ZnO Interactions with Biomatrices: Effect of Particle Size on ZnO-Protein Corona
Abstract
:1. Introduction
2. Results
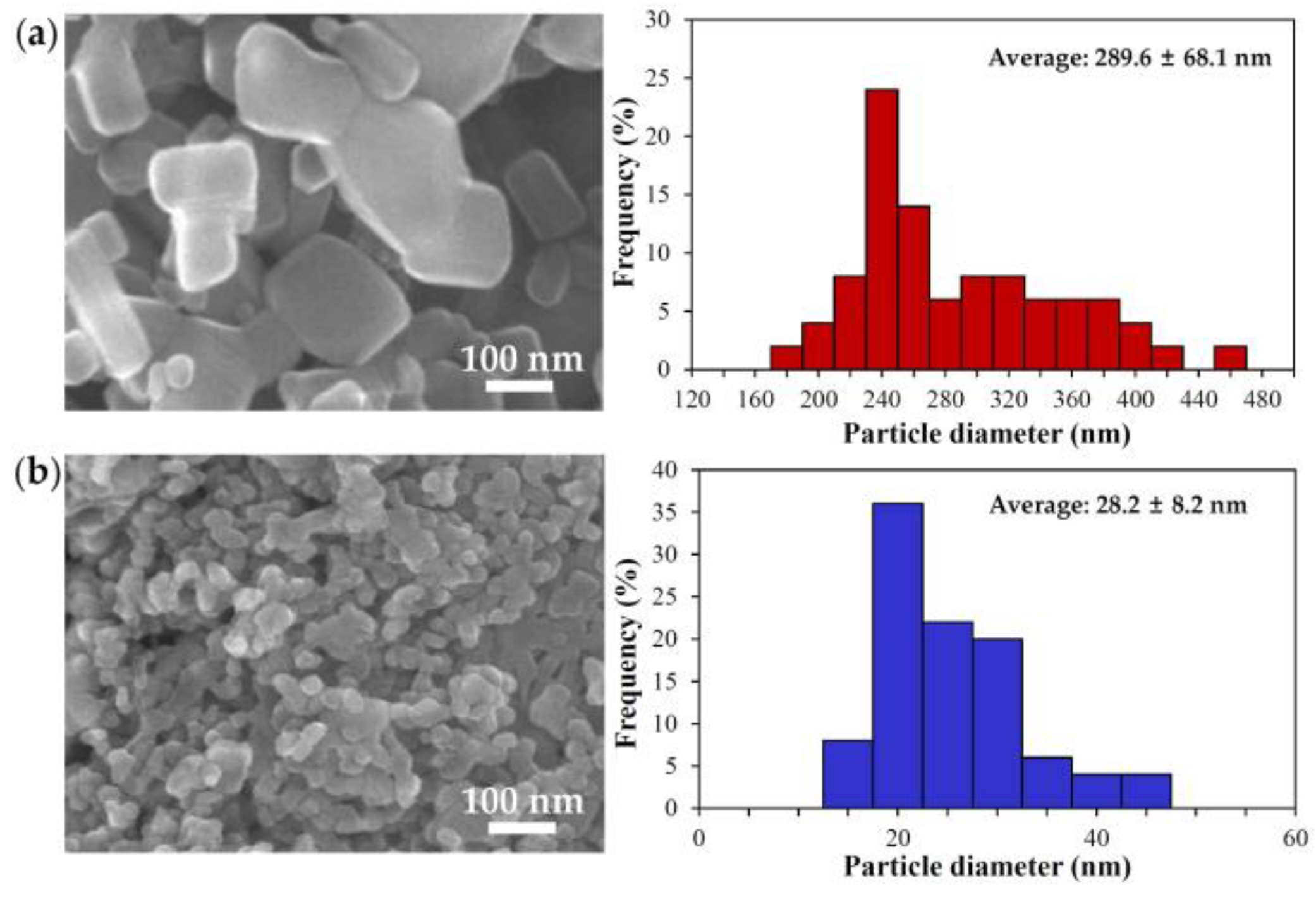
2.1. Characterization of Bulk and Nano ZnOs
2.2. Changes in the Physicochemical Properties of ZnOs in Simulated Biofluids
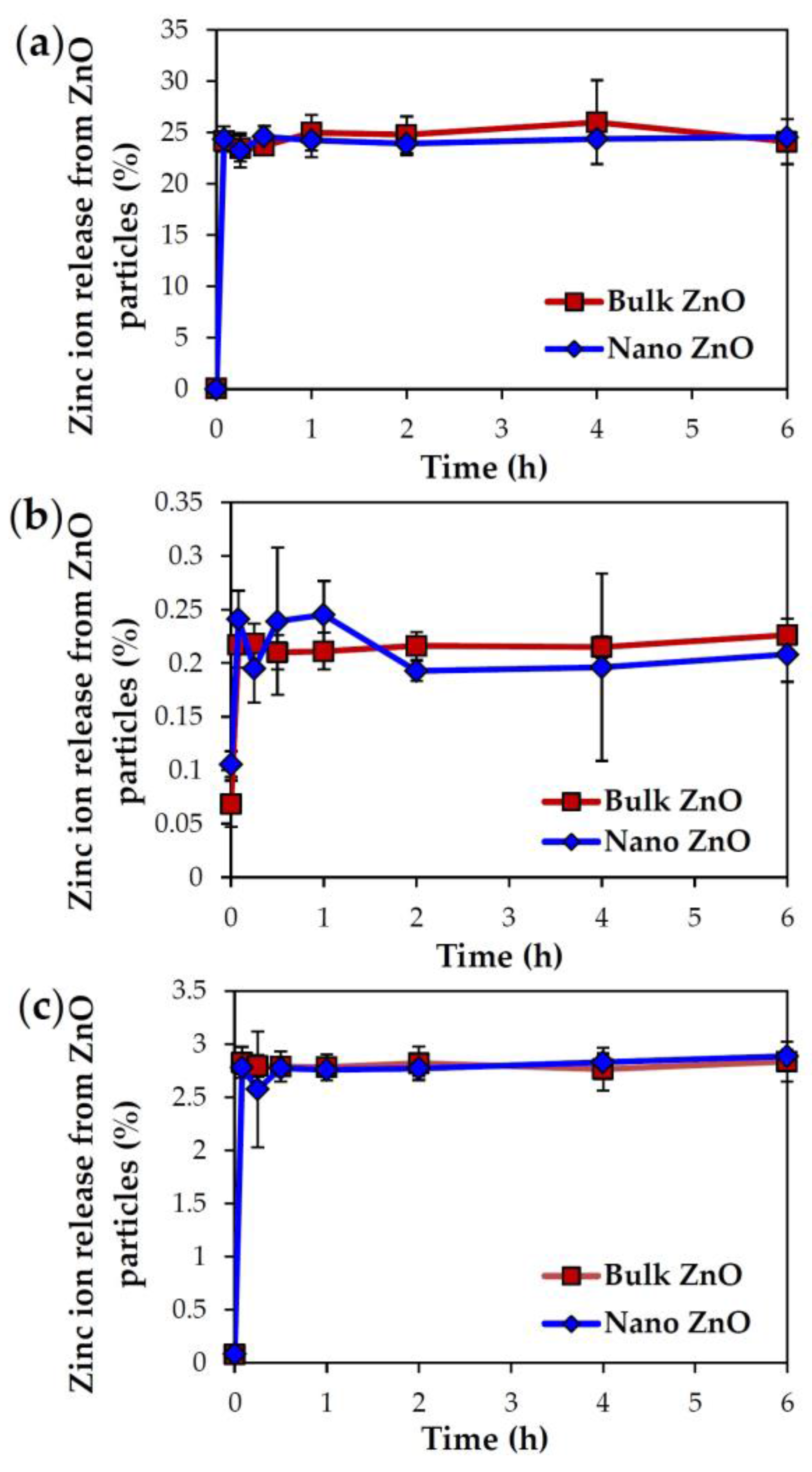
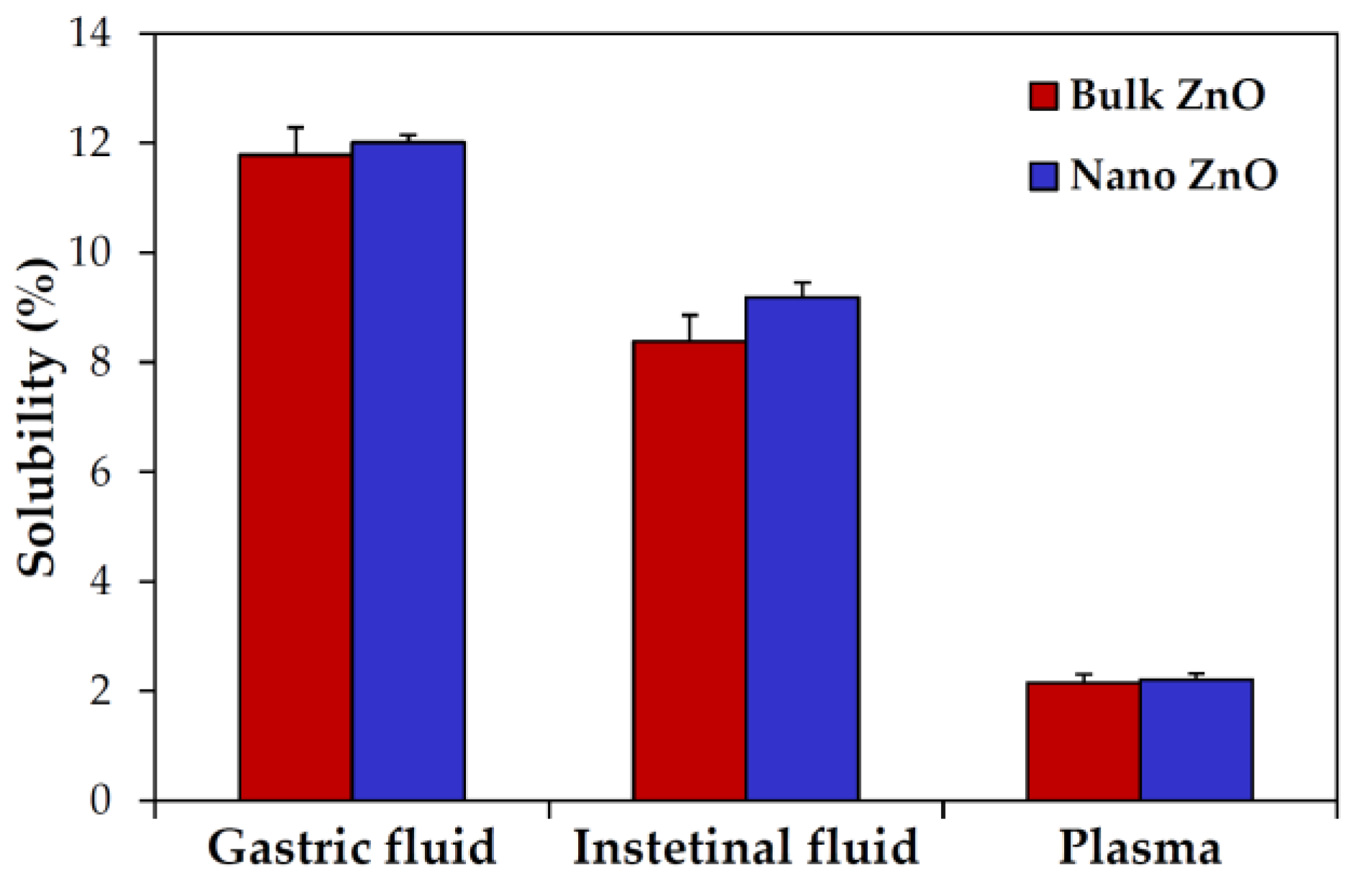
2.3. Dissolution Properties of ZnOs in Simulated Biofluids
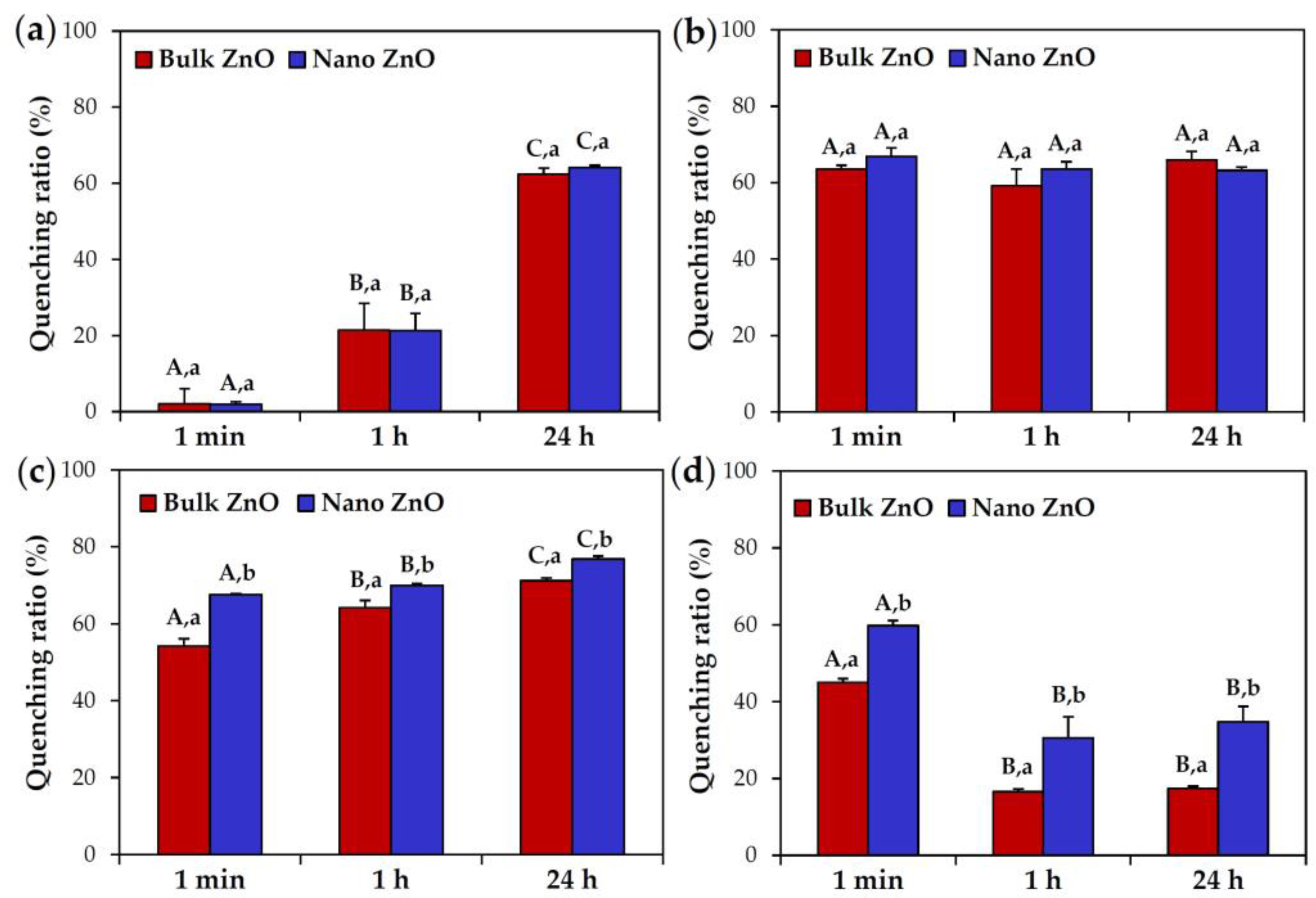
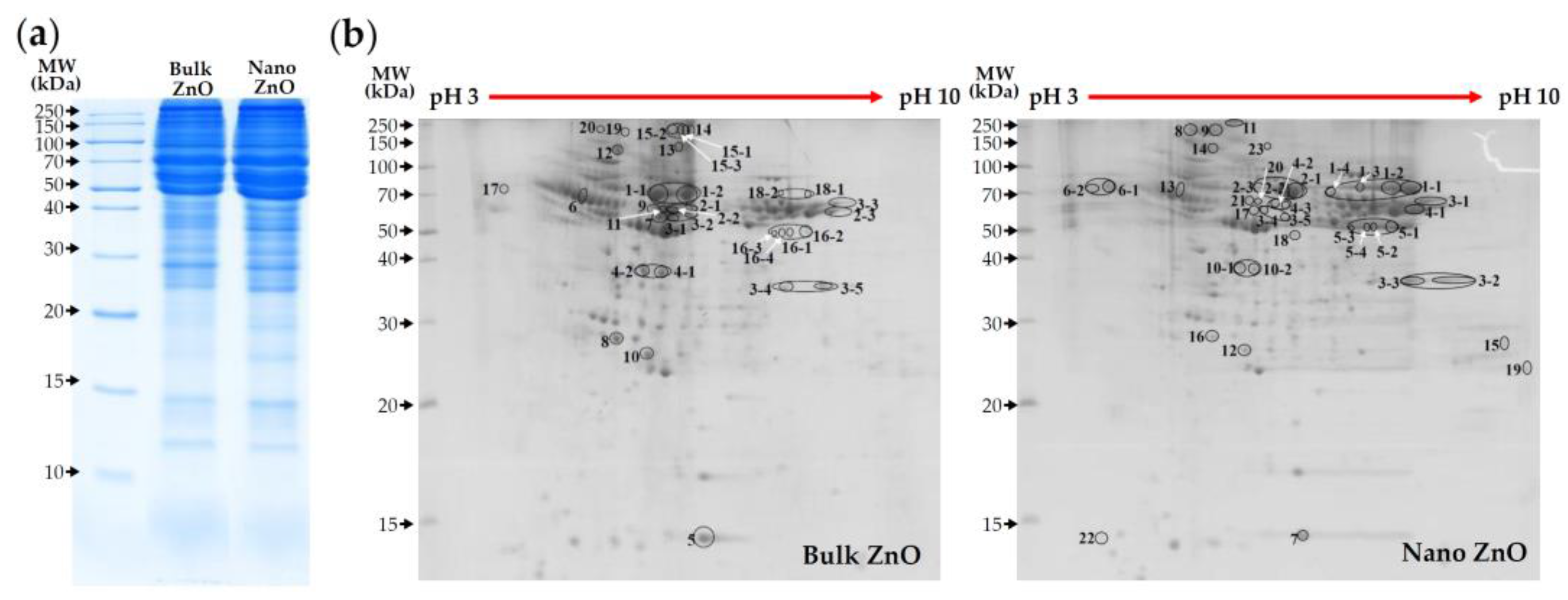
2.4. ZnO Interactions with Proteins in Simulated Biofluids
2.5. ZnO Plasma–Protein Corona
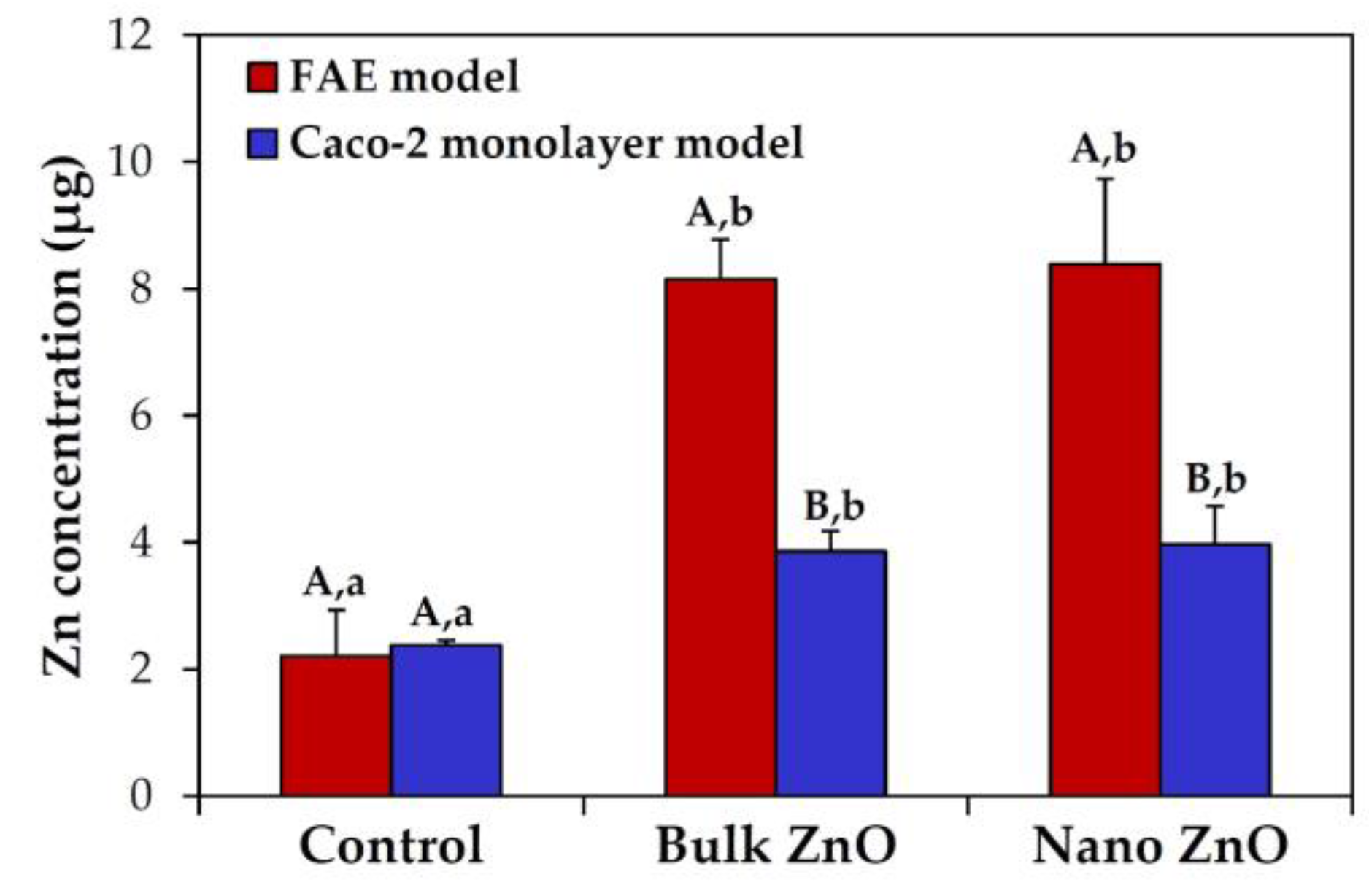
2.6. Intestinal Transport Mechanism
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characterization
4.3. Preparation of Simulated Biofuids
4.4. Animals and Preparation of Rat-Extracted Biofluids
4.5. In Vitro and Ex Vivo Dissolution Properties of ZnO in Biofluids
4.6. Fluorescence Quenching Measurement
4.7. 1D and 2D Gel Electrophoresis
4.8. Identification of Proteins by Liquid Chromatography-Mass Spectrometry/Mass Spectrometry
4.9. Three Dimensional (3D) Cell Culture for FAE Model
4.10. 3D Cell Culture for Intestinal Epithelial Monolayers
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burman, U.; Saini, M.; Praveen-Kumar. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morliere, C.; Bolzinger, M.-A. Antimicrobial activity of zinc oxide particles on five micro-organisms of the challenge tests related to their physicochemical properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Proc. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.F.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Faiz, H.; Zuberi, A.; Nazir, S.; Rauf, M.; Younus, N. Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: Comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int. J. Agric. Biol. 2015, 17, 568–574. [Google Scholar] [CrossRef]
- Raya, S.D.H.A.; Hassan, M.I.; Farroh, K.Y.; Hashim, S.A.; Salaheldin, T.A. Zinc oxide nanoparticles fortified biscuits as a nutritional supplement for zinc deficient rats. J. Nanomed. Res. 2016, 4, 2. [Google Scholar]
- Hong, T.-K.; Tripathy, N.; Son, H.-J.; Ha, K.-T.; Jeong, H.-S.; Hahn, Y.-B. A comprehensive in vitro and in vivo study of ZnO nanoparticles toxicity. J. Mater. Chem. B 2013, 1, 2985–2992. [Google Scholar] [CrossRef]
- Paek, H.-J.; Lee, Y.-J.; Chung, H.-E.; Yoo, N.-H.; Lee, J.-A.; Kim, M.-K.; Lee, J.-K.; Jeong, J.; Choi, S.-J. Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo. Nanoscale 2013, 5, 11416–11427. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.-R.; Chung, H.-E.; Kim, H.-J.; Bae, S.-H.; Go, M.-R.; Yu, J.; Choi, S.-J. Effects of zinc oxide nanoparticle dispersants on cytotoxicity and cellular uptake. Mol. Cell. Toxicol. 2016, 12, 281–288. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Treuel, L.; Nienhaus, G.U. Toward a molecular understanding of nanoparticle-protein interactions. Biophys. Rev. 2012, 4, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Choy, J.-H. Biokinetics of zinc oxide nanoparticles: Toxicokinetics, biological fates, and protein interaction. Int. J. Nanomed. 2014, 9, 261–269. [Google Scholar]
- Anders, C.B.; Chess, J.J.; Wingett, D.G.; Punnoose, A. Serum proteins enhance dispersion stability and influence the cytotoxicity and dosimetry of ZnO nanoparticles in suspension and adherent cancer cell models. Nanoscale Res. Lett. 2015, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Zukiene, R.; Snitka, V. Zinc oxide nanoparticle and bovine serum albumin interaction and nanoparticles influence on cytotoxicity in vitro. Colloids Surf. B 2015, 135, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Persson, B.; Lindner, P.; Cabane, B. Protein adsorption and complement activation for di-block copolymer nanoparticles. Biomaterials 2011, 32, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Yang, Y.; Shen, J.; Moten, A.; Chen, C.; Shen, H.; Ferrari, M.; Zhao, Y. The nano-plasma interface: Implications of the protein corona. Colloids Surf. B 2014, 124, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, I.-L.; Huang, Y.-J. Effects of serum on cytotoxicity of nano- and micro-sized ZnO particles. J. Nanopart. Res. 2013, 15, 1829. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, Y.; Huang, C.-C.; Ma, Y.; Shannon, K.B.; Chen, D.-R.; Huang, Y.-W. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J. Nanopart. Res. 2009, 11, 25–39. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R. Size-dependent effect of zinc oxide on toxicity and inflammatory potential of human monocytes. J. Toxicol. Environ. Health A 2014, 77, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; Reichel, C.A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.V.; Godwin, H.; et al. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Gosens, I.; Jacobsen, N.R.; Jensen, K.A.; Bokkers, B.; Kermanizadeh, A.; Stone, V.; Baeza-Squiban, A.; Cassee, F.R.; Tran, L.; et al. Impact of serum as a dispersion agent for in vitro and in vivo toxicological assessments of TiO2 nanoparticles. Arch. Toxicol. 2017, 91, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Churchman, A.H.; Wallace, R.; Milne, S.J.; Brown, A.P.; Brydson, R.; Beales, P.A. Serum albumin enhances the membrane activity of ZnO nanoparticles. Chem. Commun. 2013, 49, 4172–4174. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, N.P.; Chandran, P.; Khan, S.S. Interaction of colloidal zinc oxide nanoparticles with bovine serum albumin and its adsorption isotherms and kinetics. Colloids Surf. B 2013, 102, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Avramescu, M.-L.; Rasmussen, P.E.; Chenier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. 2017, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, W. Growth process, crystal size and alignment of ZnO nanorods synthesized under neutral and acid conditions. J. Alloys Compd. 2015, 629, 84–91. [Google Scholar] [CrossRef]
- Gwak, G.-H.; Lee, W.-J.; Paek, S.-M.; Oh, J.-M. Physico-chemical changes of ZnO nanoparticles with different size and surface chemistry under physiological pH conditions. Colloids Surf. B 2015, 127, 137–142. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Lee, J.-A.; Jo, M.-R.; Choi, S.-J. Bioavailability of silica, titanium dioxide, and zinc oxide nanoparticles in rats. J. Nanosci. Nanotechnol. 2016, 16, 6580–6586. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Young, D.; Young, A.; Wu, C.-Y.; Chen, C.-D.; Yu, J.-S.; Young, J.D. Comprehensive proteomic analysis of mineral nanoparticles derived from human body fluids and analyzed by liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2011, 418, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Neun, B.W.; Man, S.; Ye, X.; Hansen, M.; Patri, A.K.; Crist, R.M.; McNeil, S.E. Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles. Nanomedcine 2014, 10, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, S.; Yang, Q.; Zhang, T.; Wei, X.-Q.; Jiang, L.; Zhang, C.-L.; Chen, Q.-M.; Zhang, Z.-R.; Lin, Y.-F. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials 2013, 34, 8521–8530. [Google Scholar] [CrossRef] [PubMed]
- Drew, A.F.; Liu, H.; Davidson, J.M.; Daugherty, C.C.; Degen, J.L. Wound-healing defects in mice lacking fibrinogen. Blood 2001, 97, 3691–3698. [Google Scholar] [CrossRef] [PubMed]
- Smiley, S.T.; King, J.A.; Hancock, W.W. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol. 2001, 167, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F.; Billingham, R.E.; Burgess, L. Distribution of fibronectin during wound healing in vivo. J. Investig. Dermatol. 1981, 76, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Andersen, A.J.; Ahmadvand, D.; Wibroe, P.P.; Andresen, T.L.; Hunter, A.C. Material properties in complement activation. Adv. Drug Deliv. Rev. 2011, 63, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Moyano, D.F.; Liu, Y.; Peer, D.; Rotello, V.M. Modulation of immune response using engineered nanoparticle surfaces. Small 2016, 12, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Mi, F.-L.; Liao, Z.-X.; Hsiao, C.-W.; Sonaje, K.; Chung, M.-F.; Hsu, L.-W.; Sung, H.-W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.M.; Leong, K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Plapied, L.; Plaideau, C.; Legendre, D.; des Rieux, A.; Pourcelle, V.; Freichels, H.; Jerome, C.; Marchand, J.; Preat, V.; et al. In vitro identification of targeting ligands of human M cells by phage display. Int. J. Pharm. 2010, 394, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-H.; Yu, J.; Go, M.-R.; Kim, H.-J.; Hwang, Y.-G.; Choi, S.-J. Oral toxicity and intestinal transport mechanism of colloidal gold nanoparticle-treated red ginseng. Nanomaterials 2016, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Bae, S.-H.; Kim, H.-J.; Kim, K.-M.; Song, J.H.; Go, M.-R.; Yu, J.; Oh, J.-M.; Choi, S.-J. Cytotoxicity, intestinal transport, and bioavailability of dispersible iron and zinc supplements. Front. Microbiol. 2017, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-A.; Kim, M.-K.; Song, J.H.; Jo, M.-R.; Yu, J.; Kim, K.-M.; Kim, Y.-R.; Oh, J.-M.; Choi, S.-J. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf. B 2017, 150, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Song, Z.-M.; Cheng, B.; Xiang, K.; Chen, X.-X.; Liu, J.-H.; Cao, A.; Wang, Y.; Liu, Y.; Wang, H. Evaluation of the toxicity of food additive silica nanoparticles on gastrointestinal cells. J. Appl. Toxicol. 2014, 34, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, M.K.; Kim, H.M.; Lee, J.K.; Jeong, J.; Kim, Y.R.; Oh, J.M.; Choi, S.J. The fate of calcium carbonate nanoparticles administered by oral route: Absorption and their interaction with biological matrices. Int. J. Nanomed. 2015, 10, 2273–2293. [Google Scholar]
- Bahk, Y.Y.; Kim, S.A.; Kim, J.S.; Euh, H.J.; Bai, G.H.; Cho, S.N.; Kim, Y.S. Antigens secreted from Mycobacterium tuberculosis: Identification by proteomics approach and test for diagnostic marker. Proteomics 2004, 4, 3299–3307. [Google Scholar] [CrossRef] [PubMed]
- Gobom, J.; Nordhoff, E.; Mirgorodskaya, E.; Ekman, R.; Roepstorff, P. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 1999, 34, 105–116. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Theate, I.; Mast, J.; Preat, V.; Schneider, Y.-J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
| Hydrodynamic radius (nm) | Size | Time | DW | Gastric Fluid | Intestinal Fluid | Plasma |
| Bulk ZnO | 1 min | 3453.3 ± 278.0 A,a | 2161.0 ± 257.0 B,b | 3224.5 ± 180.0 A,a | 3327.3 ± 268.8 A,a | |
| 1 h | 2463.0 ± 235.3 B,b,c | 2828.5 ± 158.7 A,b | 2184.3 ± 203.8 B,c | |||
| 6 h | 3176.0 ± 272.4 A,a | 2585.0 ± 84.9 B,b | 1735.8 ± 114.0 C,c | |||
| 24 h | 3237.3 ± 81.8 A,a | 2227.5 ± 139.0 C,b | 1473.8 ± 51.4 C,c | |||
| Nano ZnO | 1 min | 1976.0 ± 198.7 A,b | 2060.8 ± 41.4 A,B,a | 3009.8 ± 200.4 B,c | 2485.3 ± 226.9 B,b,* | |
| 1 h | 2365.0 ± 188.9 B,a,b | 3079.8 ± 206.5 B,c | 2439.0 ± 144.8 B,b | |||
| 6 h | 2993.8 ± 203.2 C,c | 2435.0 ± 162.3 C,b | 1761.8 ± 250.0 A,C,a | |||
| 24 h | 3180.8 ± 81.3 C,c | 2300.5 ± 131.7 A,C,b | 1502.3 ± 187.8 C,a |
| Zeta potential (mV) | Size | Time | DW | Gastric Fluid | Intestinal Fluid | Plasma |
| Bulk ZnO | 1 min | 17.5 ± 1.6 A,a | −17.4 ± 0.5 B,b | −23.4 ± 0.6 B,c | −27.5 ± 0.9 B,d | |
| 1 h | −16.9 ± 0.6 B,b | −25.9 ± 1.8 C,c | −27.9 ± 0.9 B,c | |||
| 6 h | −16.9 ± 0.9 B,b | −27.1 ± 0.9 C,D,c | −28.5 ± 0.4 B,c | |||
| 24 h | −16.6 ± 0.4 B,b | −28.6 ± 0.7 D,c | −27.9 ± 0.7 B,c | |||
| Nano ZnO | 1 min | 16.0 ± 1.0 A,a | −14.8 ± 0.7 B,b,* | −19.0 ± 0.6 B,c,* | −23.1 ± 1.0 B,d,* | |
| 1 h | −13.5 ± 0.8 B,b,* | −20.6 ± 0.9 C,c,* | −22.8 ± 0.9 B,d,* | |||
| 6 h | −13.8 ± 0.7 B,b,* | −20.7 ± 0.6 C,c,* | −24.6 ± 0.8 B,C,d,* | |||
| 24 h | −13.6 ± 1.0 B,b,* | −17.2 ± 2.1 B,c,* | −26.2 ± 0.7 C,d,* |
| MW (kDa) | pI | Bulk ZnO | No. | Nano ZnO | pI | MW (kDa) |
|---|---|---|---|---|---|---|
| 68.7 | 6.09 | Serum albumin | 1 | Serum albumin precursor | 6.09 | 68.8 |
| 54.3 | 7.89 | Fibrinogen B beta chain | 2 | Serum albumin | 6.09 | 68.7 |
| 60.5 | 7.56 | Fibrinogen alpha subunit | 3 | Fibrinogen alpha subunit | 7.56 | 60.5 |
| 167.2 | 6.46 | Alpha-1-macroglobulin | 4 | Fibrinogen B beta chain | 7.89 | 54.3 |
| 15.7 | 5.77 | Prealbumin | 5 | Fibrinogen gamma chain precursor | 5.85 | 49.7 |
| 45.7 | 5.37 | Serine protease inhibitor A3K | 6 | Vitronectin | 5.68 | 54.7 |
| 60.7 | 6.56 | Fibrinogen alpha chain precursor | 7 | Prealbumin | 5.77 | 15.7 |
| 26.2 | 5.50 | Serum amyloid P-component precursor | 8 | Alpha-1-inhibitor 3 | 5.70 | 163.8 |
| 87.0 | 5.57 | Fibrinogen alpha chain isoform X2 | 9 | Fibronectin isoform X3 | 5.61 | 262.8 |
| 103.6 | 6.08 | Inter-alpha-inhibitor H4 heavy chain | 10 | Alpha-1-macroglobulin | 6.46 | 167.2 |
| 54.2 | 7.90 | Fibrinogen beta chain precursor | 11 | Fibronectin isoform X2 | 5.54 | 262.8 |
| 254.4 | 5.27 | Fibronectin isoform X6 | 12 | Inter-alpha-inhibitor H4 heavy chain | 6.08 | 103.6 |
| 272.6 | 5.50 | Fibronectin | 13 | Serine protease inhibitor A3K | 5.37 | 45.7 |
| 262.8 | 5.54 | Fibronectin isoform X2 | 14 | Fibronectin isoform X6 | 5.27 | 254.4 |
| 253.2 | 5.47 | Fibronectin 1 isoform CRA-b | 15 | Complement C1q subcomponent subunit B precursor | 9.13 | 26.6 |
| 49.7 | 5.85 | Fibrinogen gamma chain precursor | 16 | Serum amyloid P-component precursor | 5.50 | 26.2 |
| 54.7 | 5.68 | Vitronectin | 17 | Fibrinogen alpha chain precursor | 6.56 | 60.7 |
| 68.8 | 6.09 | Serum albumin precursor | 18 | Complement C3 precursor | 6.06 | 186.4 |
| 262.8 | 5.61 | Fibronectin isoform X3 | 19 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily D member 3 | 9.36 | 53.6 |
| 163.8 | 5.70 | Alpha-1-inhibitor 3 | 20 | Fibrinogen beta chain precursor | 7.90 | 54.2 |
| 21 | Fibrinogen alpha chain isoform X2 | 5.57 | 87.0 | |||
| 22 | Keratin K6 | 3.10 | 5.6 | |||
| 23 | Fibronectin | 5.50 | 272.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Kim, H.-J.; Go, M.-R.; Bae, S.-H.; Choi, S.-J. ZnO Interactions with Biomatrices: Effect of Particle Size on ZnO-Protein Corona. Nanomaterials 2017, 7, 377. https://doi.org/10.3390/nano7110377
Yu J, Kim H-J, Go M-R, Bae S-H, Choi S-J. ZnO Interactions with Biomatrices: Effect of Particle Size on ZnO-Protein Corona. Nanomaterials. 2017; 7(11):377. https://doi.org/10.3390/nano7110377
Chicago/Turabian StyleYu, Jin, Hyeon-Jin Kim, Mi-Ran Go, Song-Hwa Bae, and Soo-Jin Choi. 2017. "ZnO Interactions with Biomatrices: Effect of Particle Size on ZnO-Protein Corona" Nanomaterials 7, no. 11: 377. https://doi.org/10.3390/nano7110377




