An In Vitro Study of the Photodynamic Effectiveness of GO-Ag Nanocomposites against Human Breast Cancer Cells
Abstract
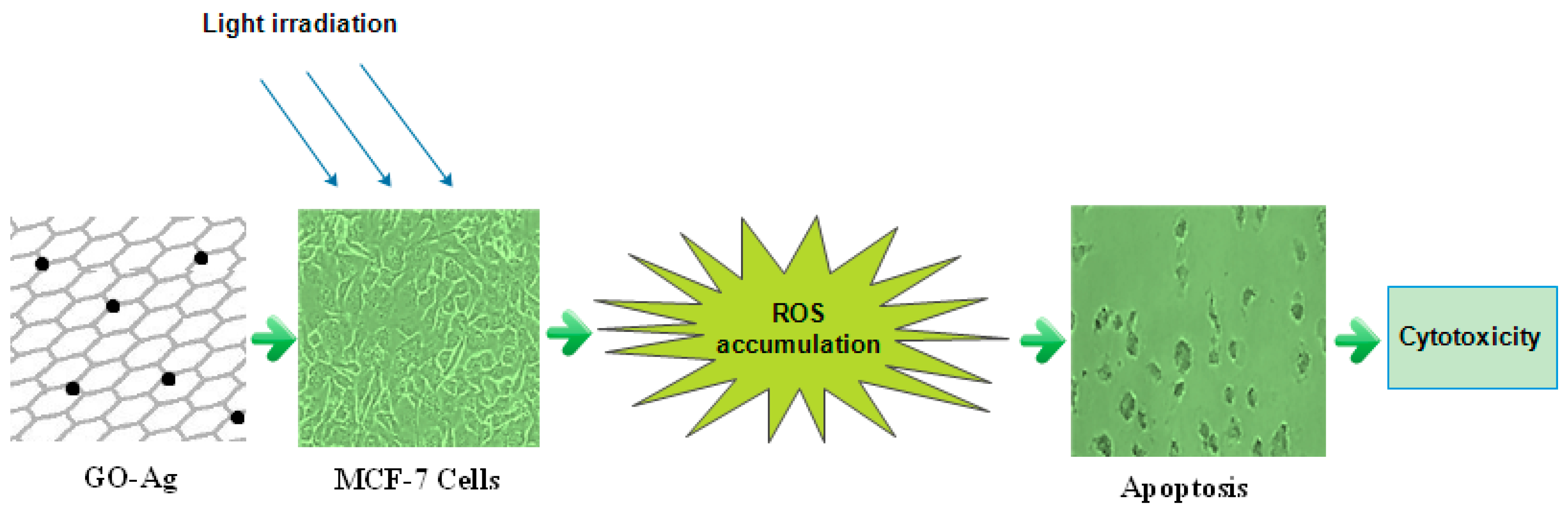
:1. Introduction
2. Materials and Methods
2.1. GO-Ag Nanocomposites Preparation
2.2. Cell Culturing (MCF-7, Breast Cancer Cell Line)
2.3. Labeling of MCF-7 Cells
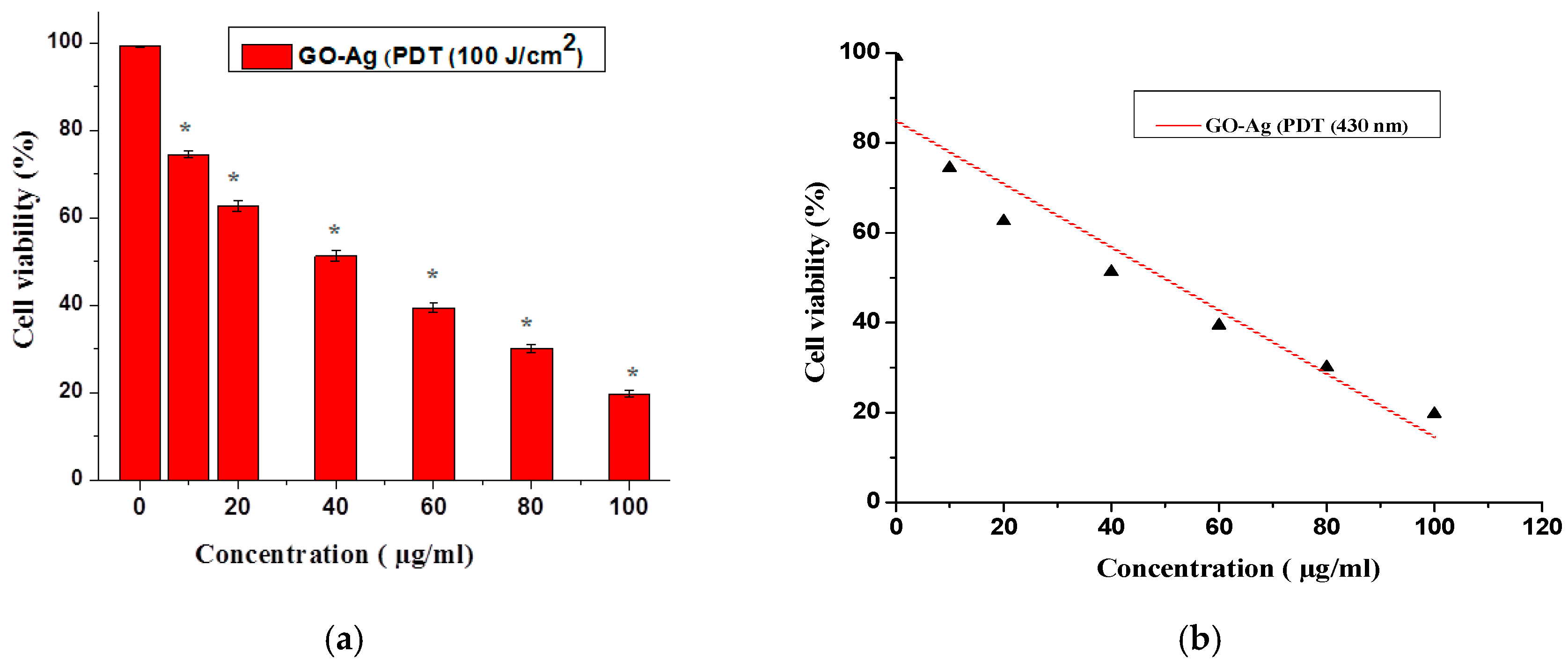
2.4. Photodynamic Therapy of MCF-7 Cells
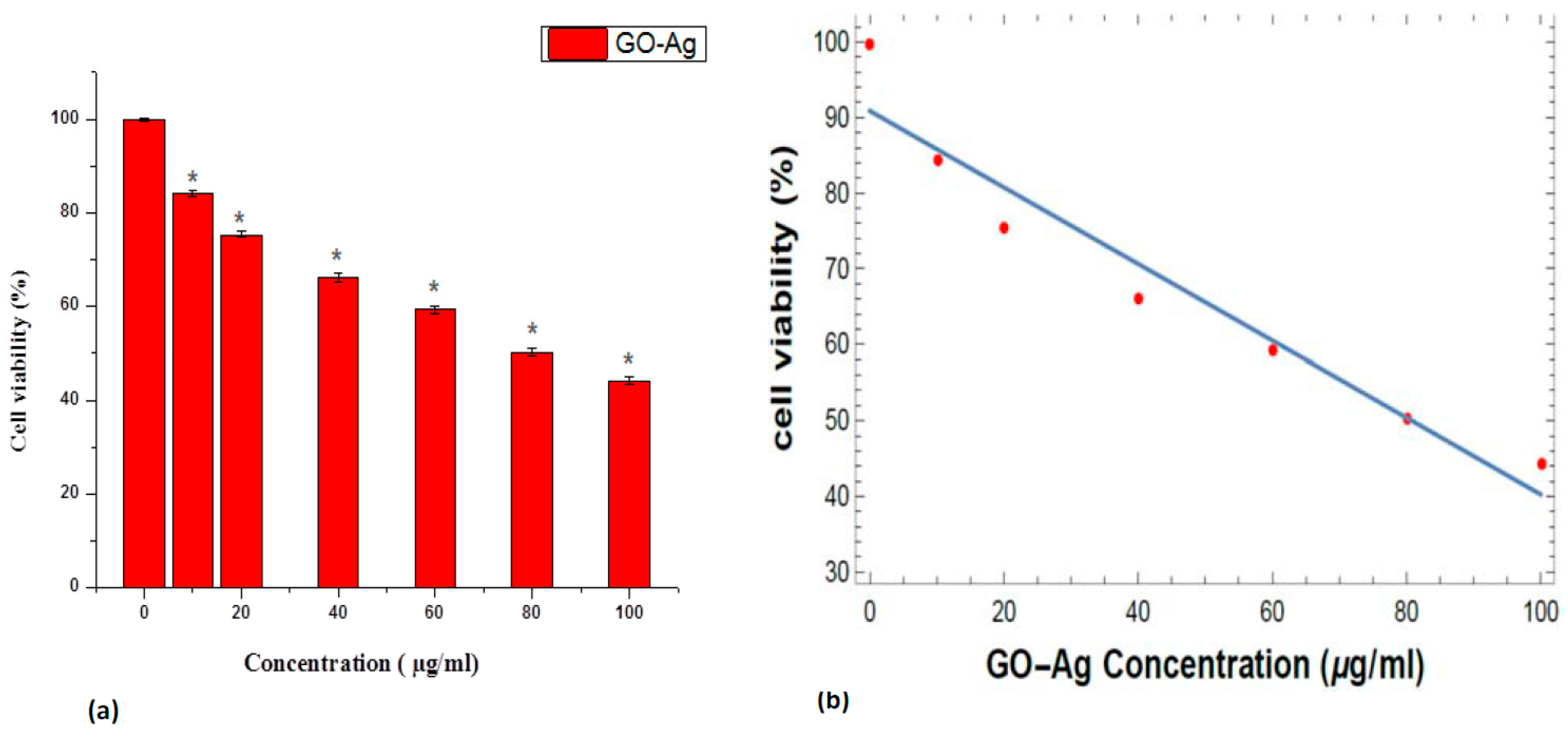
2.5. In Vitro Cellular Cytotoxicity MTT Assay
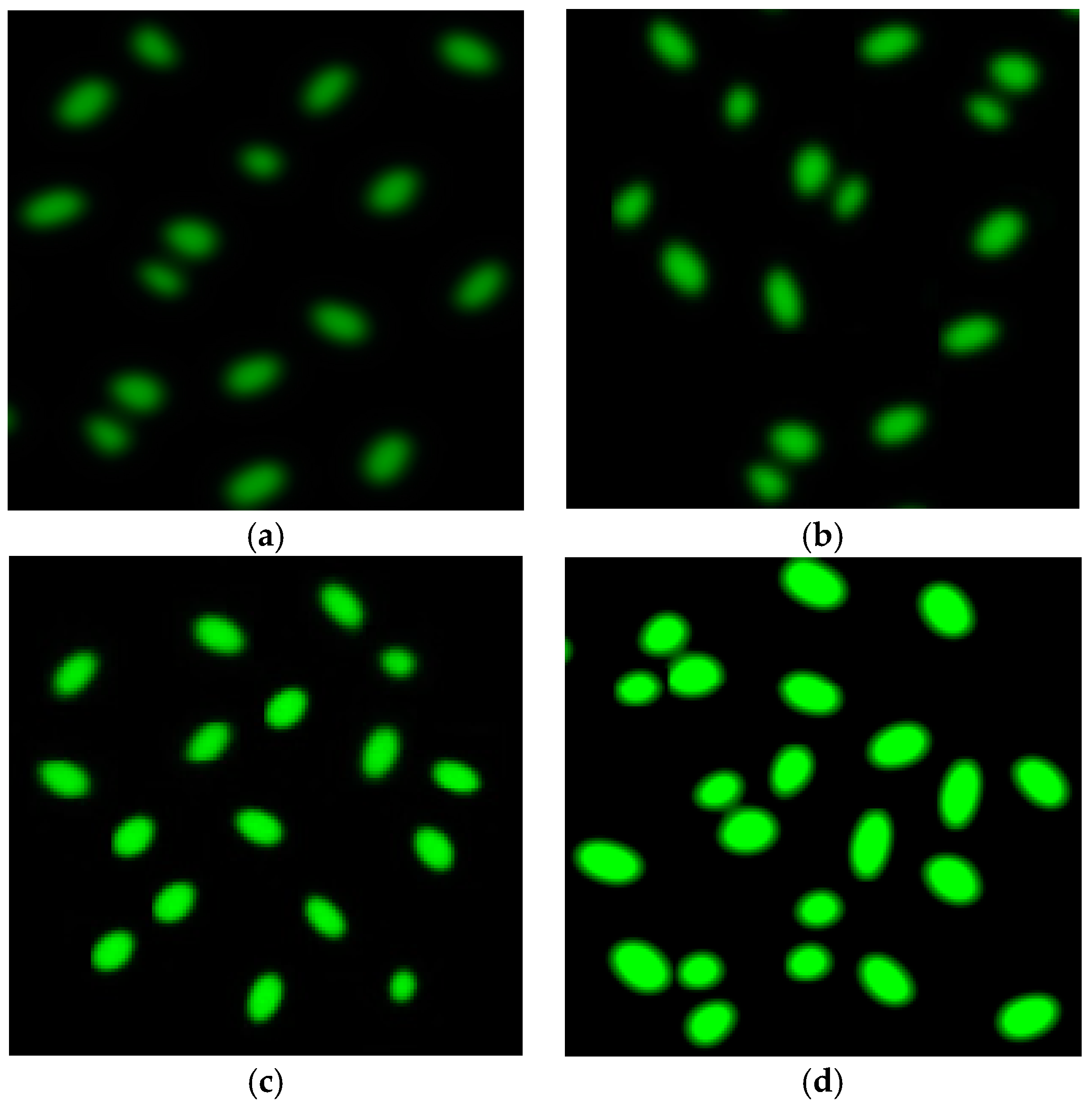
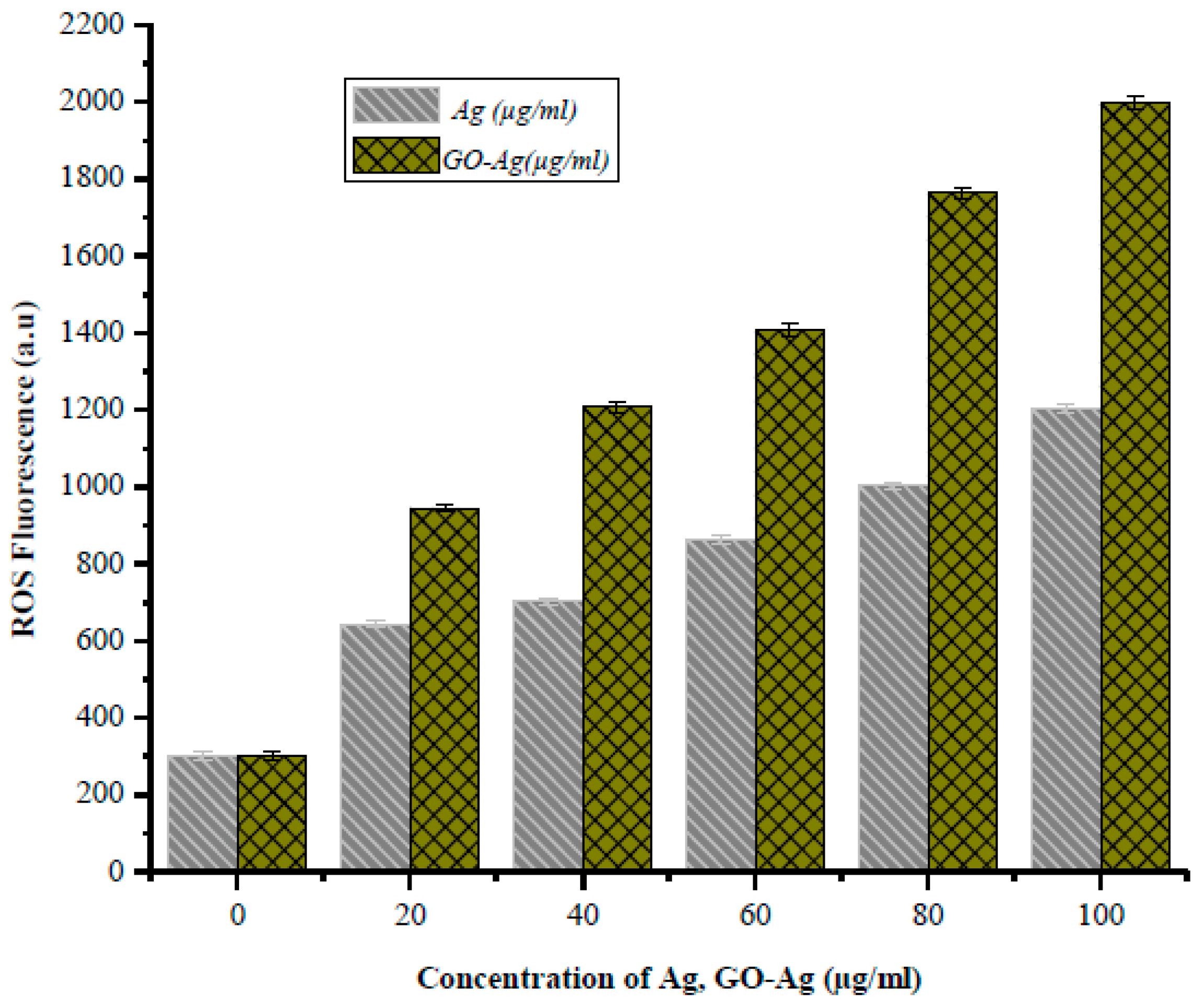
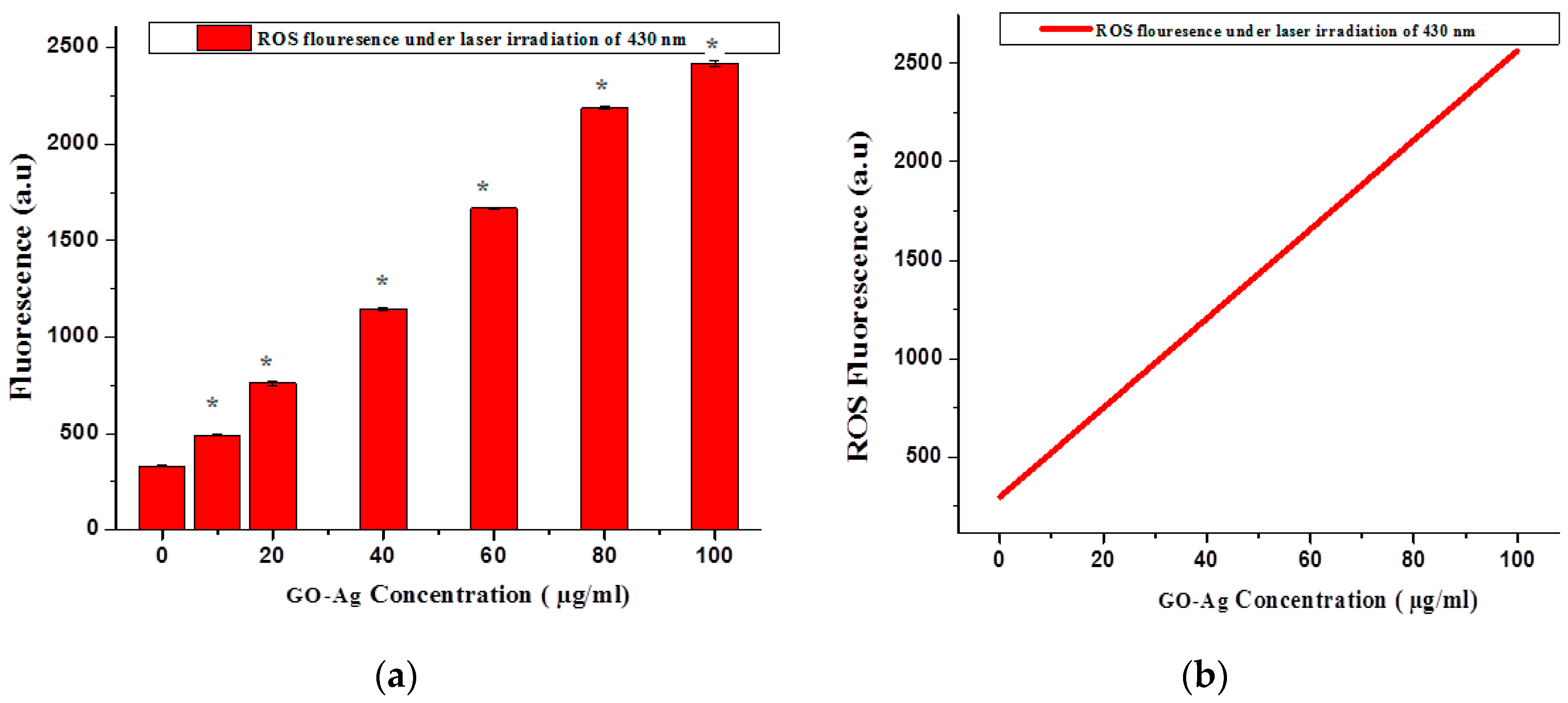
2.6. Reactive Oxygen Species Fluorescence
2.7. Characterization
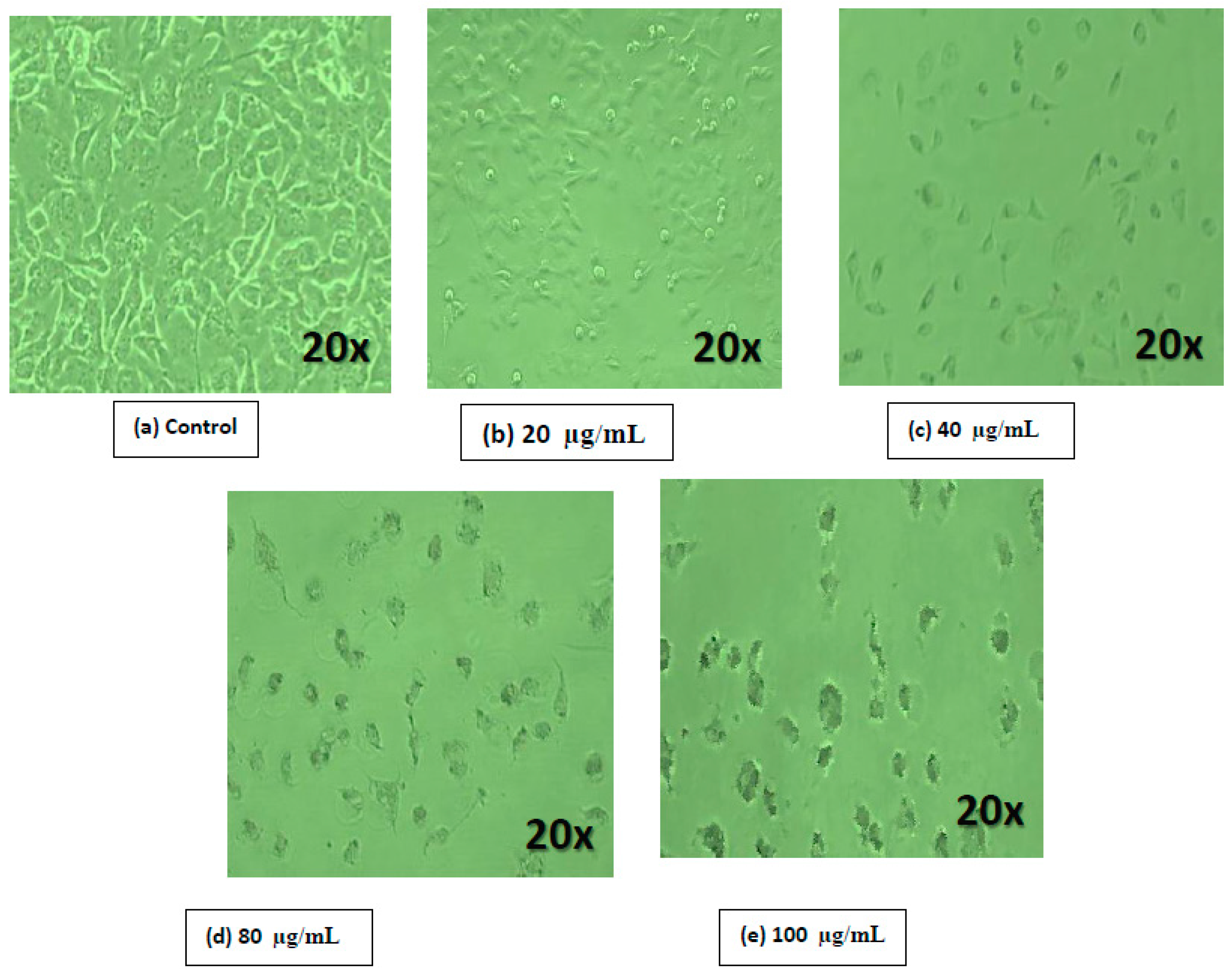
2.8. Cell Morphological Analysis
2.9. Statistical Analysis
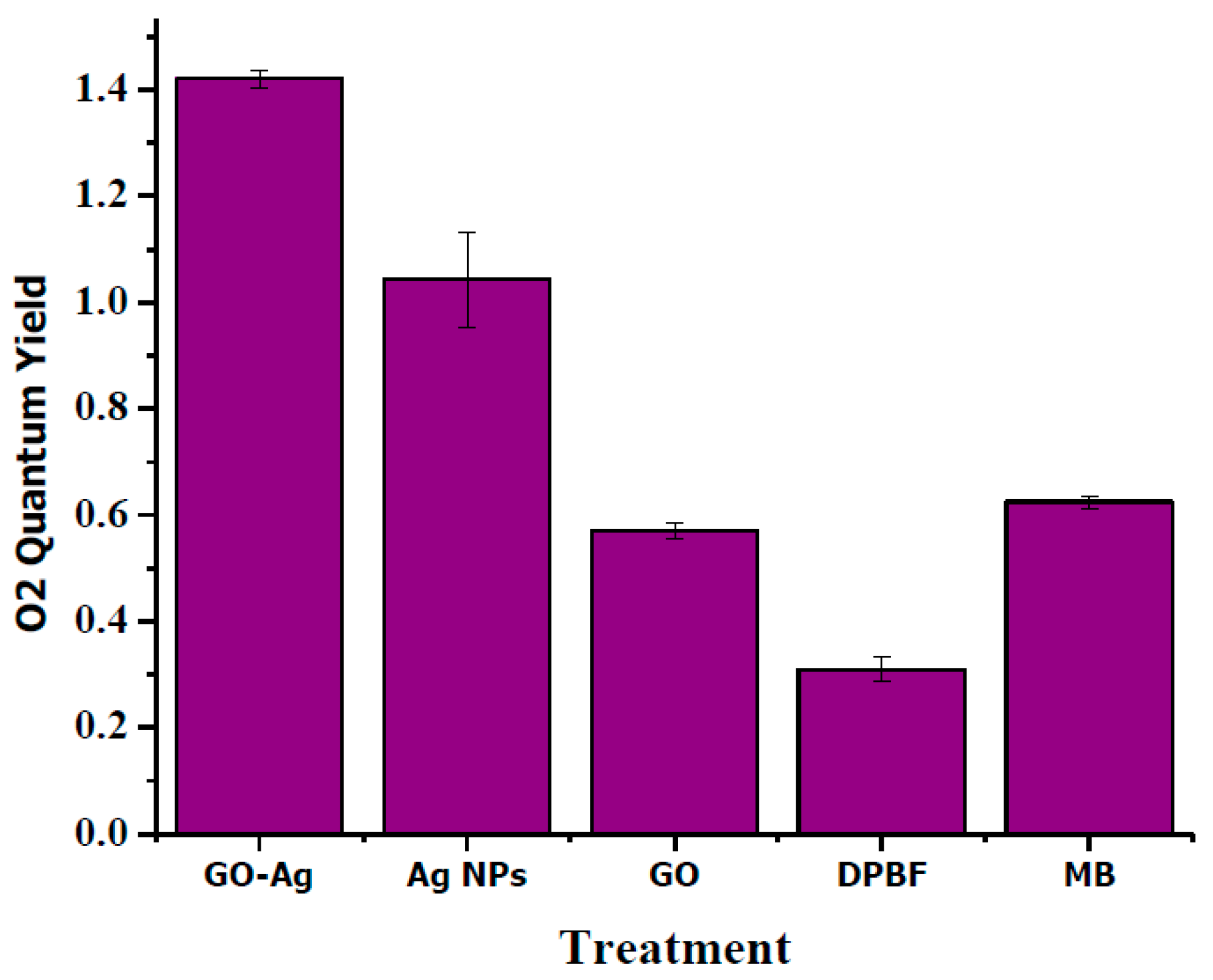
2.10. Exposure of Singlet Oxygen by Chemical Trapping
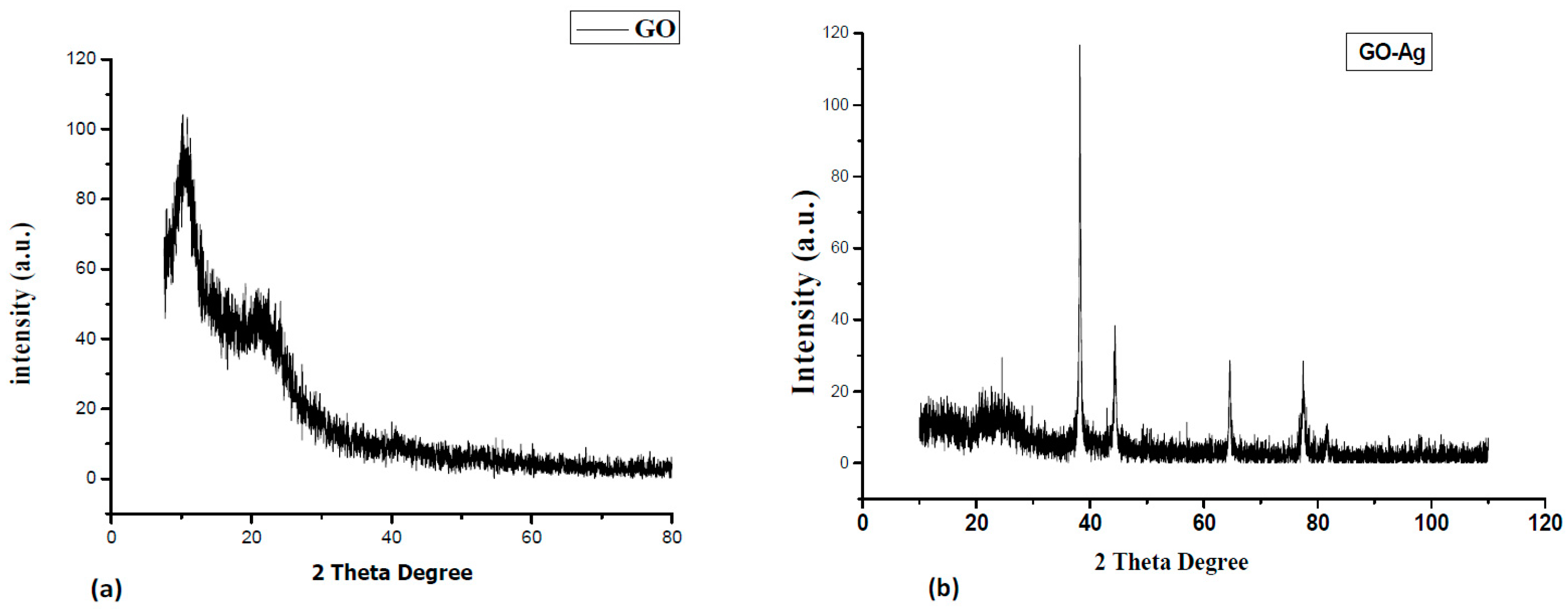
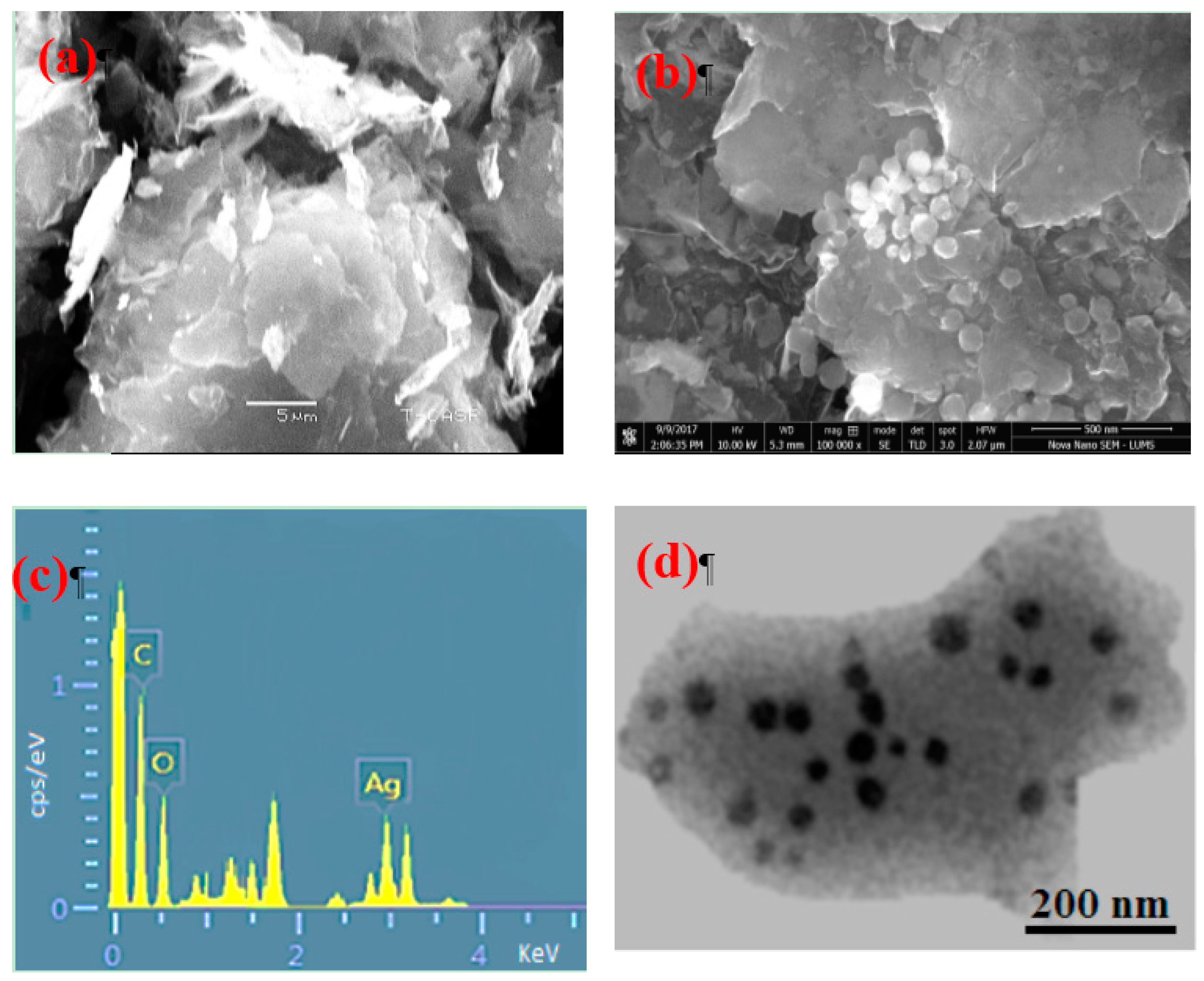
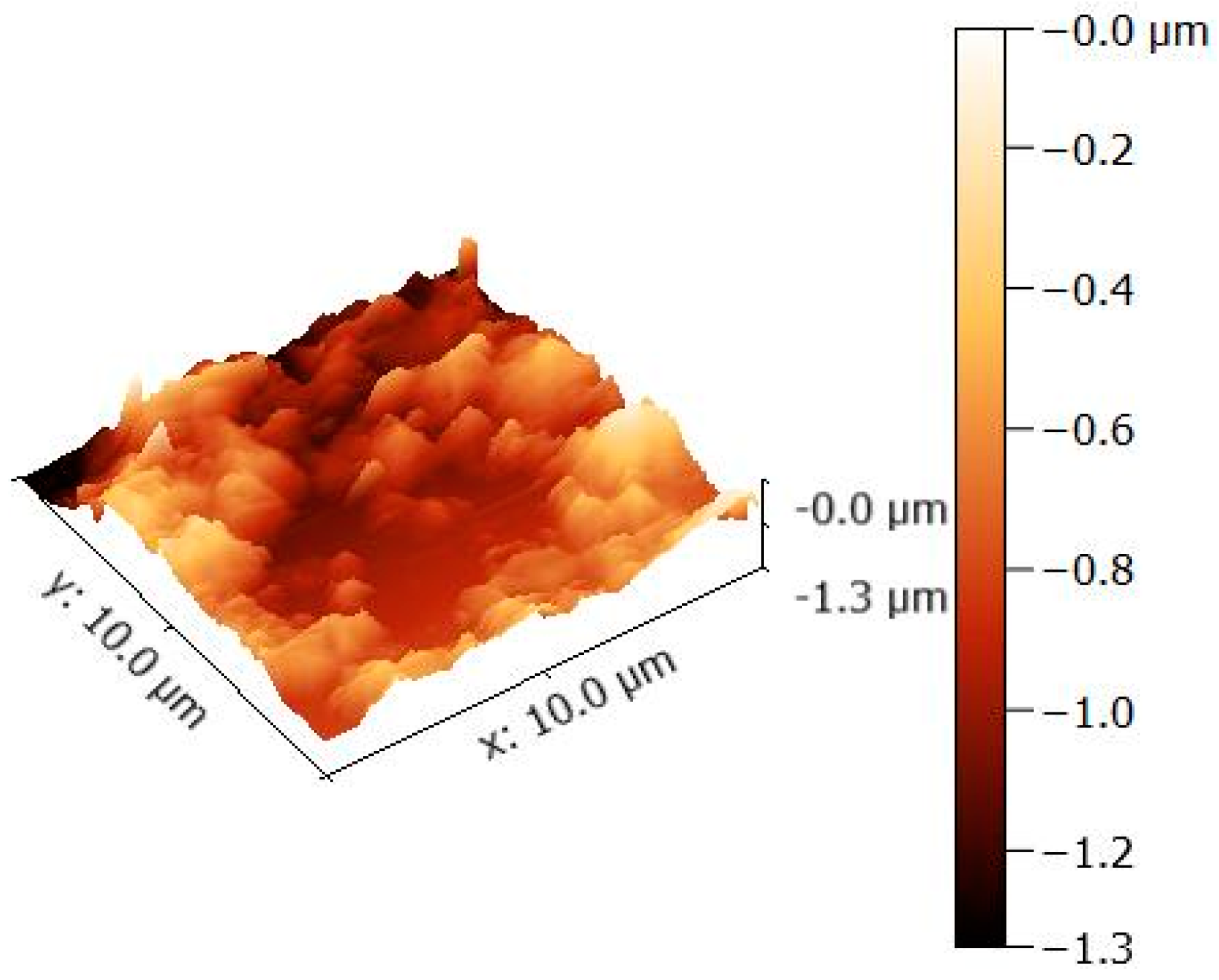
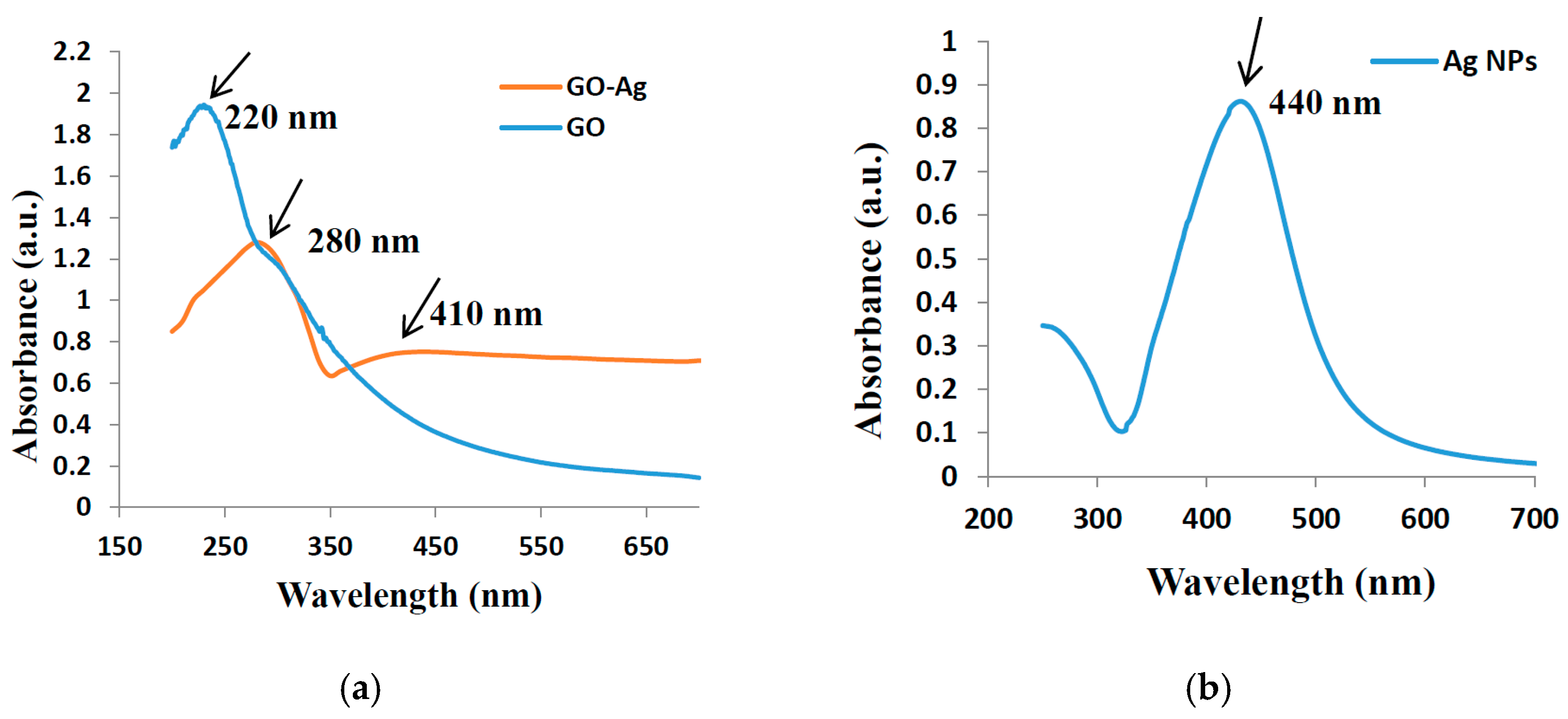
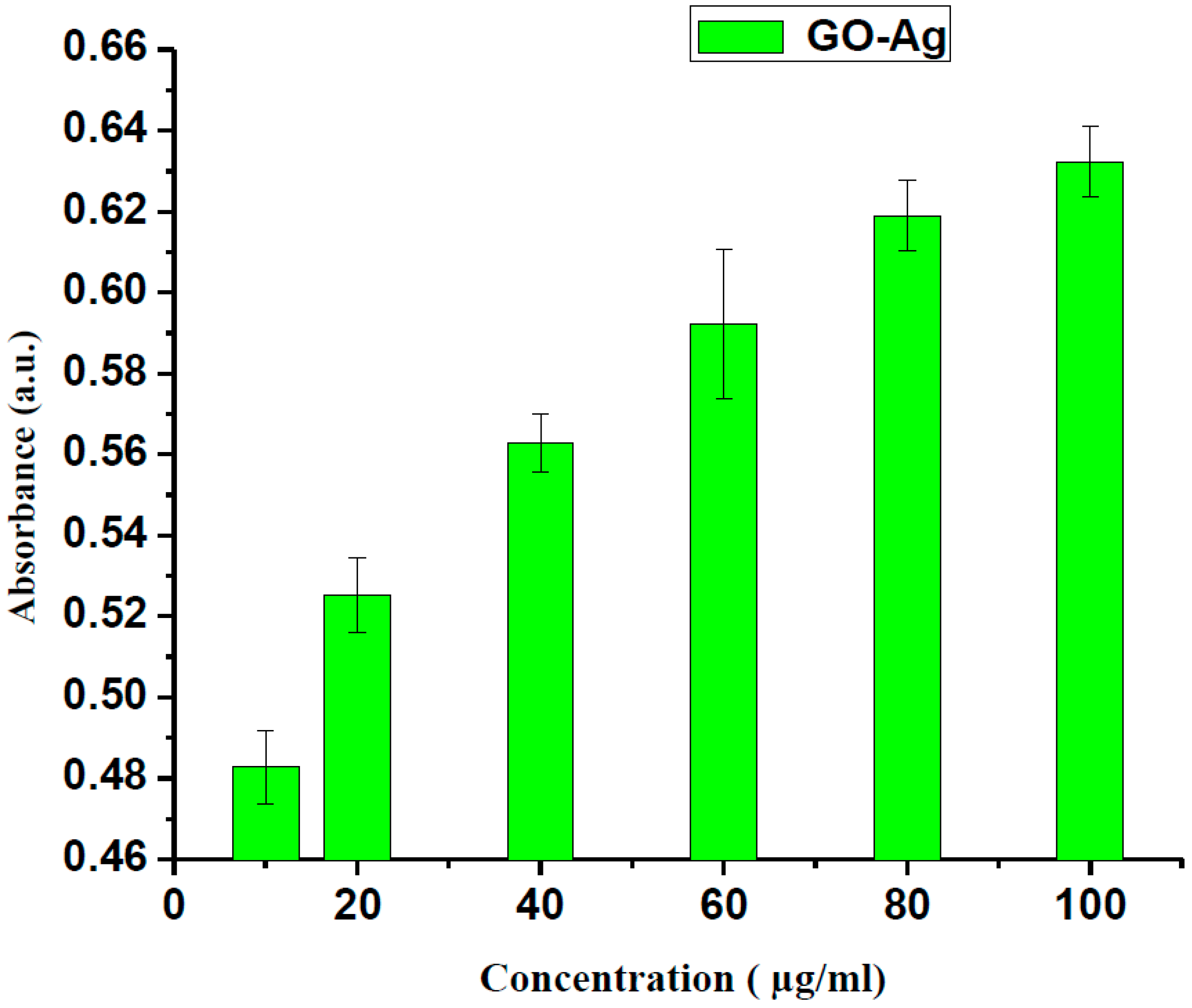
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Tria, M.C.; Vergara, R.A.; Ahmed, F.; Advincula, R.C.; Rodrigues, D.F. Antimicrobial graphene polymer (PVK-GO) nanocomposite films Chem. Chem. Commun. 2011, 47, 8892–8894. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene-based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Tian, M.; Qu, L.; Zhang, X.; Zhang, K.; Zhu, S.; Guo, X.; Han, G.; Tang, X.; Sun, Y. Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Pircheraghi, G.; Monemian, S.A.; Manas-Zloczower, I. Epoxy composites with carbon nanotubes and graphene nanoplatelets—Dispersion and synergy effects. Carbon 2014, 78, 268–278. [Google Scholar] [CrossRef]
- Ang, P.K.; Jaiswal, M.; Lim, C.H.Y.X.; Wang, Y.; Sankaran, J.; Li, A.; Lim, C.T.; Wohland, T.; Barbaros, O.; Loh, K.P. A bioelectronic platform using a graphene-lipid bilayer interface. ACS Nano 2010, 4, 7387–7394. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, J.; Shan, M.; Li, Y.; Li, B.; Niu, J.; Zhou, B.; Qian, X. Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2014, 458, 1–13. [Google Scholar] [CrossRef]
- Aziz, A.; Lim, H.N.; Girei, S.H.; Yaacob, M.H.; Mahdi, M.A.; Huang, N.M.; Pandikumar, A. Silver/graphene nanocomposite-modified optical fiber sensor platform for ethanol detection in water medium. Sens. Actuators B Chem. 2015, 206, 119–125. [Google Scholar]
- Shi, S.; Yang, K.; Hong, H.; Chen, F.; Valdovinos, H.F.; Goel, S.; Barnhart, T.E.; Liu, Z.; Cai, W. VEGFR targeting leads to significantly enhanced tumor uptake of nanographene oxide in vivo. Biomaterials 2015, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ali-Boucetta, H.; Bitounis, D.; Raveendran-Nair, R.; Servant, A.; Van Den Bossche, J.; Kostarelos, K. Purified graphene oxide dispersions lack in vitro cytotoxicity and in vivo pathogenicity. Adv. Healthc. Mater. 2013, 2, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.G.; Koch, B.; Bachmatiuk, A.; Ma, X.; Sanchez, S.; Damm, C.; Schmidt, O.G.; Gemming, T.; Eckert, J.; Rümmeli, M.H. A size dependent evaluation of the cytotoxicity and uptake of nanographene oxide. J. Mater. Chem. B 2015, 3, 2522–2529. [Google Scholar] [CrossRef]
- Russier, J.; Treossi, E.; Scarsi, A.; Perrozzi, F.; Dumortier, H.; Ottaviano, L.; Meneghetti, M.; Palermo, V.; Bianco, A. Evidencing the mask effect of graphene oxide: A comparative study on primary human and murine phagocytic cells. Nanoscale 2013, 5, 11234–11247. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.; Matesanz, M.C.; Vila, M.; Feito, M.J.; Goncalves, G.; Vallet-Regi, M.; Marques, P.A.; Portolés, M.T. Endocytic mechanisms of graphene oxide nanosheets in osteoblasts, hepatocytes and macrophages. ACS Appl. Mater. Interfaces 2014, 6, 13697–13706. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhang, Y.; Liang, L. Effect of graphene oxide on undifferentiated and retinoic acid-differentiated SH-SY5Y cells line. Nanoscale 2012, 4, 3861–3866. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Seo, J.; Cheon, J. Nanoscaling laws for magnetic nanoparticles and their applications in biomedical sciences. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, G.; Eden, H.; Ai, H.; Chen, X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in mda-mb-231 human breast cancer cells. BioMed Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.R.; Jing, L.; Valladares, A.; Mehta, S.L.; Wang, Z.Z.; Li, P.A.; Bang, J.J. Silver nanoparticle exposure induced mitochondrial stress, caspase-3 activation and cell death: Amelioration by sodium selenite. Int. J. Biol. Sci. 2015, 11, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, H.; Wei, S.; Ge, X.; Zhou, J.; Shen, J. High-efficiency loading of hypocrellin B on graphene oxide for photodynamic therapy. Carbon 2012, 50, 5594–5604. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Pan, X.; Wang, M.; Yao, L.; Liang, X.; Ma, J.; Fei, Y.; Wang, P.-N.; Mi, L. Targeting and photodynamic killing of cancer cell by nitrogen-doped titanium dioxide coupled with folic acid. Nanomaterials 2016, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Elnagheeb, M. Mesoporous silica nanoparticles loaded with cisplatin and phthalocyanine for combination chemotherapy and photodynamic therapy in vitro. Nanomaterials 2015, 5, 2302–2316. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.Q.; Teng, C.P.; Ye, E.; Loh, X.J. Effective near-infrared photodynamic therapy assisted by upconversion nanoparticles conjugated with photosensitizers. Int. J. Nanomed. 2015, 10, 419–432. [Google Scholar]
- Qiu, H.; Yang, C.; Shao, W.; Damasco, J.; Wang, X.; Ågren, H.; Prasad, P.; Chen, G. Enhanced Upconversion Luminescence in Yb3+/Tm3+-Codoped Fluoride Active Core/Active Shell/Inert Shell Nanoparticles through Directed Energy Migration. Nanomaterials 2014, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Ou, W. Photoactivation of quantum dot fluorescence following endocytosis. Nano Lett. 2005, 5, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tian, X.-J.; Yang, W.-L.; Ehrenberg, B.; Chen, J.-Y. The conjugates of gold nanorods and chlorin e6 for enhancing the fluorescence detection and photodynamic therapy of cancers. Phys. Chem. Chem. Phys. 2013, 15, 15727–15733. [Google Scholar] [CrossRef] [PubMed]
- Al-Marri, A.H.; Khan, M.; Khan, M. Pulicaria glutinosa extract: A toolbox to synthesize highly reduced graphene oxide-silver nanocomposites. Int. J. Mol. Sci. 2015, 16, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Fakhar-E-Alam, M.; Fatima, M.; Shaheen, F.; Iqbal, S.; Atif, M. Photodynamic Effect of Ni Nanotubes on an HeLa Cell Line. PLoS ONE 2016, 11, e0150295. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphtic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Fatima, M.; Fakhar-E-Alam, M.; Atif, M.; Zaidi, S.S.Z.; Suleman, R.; Shakoor, M.N. Apoptotic effect of α-Fe2O3 and SiO2 nanoparticles in human rhabdomyosarcoma cell line. Laser Phys. 2014, 24, 125602. [Google Scholar] [CrossRef]
- Atif, M.; Firdous, S.; Khurshid, A.; Noreen, L.; Zaidi, S.S.Z.; Ikram, M. In vitro study of 5-aminolevulinic acid-based photodynamic therapy for apoptosis in human cervical HeLa cell line. Laser Phys. Lett. 2009, 6, 886. [Google Scholar] [CrossRef]
- Hayashida, Y.; Ikeda, Y.; Sawada, K.; Kawai, K.; Kato, T.; Kakehi, Y.; Araki, N. Invention of a novel photodynamic therapy for tumors using a photosensitizing PI3K inhibitor. Int. J. Cancer 2016, 139, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Fakhar-e-Alam, M.; Kishwar, S.; Khan, Y.; Siddique, M.; Atif, M.; Nur, O. Tumoricidal effects of nanomaterials in HeLa cell line. Laser Phys. 2011, 21, 1978–1988. [Google Scholar] [CrossRef]
- Fakhar-e-Alam, M.; Kishwar, S.; Siddique, M.; Nur, O.; Willander, M. The photodynamic effect of ZnO nanorods and their ligands with different photosensitizers. Rev. Nanosci. Nanotechnol. 2012, 1, 40–51. [Google Scholar] [CrossRef]
- Morais, V.K.; Garg, A.C.; Oliveira, L.P.; Silva, R.B.; Azevedo, A.M.L.; Silva, E.C.D. Lima, Synthesis and characterization of size-controlled cobalt-ferrite-based ionic ferrofluids. J. Magn. Magn. Mater. 2001, 225, 37–40. [Google Scholar] [CrossRef]
- Arooj, S.; Nazir, S.; Nadhman, A.; Ahmad, N.; Muhammad, B.; Ahmad, I.; Mazhar, K.; Abbasi, R. Novel ZnO:Ag nanocomposites induce significant oxidative stress in human fibroblast malignant melanoma (Ht144) cells. Beilstein J. Nanotechnol. 2015, 6, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gu, L.; Howell, S.B.; Sailor, M.J. Porous Silicon Nanoparticle Photosensitizers for Singlet Oxygen and Their Phototoxicity against Cancer Cells. ACS Nano 2011, 5, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Redmond, R.W.; Gamlin, J.N. A Compilation of Singlet Oxygen Yields from Biologically Relevant Molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yang, Y.; Zheng, M.; Liu, Y.; Xiao, Y.; Lei, B.; Chen, W. Facile fabrication of graphene oxide loaded with silver nanoparticles as antifungal materials. Mater. Res. Express 2014, 1, 045007. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.W.; Liu, T.M.; Chung, K.Y.; Huang, K.S.; Hsieh, C.T.; Sun, C.K.; Yeh, C.S. Efficient near-IR hyperthermia and intense nonlinear optical imaging contrast on the gold nanorod-in-shell nanostructures. J. Am. Chem. Soc. 2009, 40, 14186–14187. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X. Gold Nanocages: Synthesis, Properties, and Applications. Acc. Chem. Res. 2008, 12, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.S.; Pandey, A.P.; Patil, P.O.; Tekade, A.R.; Bari, S.B.; Deshmukh, P.K. Graphene oxide based magnetic nanocomposites for efficient treatment of breast cancer. Mater. Sci. Eng. C 2014, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aburto, R.; Narayanan, T.N.; Nagaoka, Y.; Hasumura, T.; Mitcham, T.M.; Fukuda, T.; Cox, P.J.; Bouchard, R.R.; Maekawa, T.; Sakthi Kumar, D.; et al. Fluorinated graphene oxide; a new multimodal material for biological applications. Adv. Mater. 2013, 25, 5632–5637. [Google Scholar] [CrossRef] [PubMed]
- Waiwijit, U.; Kandhavivorn, W.; Oonkhanond, B.; Lomas, T.; Phokaratkul, D.; Wisitsoraat, A.; Tuantranont, A. Cytotoxicity assessment of MDA-MB-231 breast cancer cells on screen-printed graphene-carbon paste substrate. Colloids Surf. B Biointerfaces 2014, 113, 190–197. [Google Scholar] [CrossRef] [PubMed]
- De Marzi, L.; Ottaviano, L.; Perrozzi, F.; Nardone, M.; Santucci, S.; De Lapuente, J.; Borras, M.; Treossi, E.; Palermo, V.; Poma, A. Flake size-dependent cyto and genotoxic evaluation of graphene oxide on in vitro A549, CaCo2 and vero cell lines. J. Biol. Regul. Homeost. Agents 2014, 28, 281–289. [Google Scholar] [PubMed]
- Vallabani, N.V.; Mittal, S.; Shukla, R.K.; Pandey, A.K.; Dhakate, S.R.; Pasricha, R.; Dhawan, A. Toxicity of graphene in normal human lung cells (BEAS-2B). J. Biomed. Nanotechnol. 2011, 7, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Kim, T.H.; Choi, J.W. Cell chip to detect effects of graphene oxide nanopellet on human neural stem cell. J. Nanosci. Nanotechnol. 2012, 12, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphenenanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Dorn, M.; Vogt, J.; Spemann, D.; Yu, W.; Mao, Z.W. A quantitative study of the intracellular concentration of graphene/noble metal nanoparticle composites and their cytotoxicity. Nanoscale 2014, 6, 8535–8542. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Park, J.H. Reduced graphene oxide–silver nanoparticle nanocomposite: A potential anticancer nanotherapy. Int. J. Nanomed. 2015, 10, 6257–6276. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Sawosz, E.; Grodzik, M.; Winnicka, A.; Prasek, M.; Wierzbicki, M.; Chwalibog, A. In vitro evaluation of the effects of graphene platelets on glioblastoma multiforme cells. Int. J. Nanomed. 2013, 8, 413–420. [Google Scholar]
- Wu, D.; Yotnda, P. Production and Detection of Reactive Oxygen Species (ROS) in Cancers. J. Vis. Exp. 2011, 57, 3357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, L.; Li, M.; Zhao, R.; Yang, X.; Ji, T.; Gu, Z.; Yin, J.J.; Gao, X.; Nie, G. Deciphering the underlying mechanisms of oxidation-state dependent cytotoxicity of graphene oxide on mammalian cells. Toxicol. Lett. 2015, 237, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ouyang, S.; Mu, L.; An, J.; Zhou, Q. Effects of graphene oxide and oxidized carbon nanotubes on the cellular division, microstructure, uptake, oxidative stress, and metabolic profiles. Environ. Sci. Technol. 2015, 49, 10825–10833. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Weim, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, H.; Li, Y.; Shi, D. Graphene-based nanovehicles for photodynamic medical therapy. Int. J. Nanomed. 2015, 10, 2451–2459. [Google Scholar]
- Dong, H.Q.; Zhao, Z.; Wen, H.; Li, Y.; Guo, F.; Shen, A.; Pilger, F.; Lin, C.; Shi, D. Poly(ethylene glycol) conjugated nano-graphene oxide for photodynamic therapy. Sci. China Chem. 2010, 53, 2265–2271. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, Y.D.; Sun, S.F.; Guan, W.C.; Yao, Y.H.; Tang, P.Y. Visible light driven photodynamic anticancer activity of graphene oxide/TiO2 hybrid. Carbon 2012, 50, 994–1004. [Google Scholar] [CrossRef]
- Kolarova, H.; Nevrelova, P.; Tomankova, K.; Kolar, P.; Bajgar, R.; Mosinger, J. Production of reactive oxygen species after photodynamic therapy by porphyrin sensitizers. Gen. Physiol. Biophys. 2008, 27, 101. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaheen, F.; Hammad Aziz, M.; Fakhar-e-Alam, M.; Atif, M.; Fatima, M.; Ahmad, R.; Hanif, A.; Anwar, S.; Zafar, F.; Abbas, G.; et al. An In Vitro Study of the Photodynamic Effectiveness of GO-Ag Nanocomposites against Human Breast Cancer Cells. Nanomaterials 2017, 7, 401. https://doi.org/10.3390/nano7110401
Shaheen F, Hammad Aziz M, Fakhar-e-Alam M, Atif M, Fatima M, Ahmad R, Hanif A, Anwar S, Zafar F, Abbas G, et al. An In Vitro Study of the Photodynamic Effectiveness of GO-Ag Nanocomposites against Human Breast Cancer Cells. Nanomaterials. 2017; 7(11):401. https://doi.org/10.3390/nano7110401
Chicago/Turabian StyleShaheen, Fozia, Muhammad Hammad Aziz, Muhammad Fakhar-e-Alam, Muhammad Atif, Mahvish Fatima, Riaz Ahmad, Atif Hanif, Saqib Anwar, Fatima Zafar, Ghazanfar Abbas, and et al. 2017. "An In Vitro Study of the Photodynamic Effectiveness of GO-Ag Nanocomposites against Human Breast Cancer Cells" Nanomaterials 7, no. 11: 401. https://doi.org/10.3390/nano7110401




