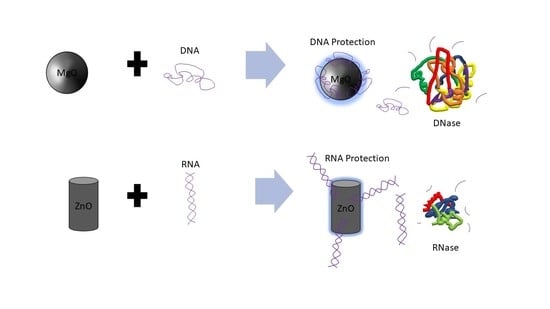
ZnO Nanoparticles Protect RNA from Degradation Better than DNA
Abstract
:1. Introduction
2. Results and Discussion
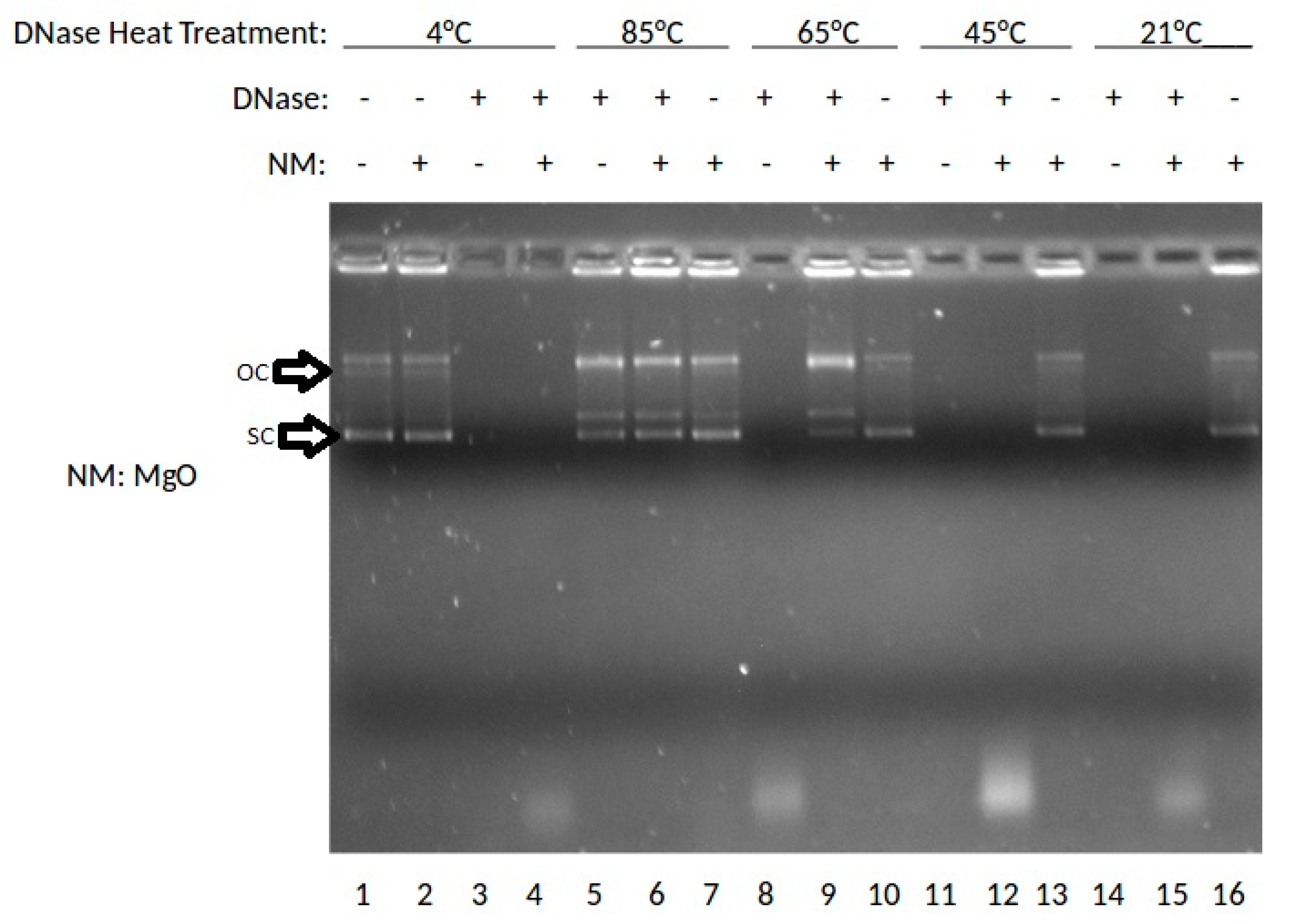
2.1. DNA Stability in the Presence of MgO NP
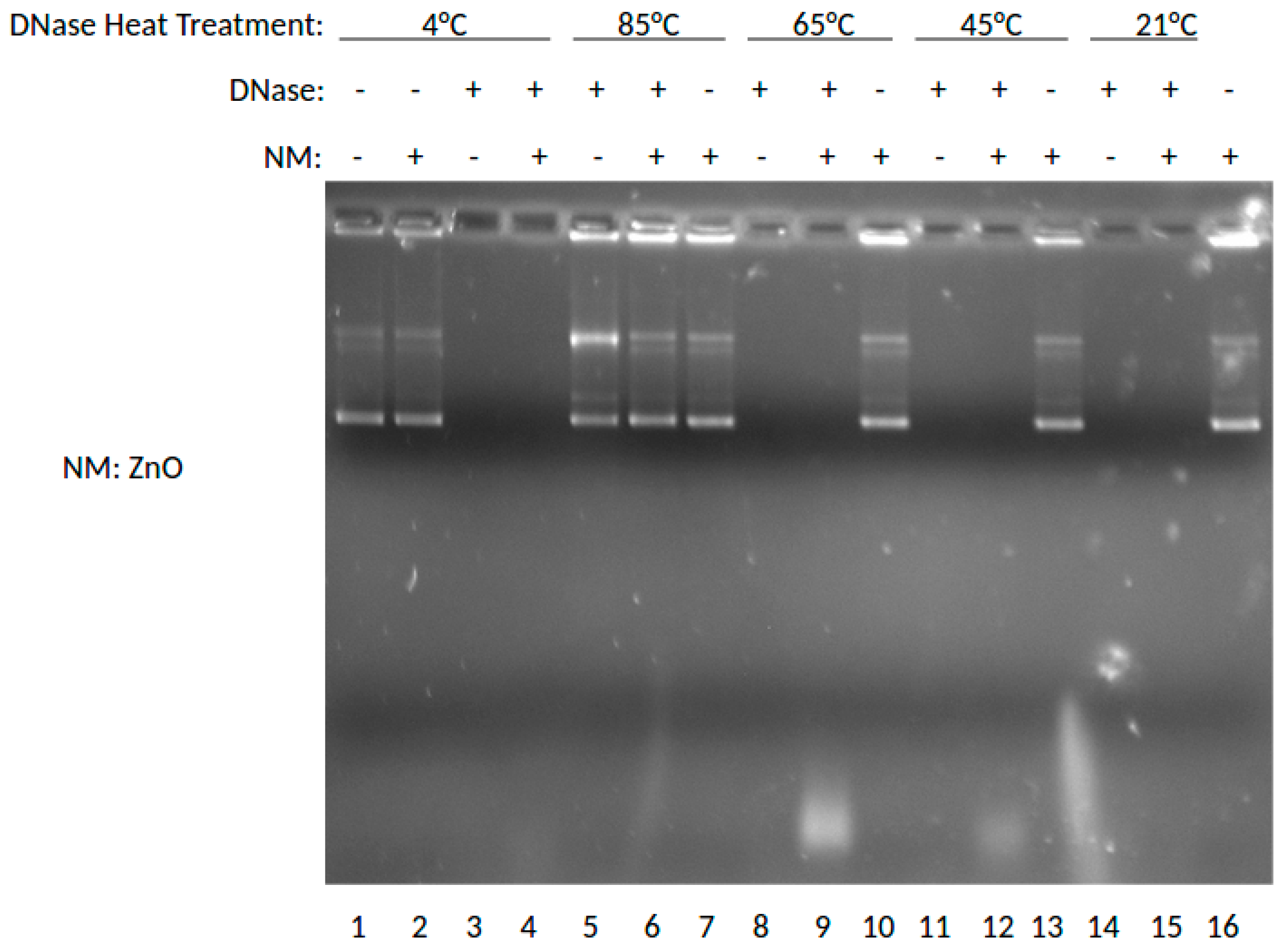
2.2. DNA Stability in the Presence of ZnO NP
2.3. RNA Stability in the Presence of Serum Provided by ZnO but Not MgO NP
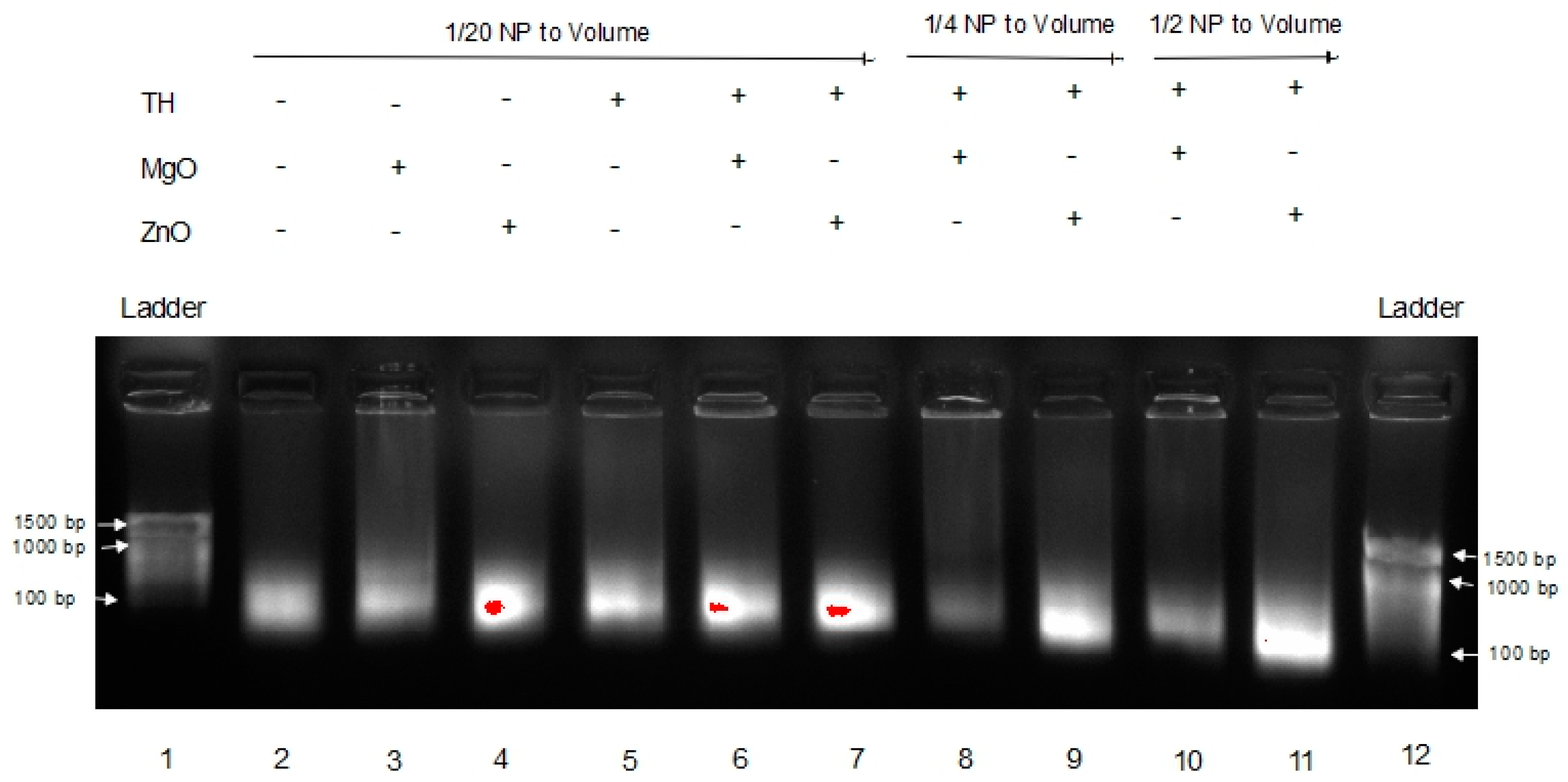
2.4. RNA Stability in the Presence of Tumor Homogenate Provided by ZnO but Not MgO NP
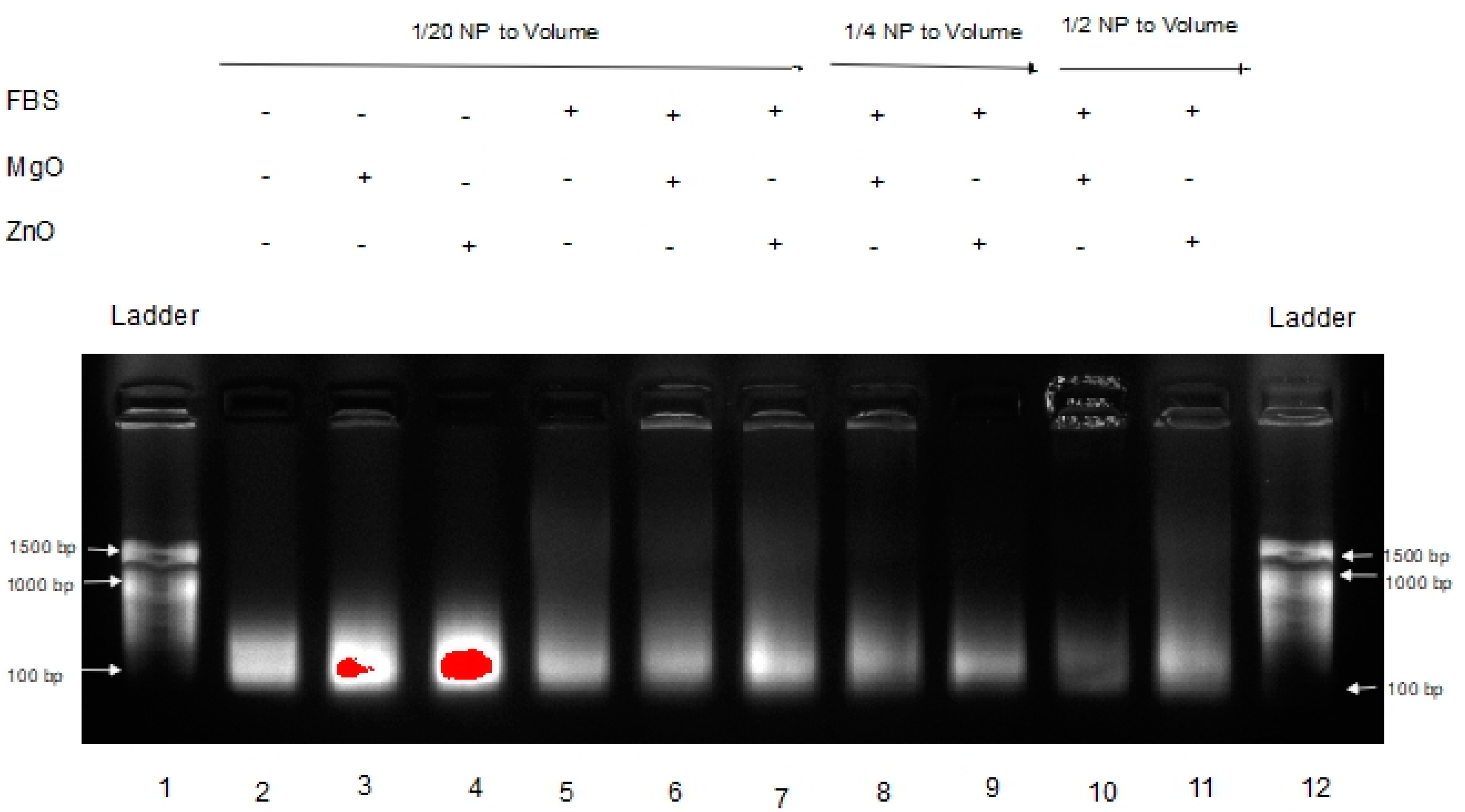
2.5. RNA Compatibility of NP
3. Materials and Methods
3.1. Materials
3.2. DNA Degradation with Heat-Killed DNase
3.3. RNA Degradation
3.4. RNA Compatibility
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.P.; et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Choi, W.J.; Choi, S.W.; Kim, E.H.; Kim, J.; Lee, J.O.; Kim, S.H. Anti-cancer activity of ZnO chips by sustained zinc ion release. Toxicol. Rep. 2016, 3, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Singh, S.K.; Chauhan, L.K.; Das, M.; Tripathi, A.; Dwivedi, P.D. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol. Lett. 2014, 227, 29–40. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.K.; Mitchell, J.A.; Morris, R.T.; Comer, J.; Hurst, M.N.; Ghosh, K.; Wanekaya, A.; Mudge, M.; Schaeffer, A.; Washington, L.L.; et al. Enzyme and cancer cell selectivity of nanoparticles: Inhibition of 3-D metastatic phenotype and experimental melanoma by zinc oxide. J. Biomed. Nanotechnol. 2017, 13, 221–231. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Xu, F. Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol. Ther. 2010, 10, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Cobaleda-Siles, M.; Henriksen-Lacey, M.; Ruiz de Angulo, A.; Bernecker, A.; Gómez Vallejo, V.; Szczupak, B.; Llop, J.; Pastor, G.; Plaza-Garcia, S.; Jauregui-Osoro, M.; et al. An iron oxide nanocarrier for dsRNA to target lymph nodes and strongly activate cells of the immune system. Small 2014, 10, 5054–5067. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Rega, A.; Morello, S.; Luciano, A.; Arra, C.; Pinto, A.; Sorrentino, R. Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J. Immunol. 2012, 188, 5357–5364. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Mudge, M.C.; Morris, R.T.; Zhang, Y.; Warcholek, S.A.; Hurst, M.N.; Riviere, J.E.; DeLong, R.K. Zinc Oxide Nanoparticle-Poly I:C RNA Complexes: Implication as Therapeutics against Experimental Melanoma. Mol. Pharm. 2017, 14, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Nguyen, T.D.T.; Aryal, S.; Ghosh, K.C.; DeLong, R.K. Elucidating the RNA Nano–Bio Interface: Mechanisms of Anticancer Poly I:C RNA and Zinc Oxide Nanoparticle Interaction. J. Phys. Chem. C 2017, 121, 15702–15710. [Google Scholar] [CrossRef]
- He, Y.; Ingudam, S.; Reed, S.; Gehring, A.; Strobaugh, T.P., Jr.; Irwin, P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J. Nanobiotechnol. 2016, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.H.; Ng, A.M.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B.; et al. Mechanisms of antibacterial activity of MgO: Non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; DeLong, R.; Fisher, M.H.; Juliano, R.L. Tat-conjugated PAMAM dendrimers as delivery agents for antisense and siRNA oligonucleotides. Pharm. Res. 2005, 22, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reveles, J.; Sedaghat-Herati, R.; Gilley, D.R.; Schaeffer, A.M.; Ghosh, K.C.; Greene, T.D.; Gann, H.E.; Dowler, W.A.; Kramer, S.; Dean, J.M.; et al. mPEG-PAMAM-G4 nucleic acid nanocomplexes: Enhanced stability, RNase protection, and activity of splice switching oligomer and poly I: C RNA. Biomacromolecules 2013, 14, 4108–4115. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Hong, J.; McGuffie, M.; Yeom, B.; Van Epps, J.S.; Kotov, N.A. Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity. ACS Nano 2015, 9, 9097–9105. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Li, B.; Li, C.X.; Fan, J.X.; Lei, Q.; Li, C.; Xu, Z.; Zhang, X.Z. Carbon-Dot-Decorated Carbon Nitride Nanoparticles for Enhanced Photodynamic Therapy against Hypoxic Tumor via Water Splitting. ACS Nano 2016, 10, 8715–8722. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCall, J.; Smith, J.J.; Marquardt, K.N.; Knight, K.R.; Bane, H.; Barber, A.; DeLong, R.K. ZnO Nanoparticles Protect RNA from Degradation Better than DNA. Nanomaterials 2017, 7, 378. https://doi.org/10.3390/nano7110378
McCall J, Smith JJ, Marquardt KN, Knight KR, Bane H, Barber A, DeLong RK. ZnO Nanoparticles Protect RNA from Degradation Better than DNA. Nanomaterials. 2017; 7(11):378. https://doi.org/10.3390/nano7110378
Chicago/Turabian StyleMcCall, Jayden, Joshua J. Smith, Kelsey N. Marquardt, Katelin R. Knight, Hunter Bane, Alice Barber, and Robert K. DeLong. 2017. "ZnO Nanoparticles Protect RNA from Degradation Better than DNA" Nanomaterials 7, no. 11: 378. https://doi.org/10.3390/nano7110378