Resistive Switching of Sub-10 nm TiO2 Nanoparticle Self-Assembled Monolayers
Abstract
:1. Introduction
2. Results
2.1. Solvothermal Synthesis of TiO2 Nanoparticles
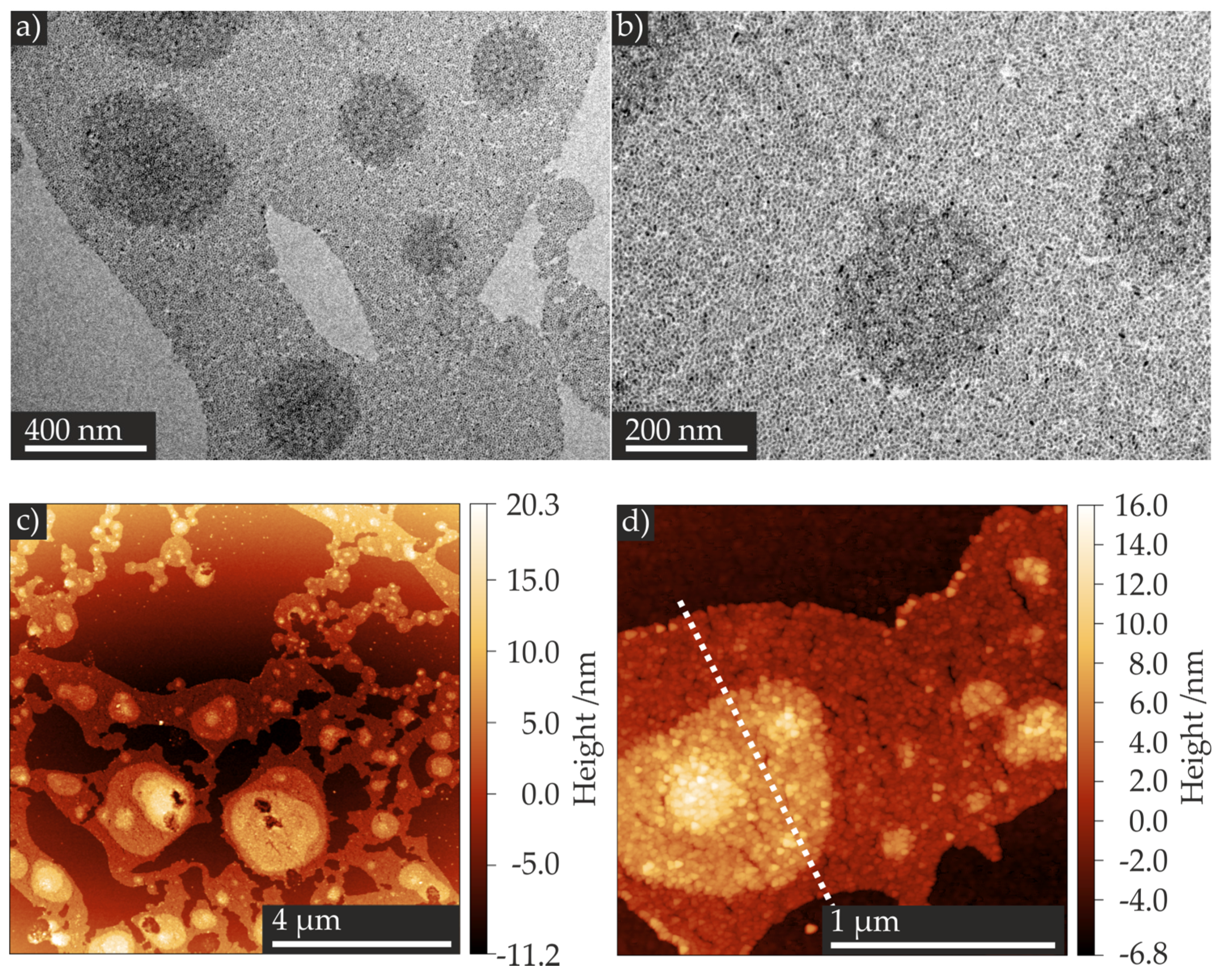
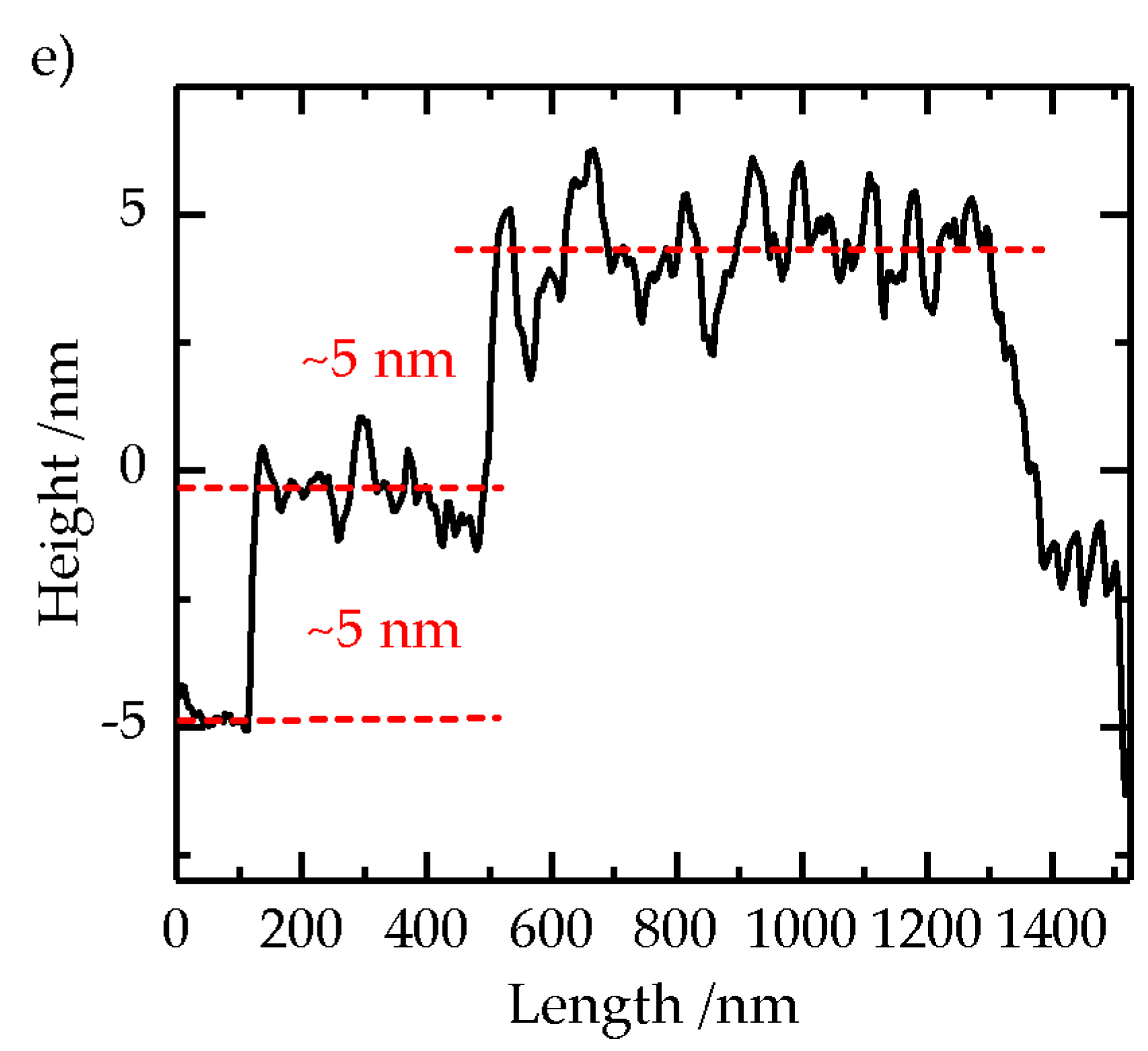
2.2. Formation of Self-Assembled TiO2 Nanoparticle Monolayers
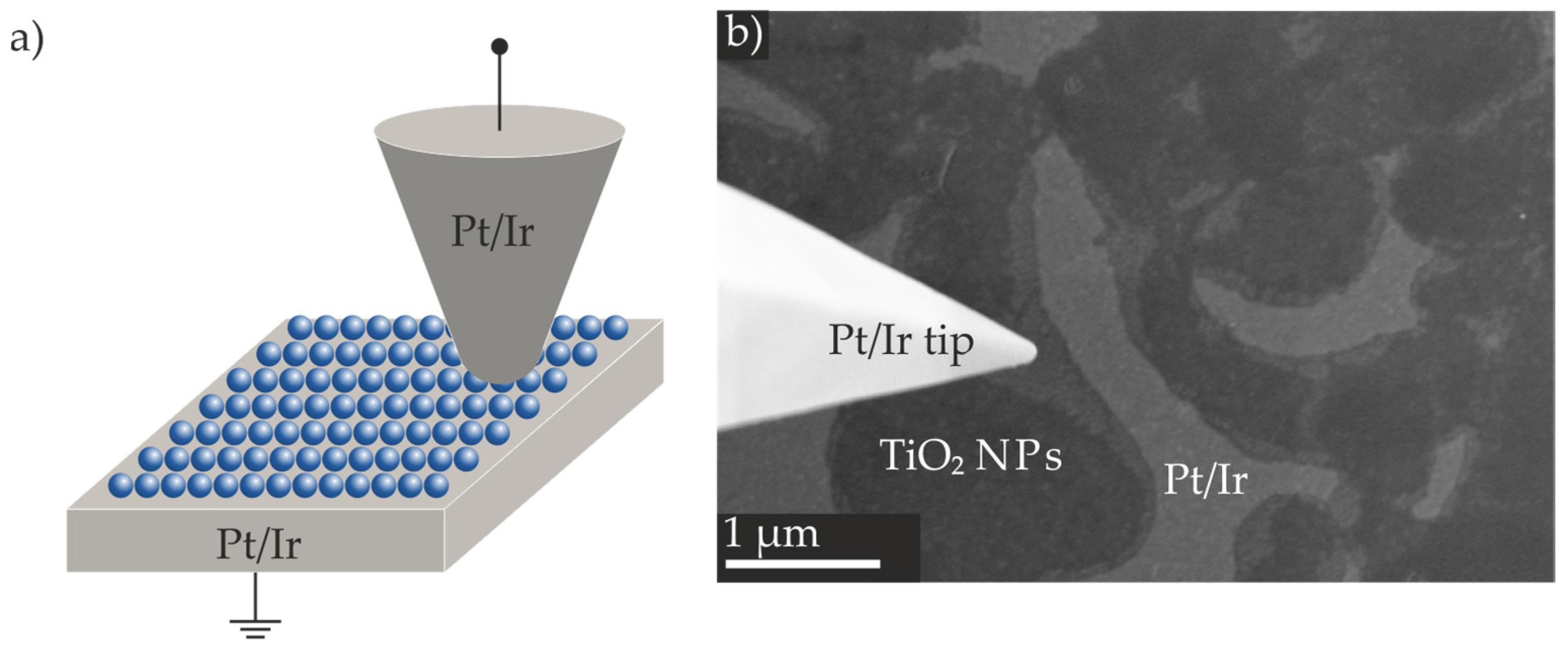
2.3. Preparation of Resistive Switching Devices
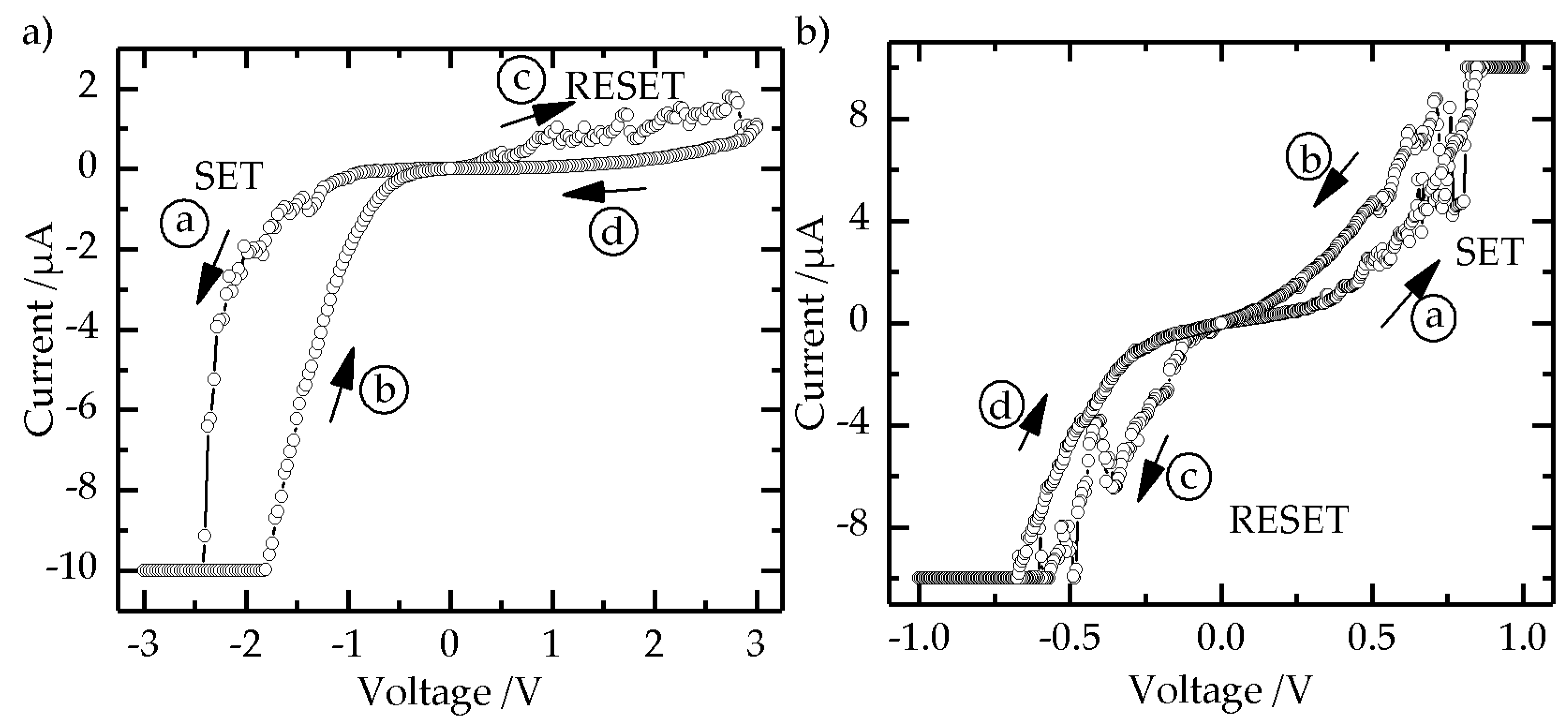
2.4. Electrical Characterization
2.5. Summary
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moore, G.E. Cramming more components onto Integrated circuits. Electronics 1965, 38, 114–117. [Google Scholar] [CrossRef]
- Moore, G.E. Progress in Digital Integrated Electronics. IEDM Tech. Dig. 1975, 21, 11–13. [Google Scholar]
- Waldrop, M.M. The chips are down for Moore’s law. Nature 2016, 530, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Waser, R.; Dittmann, R.; Staikov, G.; Szot, K. Redox-Based Resistive Switching Memories—Nanoionic Mechanisms, Prospects, and Challenges. Adv. Mater. 2009, 21, 2632–2663. [Google Scholar] [CrossRef]
- Ielmini, D.; Waser, R. Resistive Switching: From Fundamentals of Nanoionic Redox Processes to Memristive Device Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Ielmini, D.; Cagli, C.; Nardi, F.; Zhang, Y. Nanowire-based resistive switching memories: Devices, operation and scaling. J. Phys. D Appl. Phys. 2013, 46, 074006. [Google Scholar] [CrossRef]
- Ju, Y.C.; Kim, S.; Seong, T.-G.; Nahm, S.; Chung, H.; Hong, K.; Kim, W. Resistance Random Access Memory Based on a Thin Film of CdS Nanocrystals Prepared via Colloidal Synthesis. Small 2012, 8, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-D.; Baek, Y.-J.; Jin Choi, Y.; Jung Kang, C.; Ho Lee, H.; Kim, H.-M.; Kim, K.-B.; Yoon, T.-S. Investigation of analog memristive switching of iron oxide nanoparticle assembly between Pt electrodes. J. Appl. Phys. 2013, 114, 224505. [Google Scholar] [CrossRef]
- Kim, T.H.; Jang, E.Y.; Lee, N.J.; Choi, D.J.; Lee, K.-J.; Jang, J.; Choil, J.; Moon, S.H.; Cheon, J. Nanoparticle Assemblies as Memristors. Nano Lett. 2009, 9, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Jung, S.M.; Lee, H.H.; Kim, Y.-S.; Choi, Y.J.; Kang, D.-H.; Kim, K.-B.; Yoon, T.-S. Resistive switching characteristics of maghemite nanoparticle assembly. J. Phys. D Appl. Phys. 2011, 44, 085403. [Google Scholar] [CrossRef]
- Yoo, J.W.; Hu, Q.; Baek, Y.-J.; Choi, Y.J.; Kang, C.J.; Lee, H.H.; Lee, D.-J.; Kim, H.-M.; Kim, K.-B.; Yoon, T.-S. Resistive switching characteristics of maghemite nanoparticle assembly on Al and Pt electrodes on a flexible substrate. J. Phys. D Appl. Phys. 2012, 45, 225304. [Google Scholar] [CrossRef]
- Baek, Y.-J.; Hu, Q.; Yoo, J.W.; Choi, Y.J.; Kang, C.J.; Lee, H.H.; Min, S.-H.; Kim, H.-M.; Kim, K.-B.; Yoon, T.-S. Tunable threshold resistive switching characteristics of Pt-Fe2O3 core-shell nanoparticle assembly by space charge effect. Nanoscale 2013, 5, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Baek, Y.-J.; Hu, Q.; Choi, Y.J.; Kang, C.J.; Lee, H.H.; Kim, H.-M.; Kim, K.-B.; Yoon, T.-S. Multimode threshold and bipolar resistive switching in bi-layered Pt-Fe2O3 core-shell and Fe2O3 nanoparticle assembly. Appl. Phys. Lett. 2013, 102, 122111. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Mihail, I.; Li, S. Interface-Engineered Resistive Switching: CeO2 Nanocubes as High-Performance Memory Cells. ACS Appl. Mater. Interfaces 2013, 5, 9429–9434. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Chu, D.; Li, C.M.; Das, T.; Sehar, S.; Manefield, M.; Li, S. Interface Thermodynamic State-Induced High-Performance Memristors. Langmuir 2014, 30, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Lin, X.; Younis, A.; Li, C.M.; Dang, F.; Li, S. Growth and self-assembly of BaTiO3 nanocubes for resistive switching memory cells. J. Solid State Chem. 2014, 214, 38–41. [Google Scholar] [CrossRef]
- Li, C.; Beirne, G.J.; Kamita, G.; Lakhwani, G.; Wang, J.; Greenham, N.C. Probing the switching mechanism in ZnO nanoparticle memristors. J. Appl. Phys. 2014, 116, 114501. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, Y.-J.; Choi, Y.J.; Kang, C.J.; Lee, H.H.; Kim, H.-M.; Kim, K.-B.; Yoon, T.-S. Digital versus analog resistive switching depending on the thickness of nickel oxide nanoparticle assembly. RSC Adv. 2013, 3, 20978–20983. [Google Scholar] [CrossRef]
- Prakash, A.; Maikap, S.; Rahaman, S.Z.; Majumdar, S.; Manna, S.; Ray, S.K. Resistive switching memory characteristics of Ge/GeOx nanowires and evidence of oxygen ion migration. Nanoscale Res. Lett. 2013, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Chu, D.; Li, S. Tuneable resistive switching characteristics of In2O3 nanorods array via Co doping. RSC Adv. 2013, 3, 13422–13428. [Google Scholar] [CrossRef]
- Goren, E.; Ungureanu, M.; Zazpe, R.; Rozenberg, M.; Hueso, L.E.; Stoliar, P.; Tsur, Y.; Casanova, F. Resistive switching phenomena in TiOx nanoparticle layers for memory applications. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Uenuma, M.; Ban, T.; Okamoto, N.; Zheng, B.; Kakihara, Y.; Horita, M.; Ishikawa, Y.; Yamashita, I.; Uraoka, Y. Memristive nanoparticles formed using a biotemplate. RSC Adv. 2013, 3, 18044–18048. [Google Scholar] [CrossRef]
- Schmidt, D.O.; Hoffmann-Eifert, S.; Zhang, H.; La Torre, C.; Besmehn, A.; Noyong, M.; Waser, R.; Simon, U. Resistive Switching of Individual, Chemically Synthesized TiO2 Nanoparticles. Small 2015, 11, 6444–6456. [Google Scholar] [CrossRef] [PubMed]
- Szot, K.; Rogala, M.; Speier, W.; Klusek, Z.; Besmehn, A.; Waser, R. TiO2—A prototypical memristive material. Nanotechnology 2011, 22, 254001–254022. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Inoue, I.H.; Mikolajick, T.; Hwang, C.S. Metal oxide memories based on thermochemical and valence change mechanisms. MRS Bull. 2012, 37, 131–137. [Google Scholar] [CrossRef]
- Strachan, J.P.; Yang, J.J.; Montoro, L.A.; Ospina, C.A.; Ramirez, A.J.; Kilcoyne, A.L.D.; Medeiros-Ribeiro, G.; Williams, R.S. Characterization of electroforming-free titanium dioxide memristors. Beilstein J. Nanotechnol. 2013, 4, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hermes, C.; Bruchhaus, R.; Waser, R. Forming-Free TiO2-Based Resistive Switching Devices on CMOS-Compatible W-Plugs. IEEE Electron Device Lett. 2011, 32, 1588–1590. [Google Scholar] [CrossRef]
- Warneck, P.; Williams, J. The Atmospheric Chemist’s Companion, Numerical Data for Use in the Atmospheric Sciences; Springer: New York, NY, USA, 2012. [Google Scholar]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Giersig, M.; Mulvaney, P. Preparation of ordered colloid monolayers by electrophoretic deposition. Langmuir 1993, 9, 3408–3413. [Google Scholar] [CrossRef]
- Huie, J.C. Guided molecular self-assembly: A review of recent efforts. Smart Mater. Struct. 2003, 12, 264. [Google Scholar] [CrossRef]
- Wen, T.; Majetich, S.A. Ultra-Large-Area Self-Assembled Monolayers of Nanoparticles. ACS Nano 2011, 5, 8868–8876. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, V.; Liu, J.; Agarwal, R.; Andres, R.P. Self-Assembly of Uniform Monolayer Arrays of Nanoparticles. Langmuir 2003, 19, 7881–7887. [Google Scholar] [CrossRef]
- Davi, M.; Keßler, D.; Slabon, A. Electrochemical oxidation of methanol and ethanol on two-dimensional self-assembled palladium nanocrystal arrays. Thin Solid Films 2016, 615, 221–225. [Google Scholar] [CrossRef]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed Self-Assembly of Nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.-T.; Nguyen, T.-D.; Kleitz, F.; Do, T.-O. Shape-Controlled Synthesis of Highly Crystalline Titania Nanocrystals. ACS Nano 2009, 3, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Shircliff, R.A.; Stradins, P.; Moutinho, H.; Fennell, J.; Ghirardi, M.L.; Cowley, S.W.; Branz, H.M.; Martin, I.T. Angle-Resolved XPS Analysis and Characterization of Monolayer and Multilayer Silane Films for DNA Coupling to Silica. Langmuir 2013, 29, 4057–4067. [Google Scholar] [CrossRef] [PubMed]
- Inorganic Crystal Structure Database, ICSD #9852; FIZ Karlsruhe.
- Santhanam, V.; Andres, R.P. Microcontact Printing of Uniform Nanoparticle Arrays. Nano Lett. 2004, 4, 41–44. [Google Scholar] [CrossRef]
- Noyong, M.; Blech, K.; Rosenberger, A.; Klocke, V.; Simon, U. In-situ nanomanipulation system for electrical measurements in SEM. Meas. Sci. Technol. 2007, 18, N84–N89. [Google Scholar] [CrossRef]
- Xiao, P.F.; Lai, M.O.; Lu, L. Electrochemical properties of nanocrystalline TiO2 synthesized via mechanochemical reaction. Electrochim. Acta 2012, 76, 185–191. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Kim, K.M.; Jang, J.H.; Jeon, J.M.; Lee, M.H.; Kim, G.H.; Li, X.-S.; Park, G.-S.; Lee, B.; Han, S.; et al. Atomic structure of conducting nanofilaments in TiO2 resistive switching memory. Nat. Nanotechnol. 2010, 5, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, R.; Muenstermann, R.; Krug, I.; Park, D.; Menke, T.; Mayer, J.; Besmehn, A.; Kronast, F.; Schneider, C.M.; Waser, R. Scaling Potential of Local Redox Processes in Memristive SrTiO3 Thin-Film Devices. Proc. IEEE 2012, 100, 1979–1990. [Google Scholar] [CrossRef]
- Cooper, D.; Baeumer, C.; Bernier, N.; Marchewka, A.; La Torre, C.; Dunin-Borkowski, R.E.; Menzel, S.; Waser, R.; Dittmann, R. Anomalous Resistance Hysteresis in Oxide ReRAM: Oxygen Evolution and Reincorporation Revealed by In Situ TEM. Adv. Mater. 2017, 29, 1700212. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Magnéli, A. Diskrete Titanoxydphasen im Zusammensetzungsbereich TiO1,75-TiO1,90. Naturwissenschaften 1956, 43, 495–496. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, D.O.; Raab, N.; Noyong, M.; Santhanam, V.; Dittmann, R.; Simon, U. Resistive Switching of Sub-10 nm TiO2 Nanoparticle Self-Assembled Monolayers. Nanomaterials 2017, 7, 370. https://doi.org/10.3390/nano7110370
Schmidt DO, Raab N, Noyong M, Santhanam V, Dittmann R, Simon U. Resistive Switching of Sub-10 nm TiO2 Nanoparticle Self-Assembled Monolayers. Nanomaterials. 2017; 7(11):370. https://doi.org/10.3390/nano7110370
Chicago/Turabian StyleSchmidt, Dirk Oliver, Nicolas Raab, Michael Noyong, Venugopal Santhanam, Regina Dittmann, and Ulrich Simon. 2017. "Resistive Switching of Sub-10 nm TiO2 Nanoparticle Self-Assembled Monolayers" Nanomaterials 7, no. 11: 370. https://doi.org/10.3390/nano7110370




