Biomass-Derived Oxygen and Nitrogen Co-Doped Porous Carbon with Hierarchical Architecture as Sulfur Hosts for High-Performance Lithium/Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
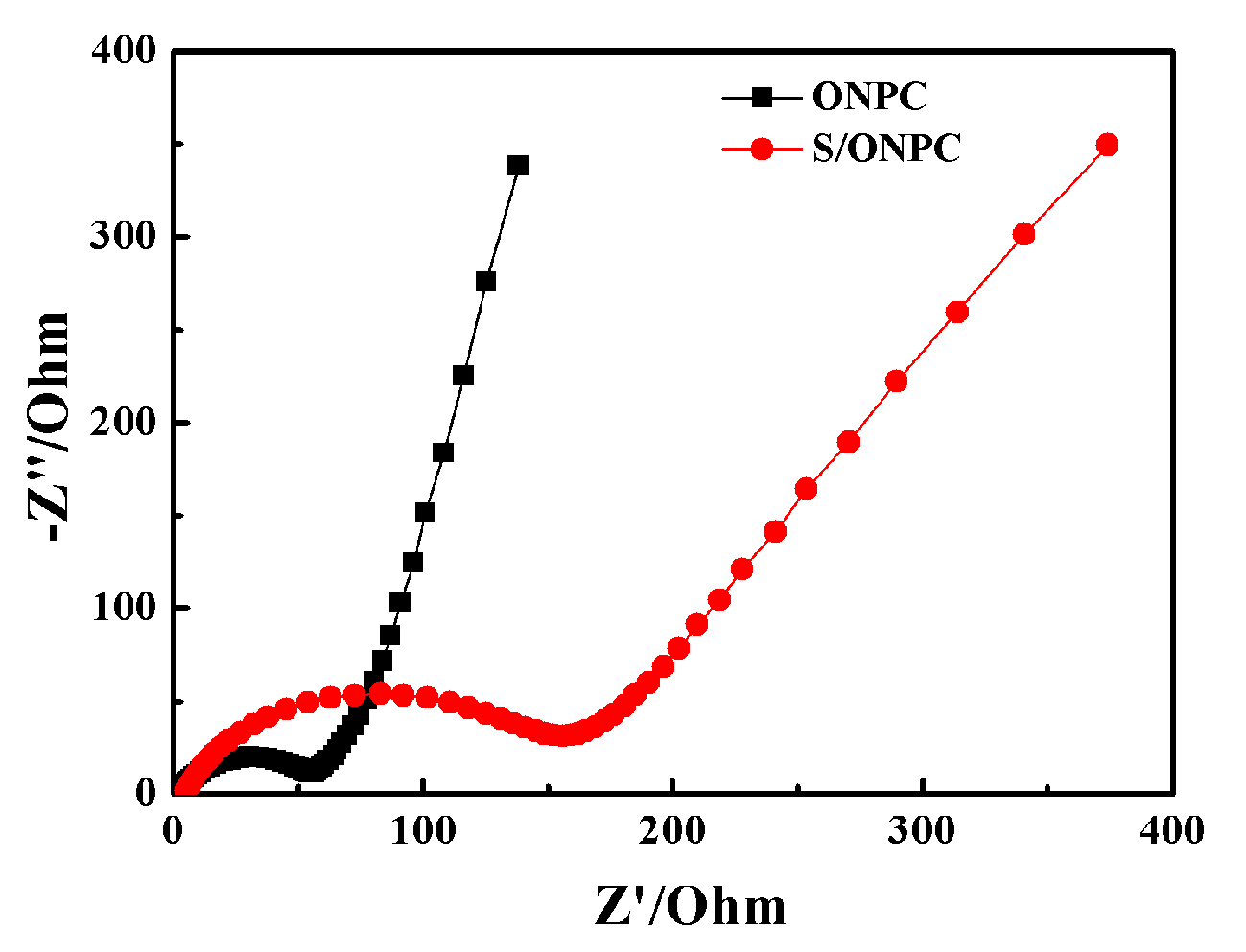
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, D.W.; Li, F.; Hou, P.X.; Yin, L.; Liu, C.; Lu, G.Q.M.; Gentle, I.R.; Cheng, H.M. A flexible nanostructured sulphur-carbon nanotube cathode with high rate performance for Li-S batteries. Energy Environ. Sci. 2014, 7, 1307–1311. [Google Scholar] [CrossRef]
- Zhang, Y.; Bakenov, Z.; Zhao, Y.; Konarov, A.; Doan, T.N.L.; Malik, M.; Paron, T.; Chen, P. One-step synthesis of branched sulfur/polypyrrole nanocomposite cathode for lithium rechargeable batteries. J. Power Sources 2012, 208, 1–8. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Zhang, Y.; Zhao, Y.; Menbayeva, A.; Bakenov, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium-sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Wu, X.; Liu, J. Flexible carbon nanotube–grapheme-sulfur composite film- free-standing cathode for high-performance lithium-sulfur batteries. Phys. Chem. C 2015, 119, 10288–10294. [Google Scholar] [CrossRef]
- Sohn, H.; Gordin, M.L.; Xu, T.; Chen, S.; Lv, D.; Song, J.; Manivannan, A.; Wang, D. Porous spherical carbon/sulfur nanocomposites by aerosol-assisted synthesis: The effect of pore structure and morphology on their electrochemical performance as lithium/sulfur battery cathodes. ACS Appl. Mater. Interfaces 2014, 6, 7596–7606. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; He, G.; Mandlmeier, B.; Yim, T.; Lee, K.T.; Bein, T.; Nazar, L.F. Spherical ordered mesoporous carbon nanoparticles with high porosity for lithium-sulfur batteries. Angew. Chem. 2012, 51, 3591–3595. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.; Cao, S.; Peng, X.; Ducati, C.; Kumar, R.V.; Cheetham, A.K. Carbon with hierarchical pores from carbonized metal-organic frameworks for lithium sulphur batteries. Chem. Commun. 2013, 49, 2192–2194. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yin, F.; Zhang, Y.; Zhang, C.; Mentbayeva, A.; Umirov, N.; Xie, H.; Bakenov, Z. A free-standing sulfur/nitrogen-doped carbon nanotube electrode for high-performance lithium/sulfur batteries. Nanoscale Res. Lett. 2015, 10, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, J.; Lv, R.; Liang, Q.; Bai, Y.; Huang, Z.-H.; Shen, W.; Kang, F. Nitrogen-enriched hierarchical porous carbon with enhanced performance in supercapacitors and lithium-sulfur batteries. RSC Adv. 2015, 5, 75403–75410. [Google Scholar] [CrossRef]
- Xu, J.; Su, D.; Zhang, W.; Bao, W.; Wang, G. A nitrogen-sulfur co-doped porous graphene matrix as a sulfur immobilizer for high performance lithium-sulfur batteries. J. Mater. Chem. A 2016, 4, 17381–17393. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Zhong, Q.; Lai, D.; Yao, J. Porous nitrogen-doped carbon derived from silk fibroin protein encapsulating sulfur as a superior cathode material for highperformance lithium-sulfur batteries. Nanoscale 2015, 7, 17791–17797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Li, Z.; Chen, P. Effect of mesoporous carbon microtube prepared by carbonizing the poplar catkin on sulfur cathode performance in Li/S batteries. J. Alloys Compd. 2015, 619, 298–302. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, N.; Lei, S.; Yan, R.; Tian, X.; Wang, J.; Song, Y.; Xu, D.; Guo, Q.; Liu, L. Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors. Electrochim. Acta 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Z.; Yin, S.; Guo, Z.; Wang, S.; Feng, C. Biomass carbon micro/nano-structures derived from ramie fibers and corncobs as anode materials for lithium-ion and sodium-ion batteries. Appl. Surf. Sci. 2016, 379, 73–82. [Google Scholar] [CrossRef]
- Ru, H.; Xiang, K.; Zhou, W.; Zhu, Y.; Zhao, X.S.; Chen, H. Bean-dreg-derived carbon materials used as superior anode material for lithium-ion batteries. Electrochim. Acta 2016, 222, 551–560. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X.; Aksay, I.A.; Lemmon, J.; Nie, Z.; Yang, Z.; Liu, J. Sandwich-type functionalized graphene sheetsulfur nanocomposite for rechargeable lithium batteries. Phys. Chem. Chem. Phys. 2011, 13, 7660–7665. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.H.; Yoon, H.; Lee, S.H.; Kim, J.E.; Lim, S.; Shin, K.H.; Park, H.S.; Jin, C.S.; Ahn, W.; Cheong, H.W.; et al. Enhanced anode performance of micro/meso-porous reduced graphene oxide prepared from carbide-derived carbon for energy storage devices. Carbon 2015, 91, 241–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Tuiyebayeva, M.; Konarov, A.; Chen, P. Synthesis of hierarchical porous sulfur/polypyrrole/multiwalled carbon nanotube composite cathode for lithium batteries. Electrochim. Acta 2014, 143, 49–55. [Google Scholar] [CrossRef]
- Fang, X.; Peng, H. A revolution in electrodes: Recent progress in rechargeable lithium-sulfur batteries. Small 2015, 11, 1488–1511. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Lv, W.; Zhang, C.; Li, F.; Tang, L.; He, Y.; Li, B.; Yang, Q.; Kang, F. A carbon sandwich electrode with graphene filling coated by ndoped porous carbon layers for lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 20218–20224. [Google Scholar] [CrossRef]
- An, Y.; Zhang, Z.; Fei, H.; Xiong, S.; Ji, B.; Feng, J. Ultrafine TiO2 confined in porous-nitrogen-doped carbon from metal-organic frameworks for high-performance lithium sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 12400–12407. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Li, X.; Cui, Y.; Xiong, D.; Yan, B.; Li, D.; Lushington, A.; Sun, X. Sulfur/nitrogen dual-doped porous graphene aerogels enhancing anode performance of lithium ion batteries. Electrochim. Acta 2016, 205, 188–197. [Google Scholar] [CrossRef]
- He, G.; Hart, C.J.; Liang, X.; Garsuch, A.; Nazar, L.F. Stable cycling of a scalable grapheme–encapsulated nanocomposite for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2014, 6, 10917–10923. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yin, L.C.; Wang, D.W.; Li, L.; Pei, S.; Gentle, I.R.; Li, F.; Cheng, H.M. Fibrous hybrid of graphene and sulfur nanocrystals for high-performance lithium-sulfur batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, H.; Huang, Y.; Wang, W.; Wei, S. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 2011, 49, 838–843. [Google Scholar] [CrossRef]
- Xu, T.; Song, J.; Gordin, M.L.; Sohn, H.; Yu, Z.; Chen, S.; Wang, D. Mesoporous carbon-carbon nanotube-sulfur composite microspheres for high-areal-capacity lithium-sulfur battery cathodes. ACS Appl. Mater. Interfaces 2013, 5, 11355–11362. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Z.; Guo, Z.; Lai, Y.; Zhang, K.; Li, J. Improved cyclability of lithium-sulfur battery cathode using encapsulated sulfur in hollow carbon nanofiber@nitrogen-doped porous carbon core-shell composite. Carbon 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Li, M.; Carter, R.; Douglas, A.; Oakes, L.; Pint, C.L. Sulfur vapor-infiltrated 3d carbon nanotube foam for binder-free high areal capacity lithium-sulfur battery composite cathodes. ACS Nano 2017, 11, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
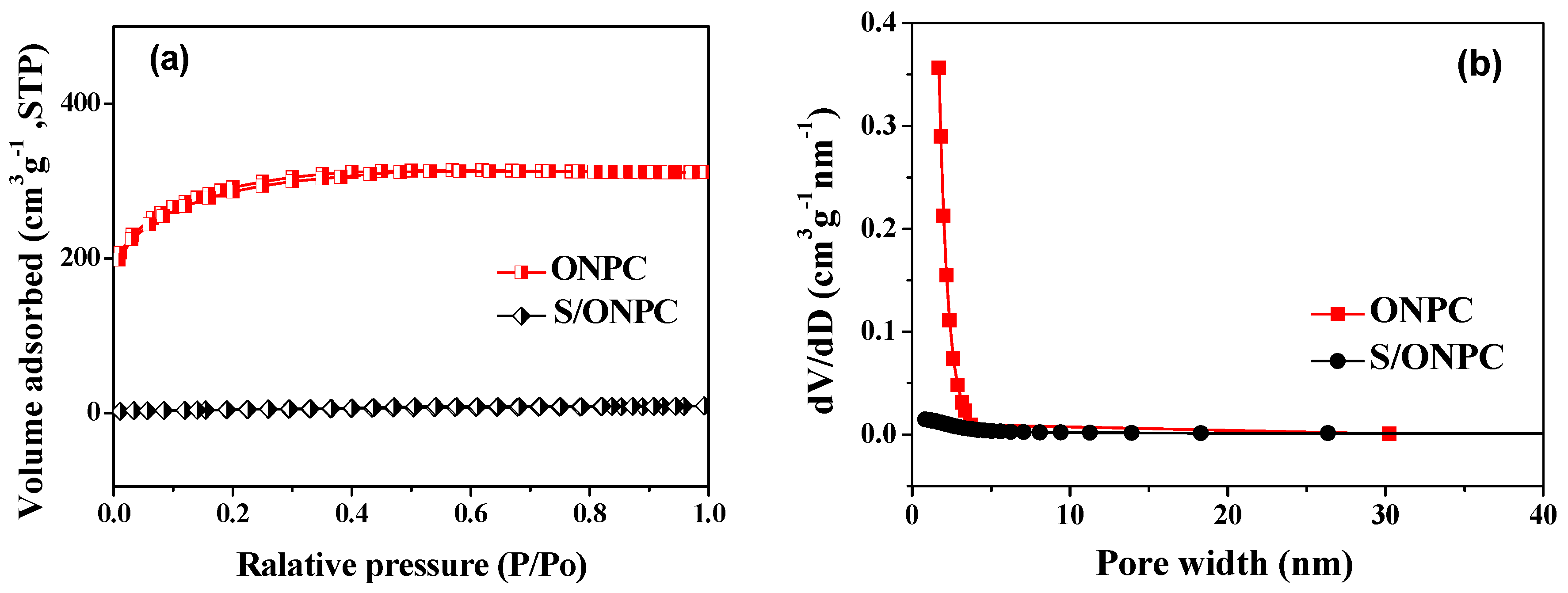

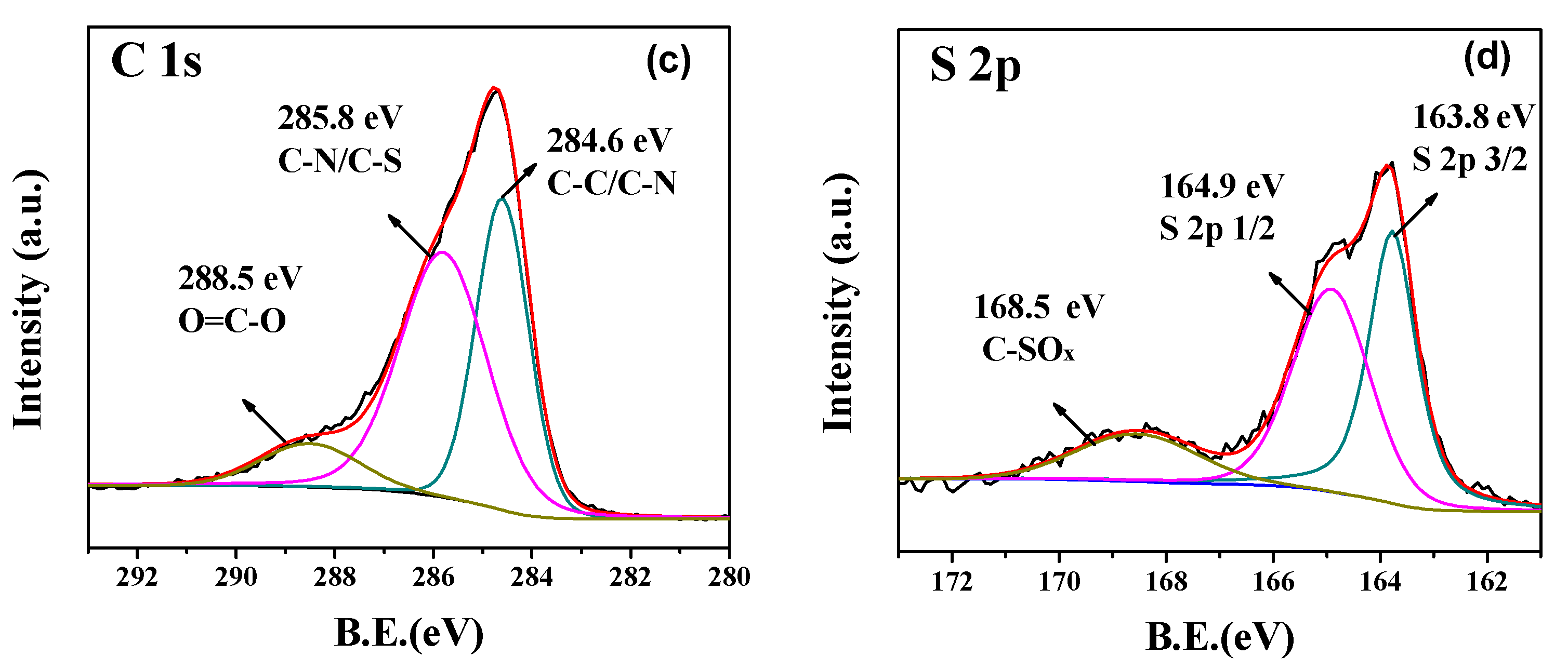
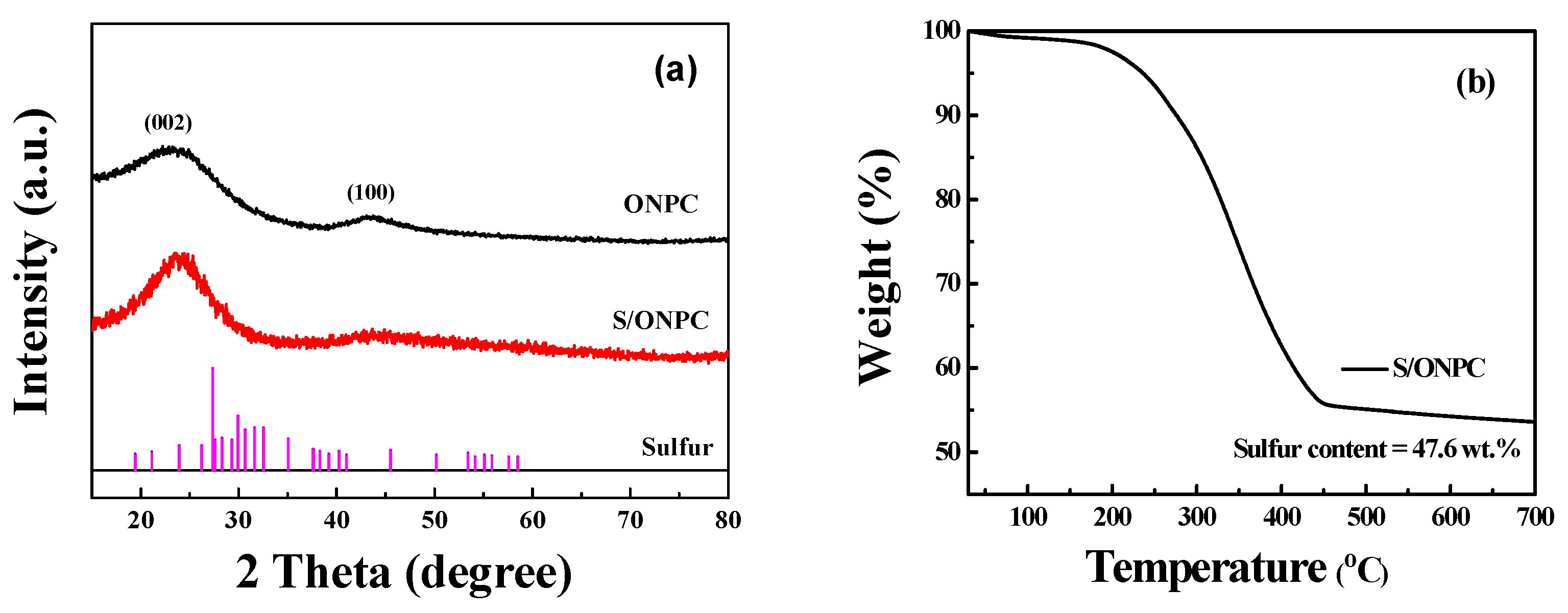
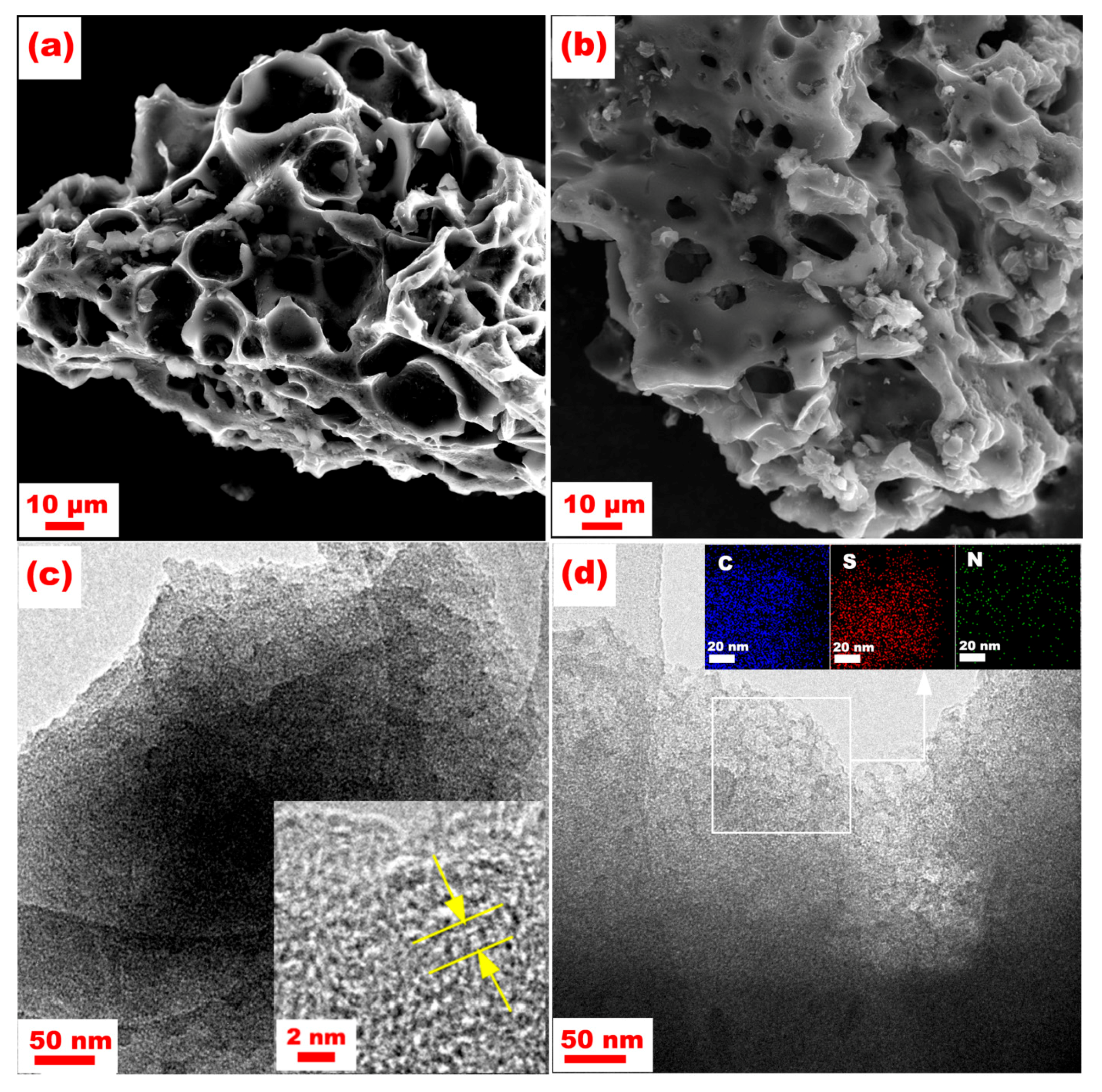

| Materials | SBET (m2·g−1) | Smicro (m2·g−1) | Vtot (cm3·g−1) | Vmicro (cm3·g−1) |
|---|---|---|---|---|
| ONPC | 1017.5 | 477.3 | 0.48 | 0.21 |
| S/ONPC | 22.4 | 3.6 | 0.015 | 0.003 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, L.; Huang, L.; Maximov, M.Y.; Jin, M.; Zhang, Y.; Wang, X.; Zhou, G. Biomass-Derived Oxygen and Nitrogen Co-Doped Porous Carbon with Hierarchical Architecture as Sulfur Hosts for High-Performance Lithium/Sulfur Batteries. Nanomaterials 2017, 7, 402. https://doi.org/10.3390/nano7110402
Zhao Y, Wang L, Huang L, Maximov MY, Jin M, Zhang Y, Wang X, Zhou G. Biomass-Derived Oxygen and Nitrogen Co-Doped Porous Carbon with Hierarchical Architecture as Sulfur Hosts for High-Performance Lithium/Sulfur Batteries. Nanomaterials. 2017; 7(11):402. https://doi.org/10.3390/nano7110402
Chicago/Turabian StyleZhao, Yan, Li Wang, Lanyan Huang, Maxim. Yu. Maximov, Mingliang Jin, Yongguang Zhang, Xin Wang, and Guofu Zhou. 2017. "Biomass-Derived Oxygen and Nitrogen Co-Doped Porous Carbon with Hierarchical Architecture as Sulfur Hosts for High-Performance Lithium/Sulfur Batteries" Nanomaterials 7, no. 11: 402. https://doi.org/10.3390/nano7110402





