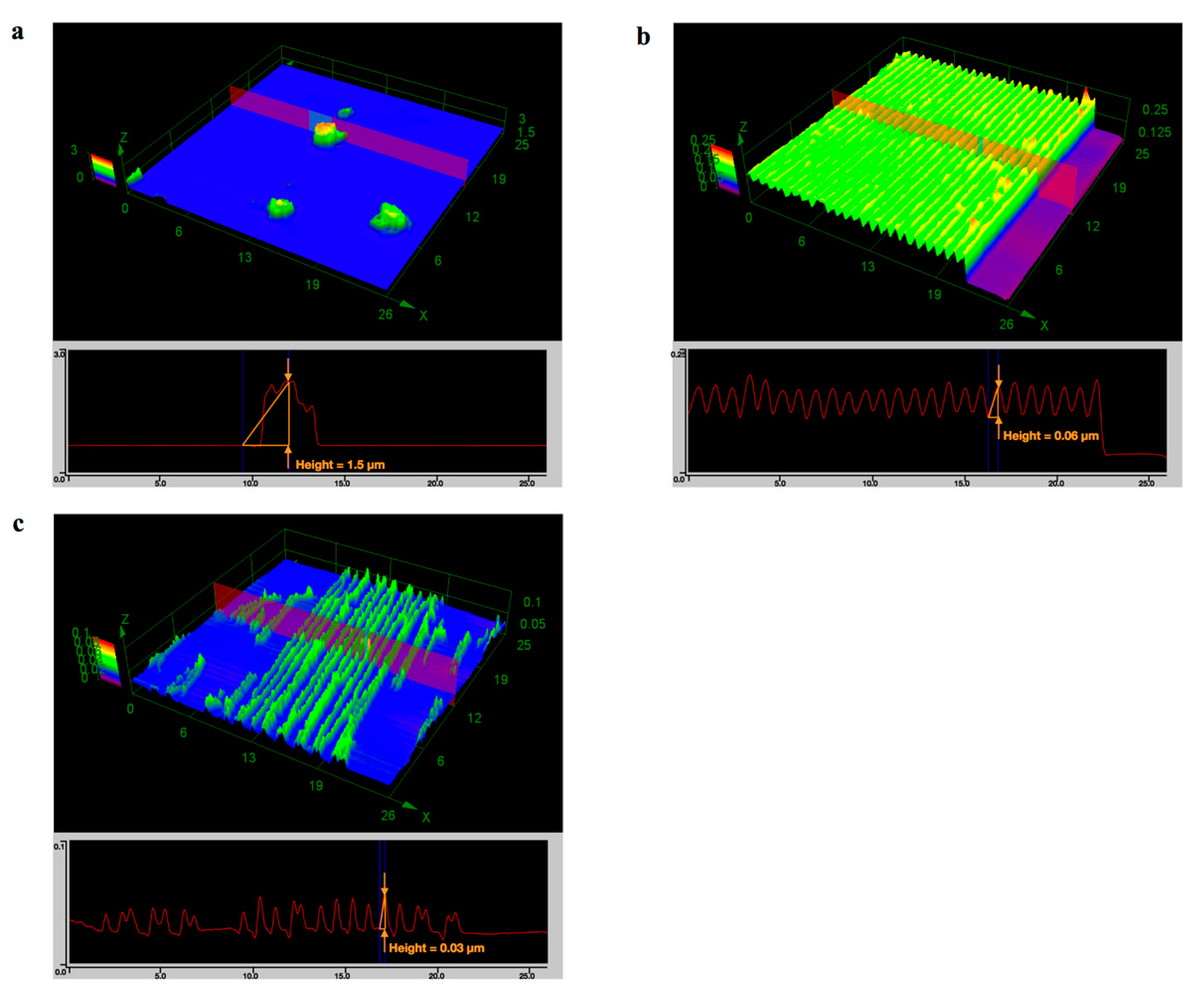
3.3. Film Formation
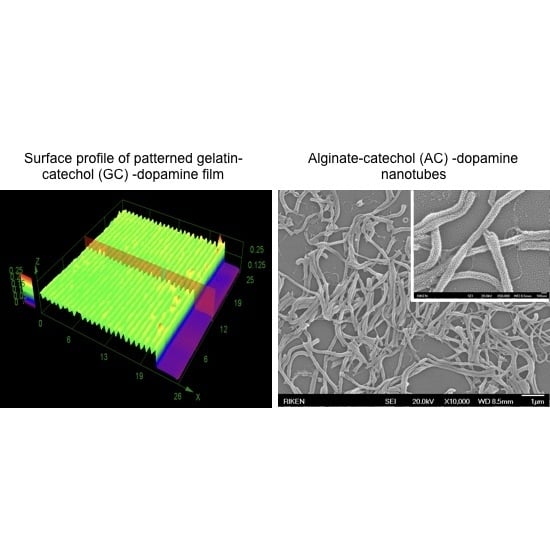
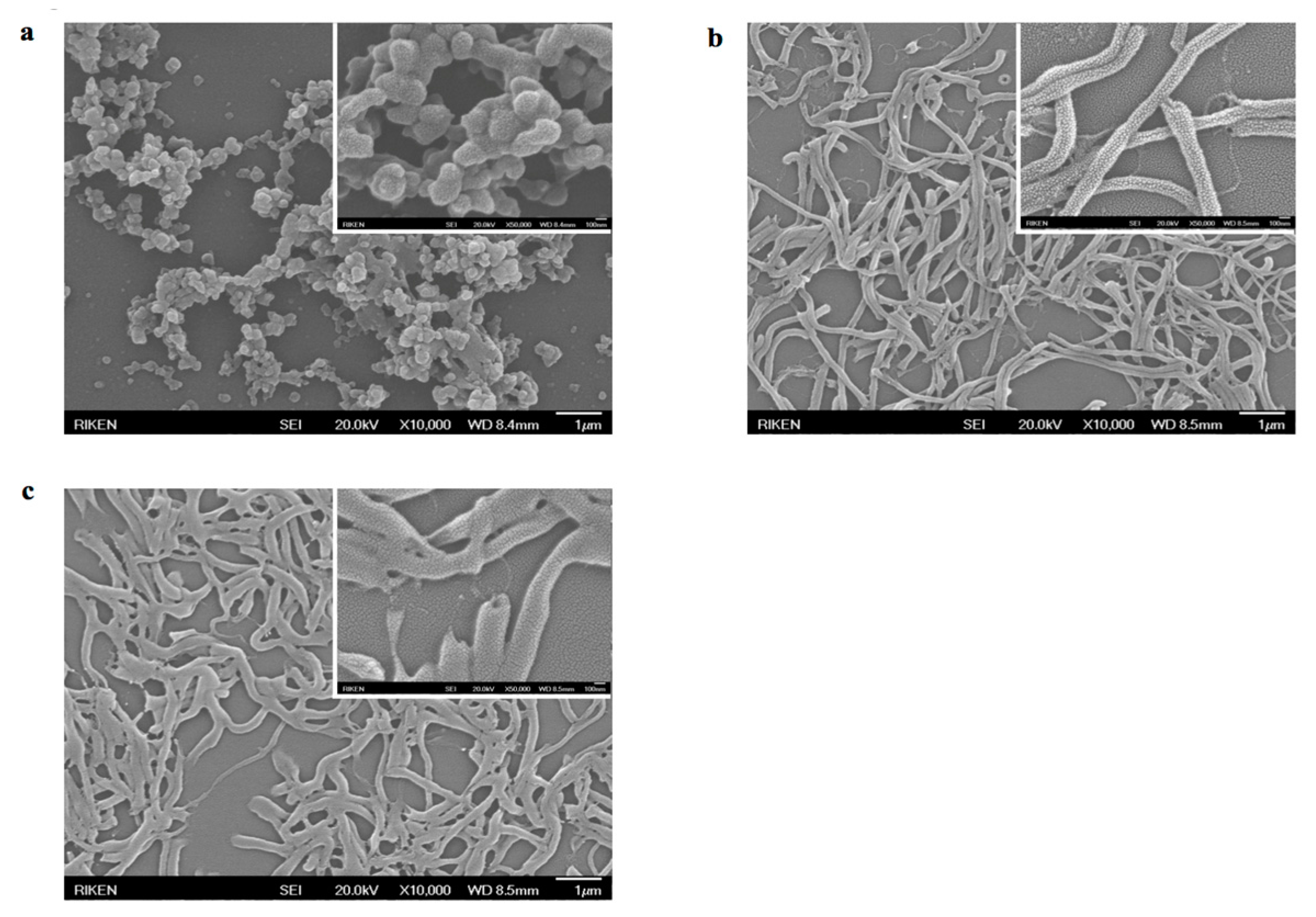
3.4. Template-Assisted Nanopattern Film Formation
3.5. Template-Assisted Nanotube Array Formation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-Step Modification of Superhydrophobic Surfaces by a Mussel-Inspired Polymer Coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed]
- Bernsmann, F.; Frisch, B.; Ringwald, C.; Ball, V. Protein Adsorption on Dopamine-Melanin Films: Role of Electrostatic Interactions Inferred from ζ-Potential Measurements versus Chemisorption. J. Colloid Interface Sci. 2010, 344, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.O.; Liu, Z.; Lau, K.H.A.; Lee, H.; Messersmith, P.B. Facile DNA Immobilization on Surfaces Through a Catecholamine Polymer. Angew. Chem. Int. Ed. 2011, 50, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine—A Nature-Inspired Polymer Coating for Biomedical Science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef] [PubMed]
- Ball, V.; Del Frari, D.; Michel, M.; Buehler, M.J.; Toniazzo, V.; Singh, M.K.; Gracio, J.; Ruch, D. Deposition Mechanism and Properties of Thin Polydopamine Films for High Added Value Applications in Surface Science at the Nanoscale. Bionanoscience 2012, 2, 16–34. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, D.J.; Lee, J.-N.; Lee, Y.M.; Park, J.-K.; Choi, J.W. Excellent Cycle Life of Lithium-Metal Anodes in Lithium-Ion Batteries with Mussel-Inspired Polydopamine-Coated Separators. Adv. Energy Mater. 2012, 2, 645–650. [Google Scholar] [CrossRef]
- Lee, M.; Rho, J.; Lee, D.-E.; Hong, S.; Choi, S.-J.; Messersmith, P.B.; Lee, H. Water Detoxification by a Substrate-Bound Catecholamine Adsorbent. ChemPlusChem 2012, 77, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Sedó, J.; Saiz-Poseu, J.; Busqué, F.; Ruiz-Molina, D. Catechol-Based Biomimetic Functional Materials. Adv. Mater. 2013, 25, 653–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5067–5115. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Luo, J.; Lv, Y.; Shen, P.; Xu, Z.K. Surface Engineering of Polymer Membranes via Mussel-Inspired Chemistry. J. Membr. Sci. 2015, 483, 42–59. [Google Scholar] [CrossRef]
- Hong, S.H.; Hong, S.; Ryou, M.-H.; Choi, J.W.; Kang, S.M.; Lee, H. Sprayable Ultrafast Polydopamine Surface Modifications. Adv. Mater. Interfaces 2016, 3, 1500857. [Google Scholar] [CrossRef]
- Zhang, C.; Ou, Y.; Lei, W.-X.; Wan, L.-S.; Ji, J.; Xu, Z.-K. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem. Int. Ed. 2016, 128, 3106–3109. [Google Scholar] [CrossRef]
- Ponzio, F.; Barthes, J.; Bour, J.; Michel, M.; Bertani, P.; Hemmerlé, J.; d’Ischia, M.; Ball, V. Oxidant Control of Polydopamine Surface Chemistry in Acids: A Mechanism-Based Entry to Superhydrophilic-Superoleophobic Coatings. Chem. Mater. 2016, 28, 4697–4705. [Google Scholar] [CrossRef]
- Krogsgard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chemistry 2016, 22, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, M.; Metz-Boutigue, M.-H.; Bertani, P.; Ball, V. Polyelectrolytes to Produce Nanosized Functional Polydopamine. J. Colloid Interface Sci. 2016, 469, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyński, R.; Bunge, A.; Liebscher, J. Polydopamine-An Organocatalyst Rather than an Innocent Polymer. Chemistry 2014, 20, 8647–8653. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Liu, Y.L.; Ruan, C.; Lu, L.; Lu, G. Sp2 C-Dominant N-Doped Carbon Sub-micrometer Spheres with a Tunable Size: A Versatile Platform for Highly Efficient Oxygen-Reduction Catalysts. Adv. Mater. 2013, 25, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Farnad, N.; Farhadi, K.; Voelcker, N.H. Polydopamine Nanoparticles as a New and Highly Selective Biosorbent for the Removal of Copper (II) Ions from Aqueous Solutions. Water Air Soil Pollut. 2012, 223, 3535–3544. [Google Scholar] [CrossRef]
- Postma, A.; Yan, Y.; Wang, Y.; Zelikin, A.N.; Tjipto, E.; Caruso, F. Self-Polymerization of Dopamine as a Versatile and Robust Technique to Prepare Polymer Capsules. Chem. Mater. 2009, 21, 3042–3044. [Google Scholar] [CrossRef]
- Xu, H.; Liu, X.; Wang, D. Interfacial Basicity-Guided Formation of Polydopamine Hollow Capsules in Pristine O/W Emulsions—Toward Understanding of Emulsion Template Roles. Chem. Mater. 2011, 23, 5105–5110. [Google Scholar] [CrossRef]
- Quignard, S.; d’Ischia, M.; Chen, Y.; Fattaccioli, J. Ultraviolet-Induced Fluorescence of Polydopamine-Coated Emulsion Droplets. ChemPlusChem 2014, 79, 1254–1257. [Google Scholar] [CrossRef]
- Cui, J.; Yan, Y.; Such, G.K.; Liang, K.; Ochs, C.J.; Postma, A.; Caruso, F. Immobilization and Intracellular Delivery of an Anticancer Drug Using Mussel-Inspired Polydopamine Capsules. Biomacromolecules 2012, 13, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.; Statz, A.R.; Rho, J.; Park, T.G.; Messersmith, P.B. Substrate-Independent Layer-by-Layer Assembly by Using Mussel-Adhesive-Inspired Polymers. Adv. Mater. 2008, 20, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, L.; Wang, Y.; Long, Y.; Gao, H.; Zhang, X.; Zhao, N.; Cai, Y.; Xu, J. Mussel-Inspired Chemistry for Robust and Surface-Modifiable Multilayer Films. Langmuir 2011, 27, 13684–13691. [Google Scholar] [CrossRef] [PubMed]
- Bernsmann, F.; Richert, L.; Senger, B.; Lavalle, P.; Voegel, J.-C.; Schaaf, P.; Ball, V. Use of Dopamine Polymerisation to Produce Free-Standing Membranes from (PLL-HA)n Exponentially Growing Multilayer Films. Soft Matter 2008, 4, 1621–1624. [Google Scholar] [CrossRef]
- Kohri, M.; Shinoda, Y.; Kohma, H.; Nannichi, Y.; Yamauchi, M.; Yagai, S.; Kojima, T.; Taniguchi, T.; Kishikawa, K. Facile Synthesis of Free-Standing Polymer Brush Films Based on a Colorless Polydopamine Thin Layer. Macromol. Rapid Commun. 2013, 34, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Xu, W.; Du, Y.; Wu, J.; Xu, Z.-K. Composite Free-Standing Films of Polydopamine/Polyethyleneimine Grown at the Air/Water Interface. RSC Adv. 2014, 4, 45415–45418. [Google Scholar] [CrossRef]
- Hong, S.; Schaber, C.F.; Dening, K.; Appel, E.; Gorb, S.N.; Lee, H. Air/Water Interfacial Formation of Freestanding, Stimuli-Responsive, Self-Healing Catecholamine Janus-Faced Microfilms. Adv. Mater. 2014, 26, 7581–7587. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, F.; Houerou, V.L.; Zafeiratos, S.; Gauthier, C.; Garnier, T.; Jierry, L.; Ball, V. Robust Alginate-Catechol@Polydopamine Free-Standing Membranes Obtained from the Water/Air Interface. Langmuir 2017, 33, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and Gelation of DOPA-Modified Poly(ethylene glycol) Hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.-I.; Lee, H.; Cho, S.-W. Bioinspired, Calcium-Free Alginate Hydrogels with Tunable Physical and Mechanical Properties and Improved Biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, L.; Tada, S.; Zhou, D.; Kitajima, T.; Isoshima, T.; Yoshida, Y.; Nakamura, M.; Yan, W.; Ito, Y. Mussel-Inspired Human Gelatin Nanocoating for Creating Biologically Adhesive Surfaces. Int. J. Nanomed. 2014, 9, 2753–2765. [Google Scholar]
- Chen, J.-T.; Zhang, M.; Russell, T.P. Instabilities in Nanoporous Media. Nano Lett. 2007, 7, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, M.; Baixe, S.; Garnier, T.; Jierry, L.; Ball, V.; Haikel, Y.; Metz-Boutigue, M.H.; Nardin, M.; Schaaf, P.; Etienne, O.; et al. Antibacterial Peptide-Based Gel for Prevention of Medical Implanted-Device Infection. PLoS ONE 2015, 10, e0145143. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.G.; Nakano, Y.; Ito, Y.; Abe, H. Transmembrane Molecular Transport through Nanopores Formed by Protein Nanotubes. Chem. Commun. 2014, 50, 602–604. [Google Scholar] [CrossRef] [PubMed]


| Dopamine-Modified Polymers | Molar Ratio in Feed Carboxyl:Catechol | Substitution Degree of Catechol Group in Carboxyl Group of Polymer | Weight of Catechol in Polymer 3 | |
|---|---|---|---|---|
| UV–vis 1 | NMR 2 | |||
| Alginate-catechol (AC) | 1:3 | 22% | 29% | 18.5% |
| Gelatin-catechol (GC) | 1:3 | 3.7% | 2% | 0.6% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Isoshima, T.; Nair, B.G.; Ito, Y. Template-Assisted Formation of Nanostructured Dopamine-Modified Polymers. Nanomaterials 2017, 7, 364. https://doi.org/10.3390/nano7110364
Zhu L, Isoshima T, Nair BG, Ito Y. Template-Assisted Formation of Nanostructured Dopamine-Modified Polymers. Nanomaterials. 2017; 7(11):364. https://doi.org/10.3390/nano7110364
Chicago/Turabian StyleZhu, Liping, Takashi Isoshima, Baiju G. Nair, and Yoshihiro Ito. 2017. "Template-Assisted Formation of Nanostructured Dopamine-Modified Polymers" Nanomaterials 7, no. 11: 364. https://doi.org/10.3390/nano7110364