Functionalized ZnO Nanoparticles with Gallic Acid for Antioxidant and Antibacterial Activity against Methicillin-Resistant S. aureus
Abstract
:1. Introduction
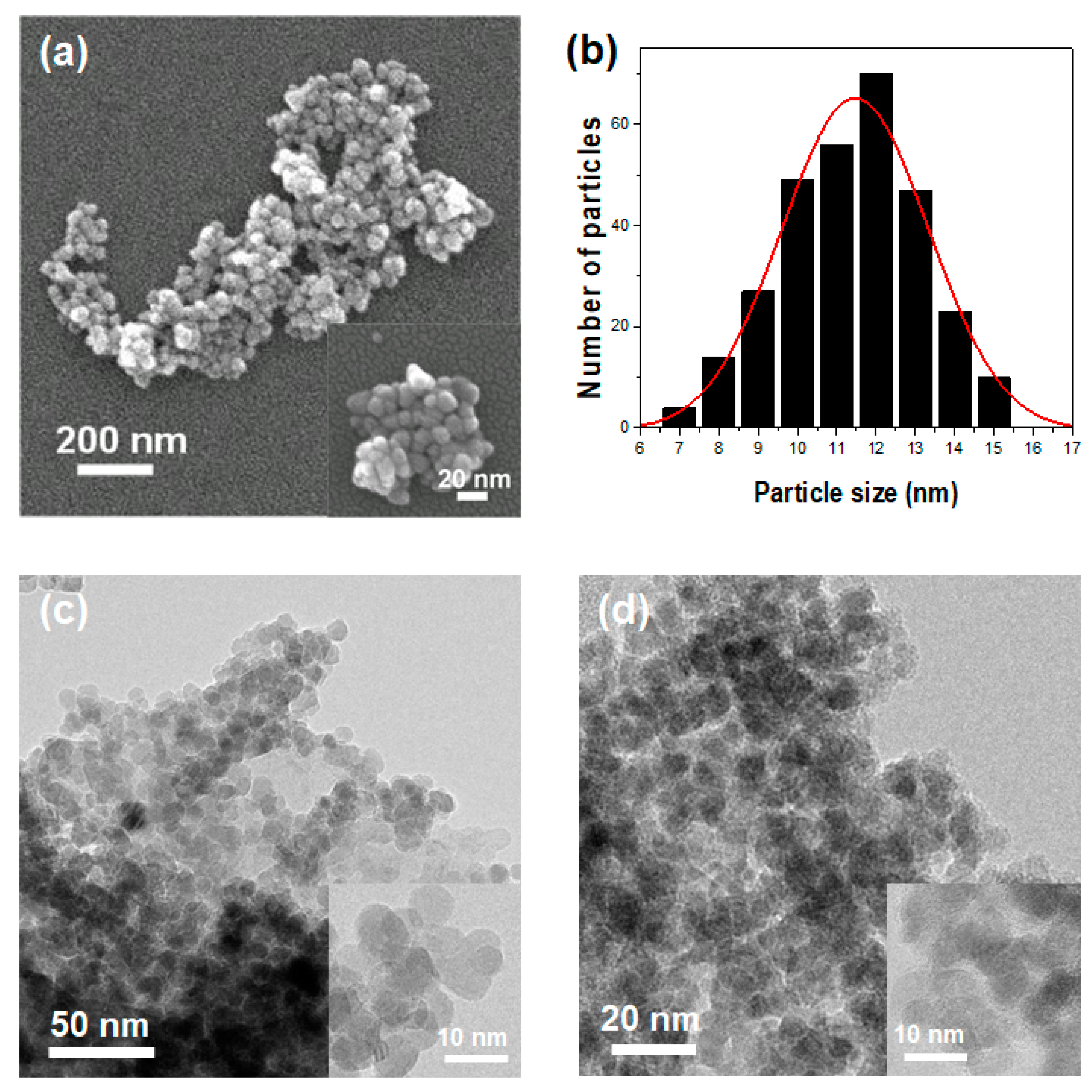
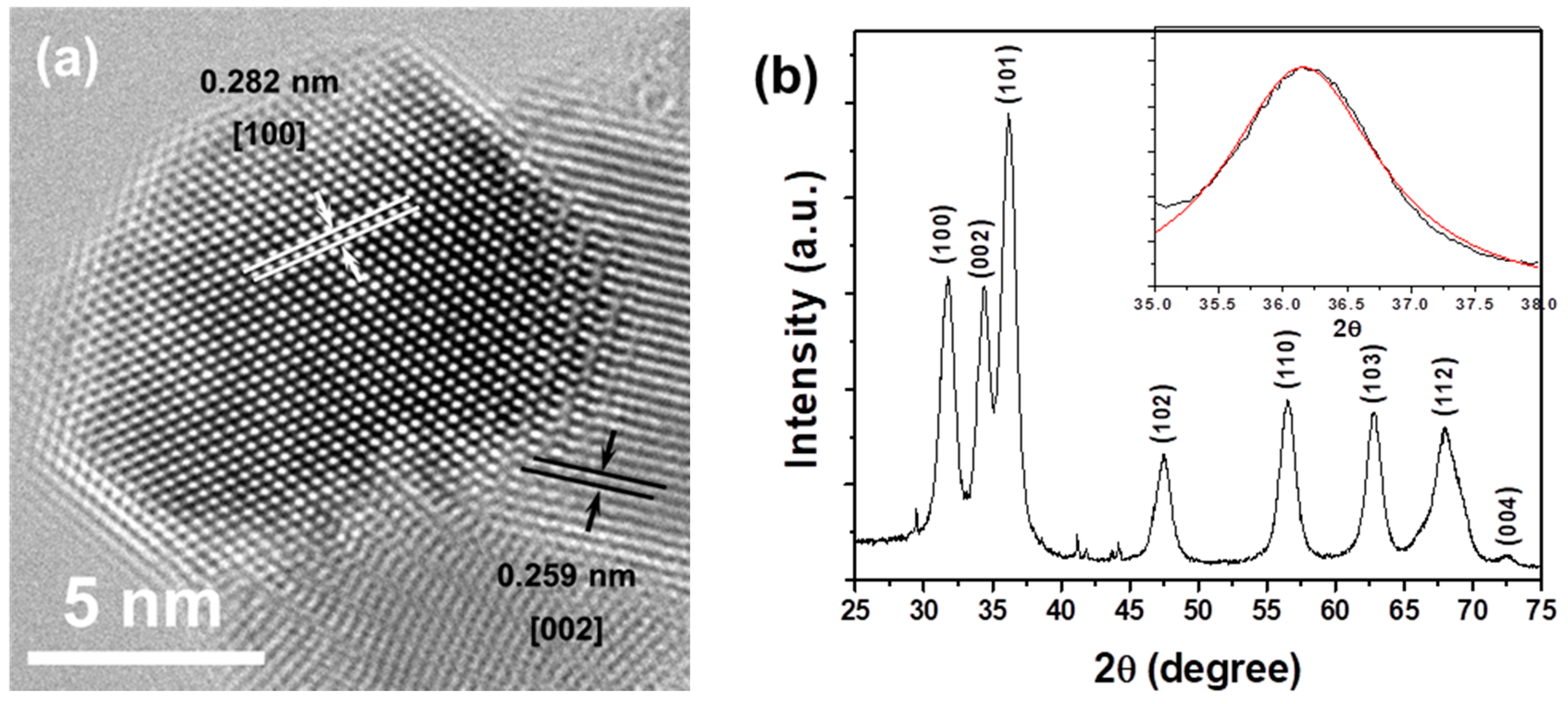
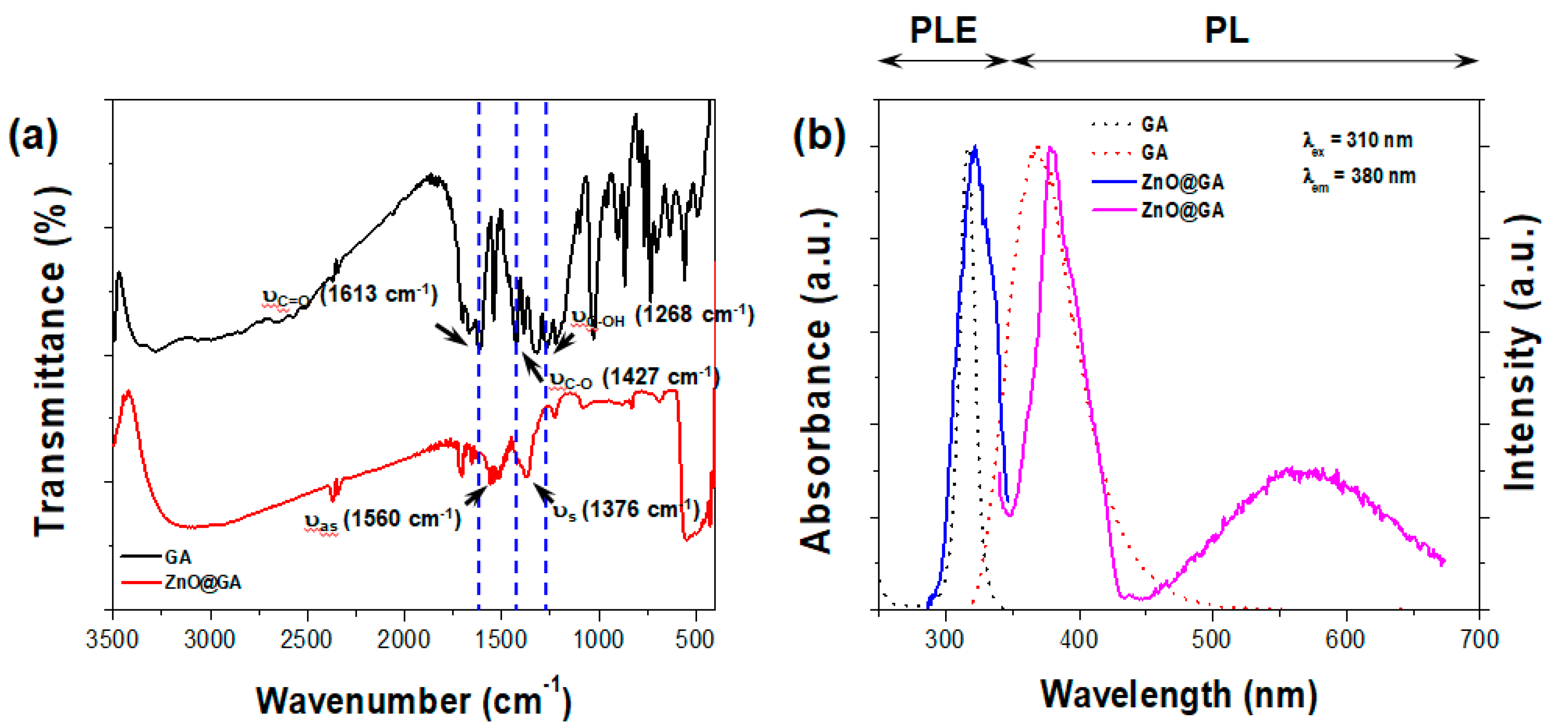
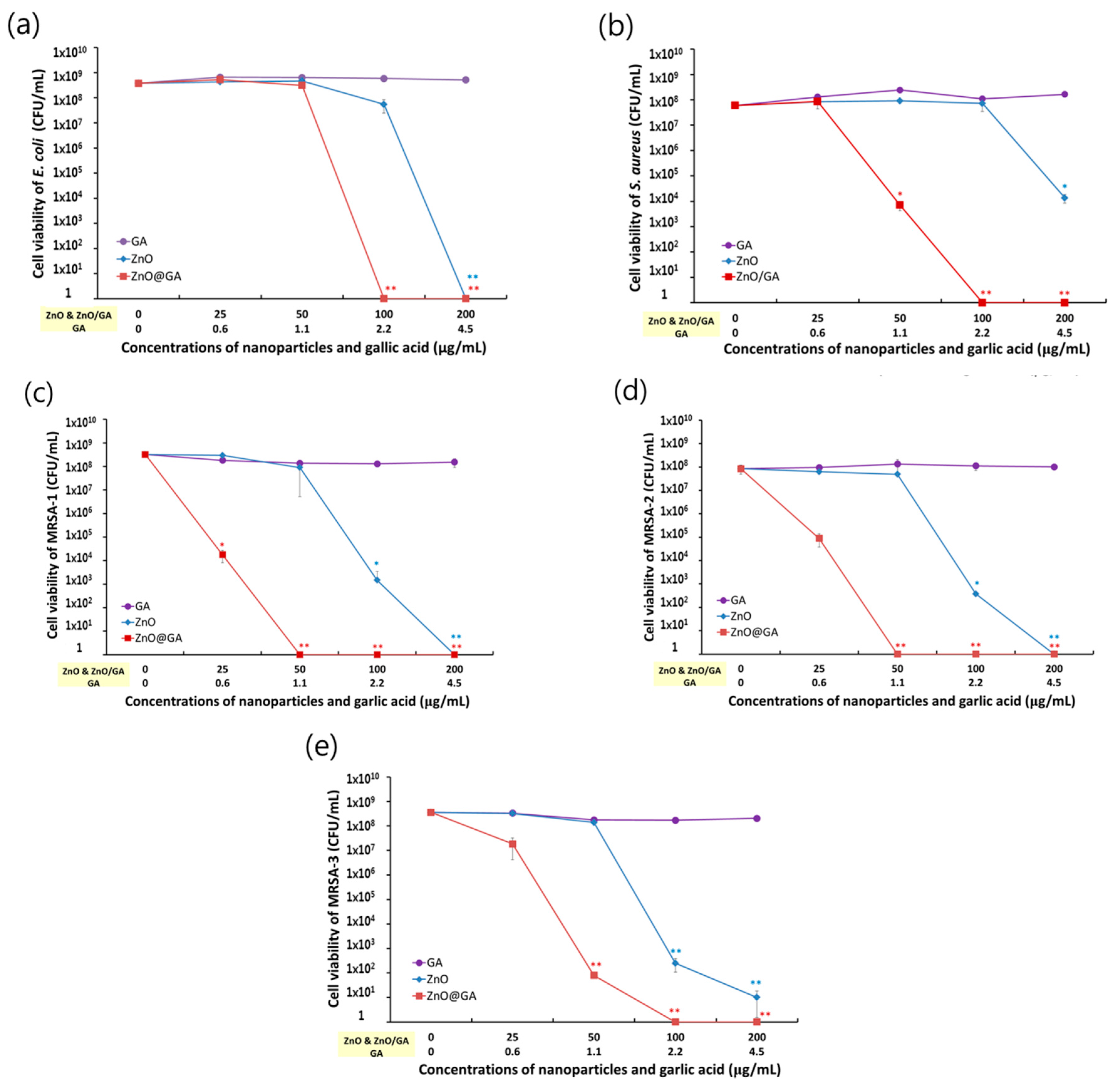
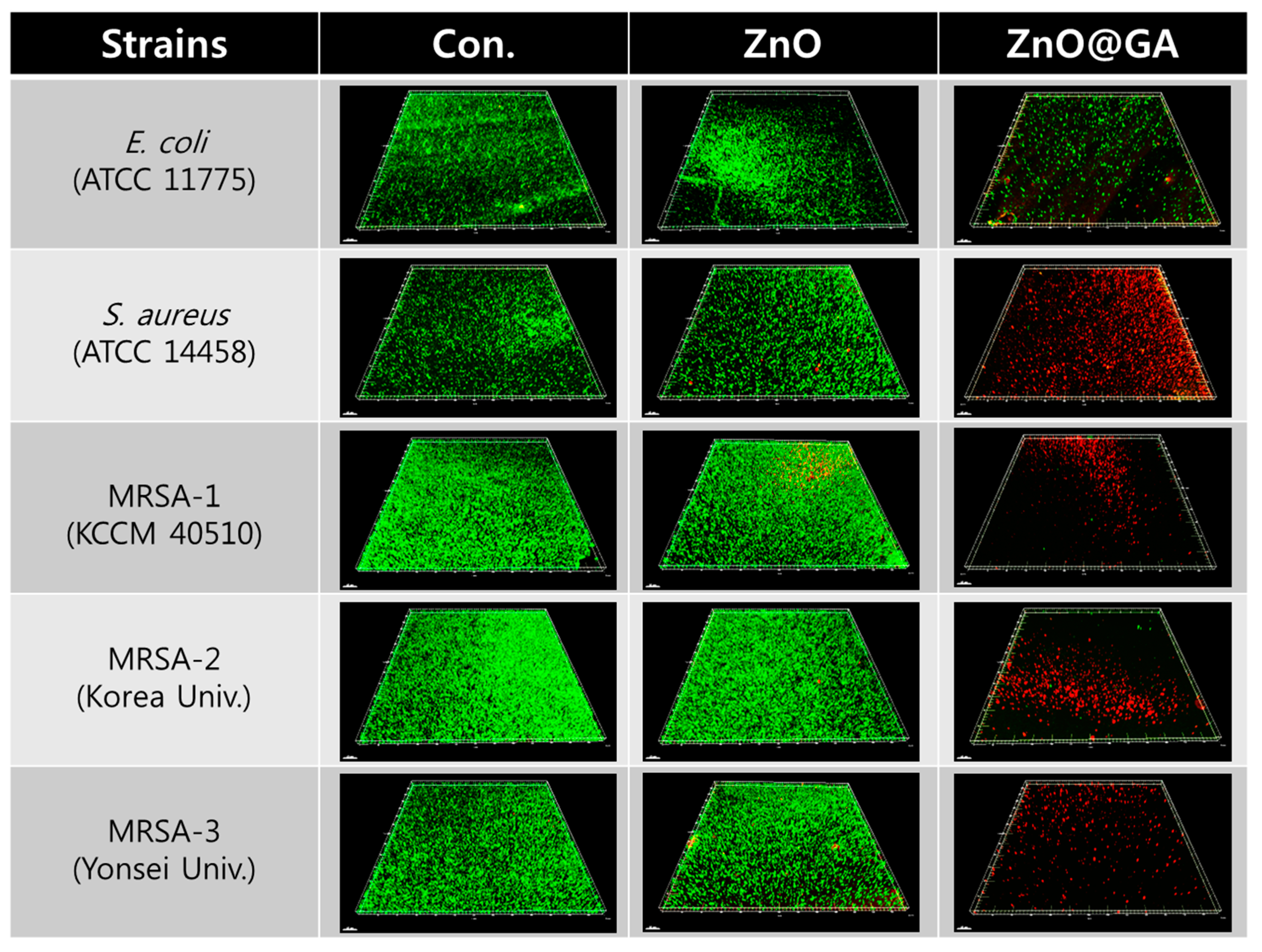
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of ZnO@GA Nanoparticles
3.2. Physical Characterization of ZnO@GA Nanoparticles
3.3. Evaluation of ZnO@GA Nanoparticle Antioxidant Activity
3.4. Evaluation of ZnO@GA Nanoparticle Antibacterial Activity
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Choi, K.-H.; Lee, H.-J.; Park, B.J.; Wang, K.-K.; Shin, E.P.; Park, J.-C.; Kim, Y.K.; Oh, M.-K.; Kim, Y.-R. Photosensitizer and vancomycin-conjugated novel multifunctional magnetic particles as photoinactivation agents for selective killing of pathogenic bacteria. Chem. Commun. 2012, 48, 4591–4593. [Google Scholar] [CrossRef] [PubMed]
- Suay-García, B.; Pérez-Gracia, M.T. The antimicrobial therapy of the future: Combating resistances. J. Infect. Dis. Ther. 2014, 2, 1000146. [Google Scholar] [CrossRef]
- Fonkwo, P.N. Pricing infectious disease; the economic and health implications of infectious diseases. EMBO Rep. 2008, 9, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Aires-de-Sousa, M.; Boyce, J.; Tiemersma, E. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368, 874–885. [Google Scholar] [CrossRef]
- Ippolito, G.; Leone, S.; Lauria, F.N.; Nicastri, E.; Wenzel, R.P. Methicillin-resistant Staphylococcus aureus: The superbug. Int. J. Infect. Dis. 2010, 14, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Cherukula, K.; Manickavasagam Lekshmi, K.; Uthaman, S.; Cho, K.; Cho, C.-S.; Park, I.-K. Multifunctional inorganic nanoparticles: Recent progress in thermal therapy and imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 893. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.L.; Ussher, A.L.; Burrell, R.E. Impact of heat on nanocrystalline silver dressings. Part I: Chemical and biological properties. Biomaterials 2005, 26, 7221–7229. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.; Gade, A.; Pierrat, S.; Sönnichsen, C.; Rai, M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr. Nanosci. 2008, 4, 141–144. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Mayers, B.; Herricks, T.; Xia, Y.N. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Navale, G.R.; Thripuranthaka, M.; Late, D.J.; Shinde, S.S. Antimicrobial activity of ZnO nanoparticles against pathogenic bacteria and fungi. JSM Nanotechnol. Nanomed. 2015, 3, 1033. [Google Scholar]
- Butler, S.; Neogi, P.; Urban, B.; Fujita, Y.; Hu, Z.; Neogi, A. ZnO nanoparticles in hydrogel polymer network for bio-imaging. Glob. J. Nanomed. 2017, 1, 555572. [Google Scholar]
- Sang, C.H.; Chou, S.J.; Pan, F.M.; Sheu, J.T. Fluorescence enhancement and multiple protein detection in ZnO nanostructure microfluidic devices. Biosens. Bioelectron. 2016, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.-J.; Wang, A.-N.; Liao, Q.-L.; Chuang, K.-Y. Non-enzymatic glucose sensor composed of carbon-coated nano-zinc oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; de Lourdes Bastos, M. Antioxidant activity of hypericum androsaemum infusion: Scavenging activity against superoxide radical, hydroxyl radical and hypochlorous acid. Biol. Pharm. Bull. 2002, 25, 1320–1323. [Google Scholar]
- Borra, S.K.; Gurumurthy, P.; Mahendra, J.; Jayamathi, K.M.; Cherian, C.N.; Chand, R. Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. J. Med. Plant Res. 2013, 7, 2680–2690. [Google Scholar] [CrossRef]
- Du, X.-W.; Fu, Y.-S.; Sun, J.; Han, X.; Liu, J. Complete UV emission of ZnO nanoparticles in a PMMA matrix. Semicond. Sci. Technol. 2006, 21, 1202–1206. [Google Scholar] [CrossRef]
- Aneesh, P.M.; Vanaja, K.A.; Jayaraj, M.K. Synthesis of ZnO nanoparticles by hydrothermal method. Proc. SPIE 2007, 6639, 66390J. [Google Scholar] [CrossRef]
- Abri, A.; Maleki, M. Isolation and identification of Gallic acid from the Elaeagnus angustifolia leaves and determination of total phenolic, flavonoids contents and investigation of antioxidant activity. Iran. Chem. Commun. 2016, 4, 146–154. [Google Scholar]
- Dutta, S.; Ganguly, B.N. Characterization of ZnO nanoparticles grown in presence of Folic acid template. J. Nanobiotechnol. 2012, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Nam, K.C.; Lee, S.Y.; Cho, G.; Jung, J.S.; Kim, H.J.; Park, B.J. Antioxidant potential and antibacterial efficiency of caffeic acid-functionalized ZnO nanoparticles. Nanomaterials 2017, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, J.; Zhu, W.; Jiang, T.; Zhang, X.; Gu, Q. Antibacterial activity of gallic acid from the flowers of Rosa chinensis Jacq. against fish pathogens. Aquacult. Res. 2007, 38, 1110–1112. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamada, Y.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y. Bactericidal action of photoirradiated gallic acid via reactive oxygen species formation. J. Agric. Food Chem. 2012, 60, 10048–10054. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Park, B.J.; Choi, K.H.; Nam, K.C.; Min, J.E.; Lee, K.D.; Uhm, H.S.; Choi, E.H.; Kim, H.J.; Jung, J.S. Photodynamic anticancer activity of CoFe2O4 nanoparticles conjugated with hematoporphyrin. J. Nanosci. Nanotechnol. 2015, 15, 7900–7906. [Google Scholar] [CrossRef] [PubMed]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
| Sample | Concentration (μM) | % Inhibition |
|---|---|---|
| ZnO@GA | 20 | 33.29 ± 0.12 |
| ZnO@GA | 40 | 57.17 ± 0.96 a |
| ZnO@GA | 100 | 69.71 ± 5.26 a |
| Gallic acid | 20 | 43.38 ± 0.48 b |
| Gallic acid | 40 | 72.76 ± 0.12 b |
| Gallic acid | 100 | 93.25 ± 0.43 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Choi, K.-H.; Min, J.; Kim, H.-J.; Jee, J.-P.; Park, B.J. Functionalized ZnO Nanoparticles with Gallic Acid for Antioxidant and Antibacterial Activity against Methicillin-Resistant S. aureus. Nanomaterials 2017, 7, 365. https://doi.org/10.3390/nano7110365
Lee J, Choi K-H, Min J, Kim H-J, Jee J-P, Park BJ. Functionalized ZnO Nanoparticles with Gallic Acid for Antioxidant and Antibacterial Activity against Methicillin-Resistant S. aureus. Nanomaterials. 2017; 7(11):365. https://doi.org/10.3390/nano7110365
Chicago/Turabian StyleLee, Joomin, Kyong-Hoon Choi, Jeeeun Min, Ho-Joong Kim, Jun-Pil Jee, and Bong Joo Park. 2017. "Functionalized ZnO Nanoparticles with Gallic Acid for Antioxidant and Antibacterial Activity against Methicillin-Resistant S. aureus" Nanomaterials 7, no. 11: 365. https://doi.org/10.3390/nano7110365




