Iron-Based Nanomaterials/Graphene Composites for Advanced Electrochemical Sensors
Abstract
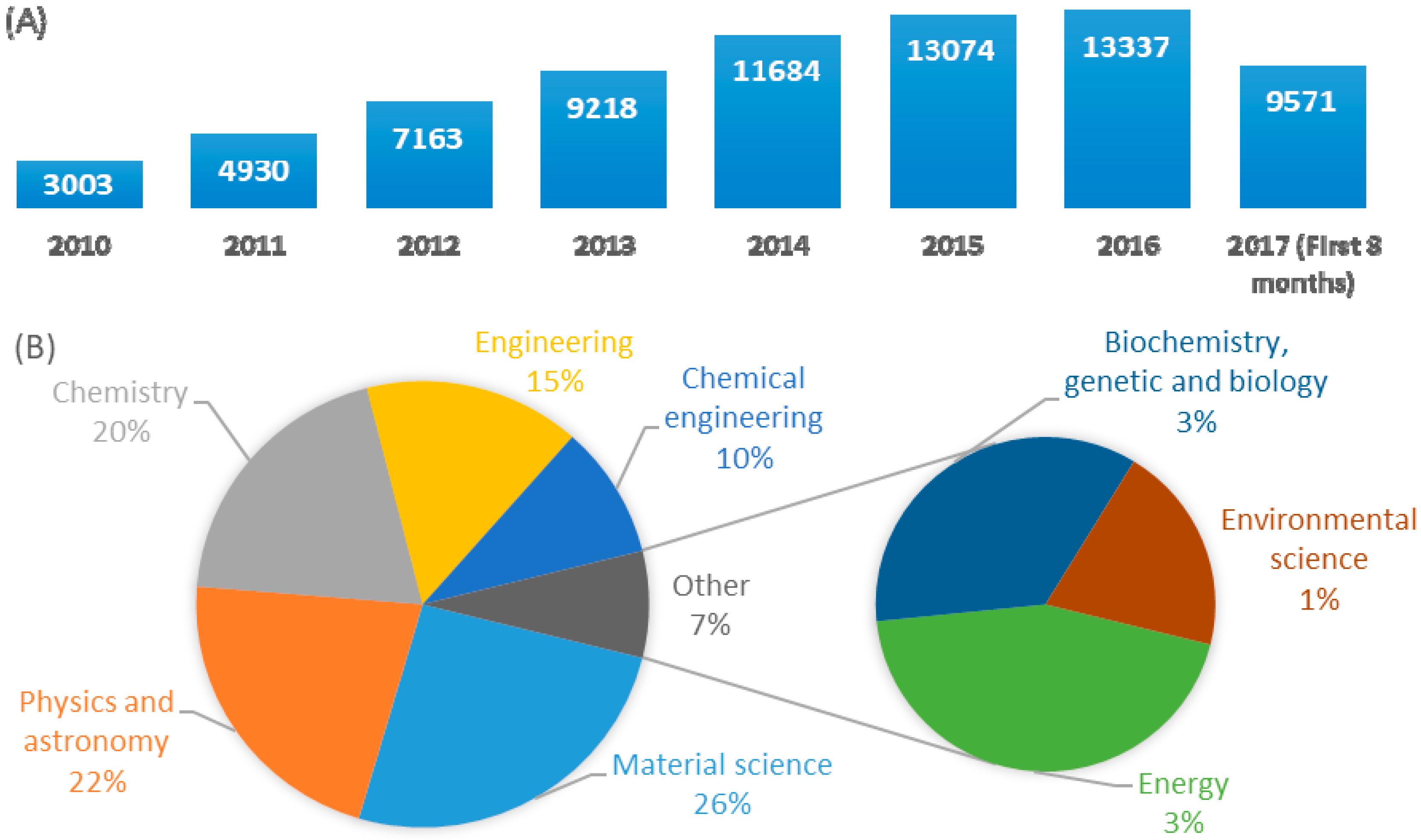
:1. Introduction
2. Iron Oxides Nanostructures
2.1. Hematite (α-Fe2O3)
2.2. Maghemite (γ-Fe2O3)
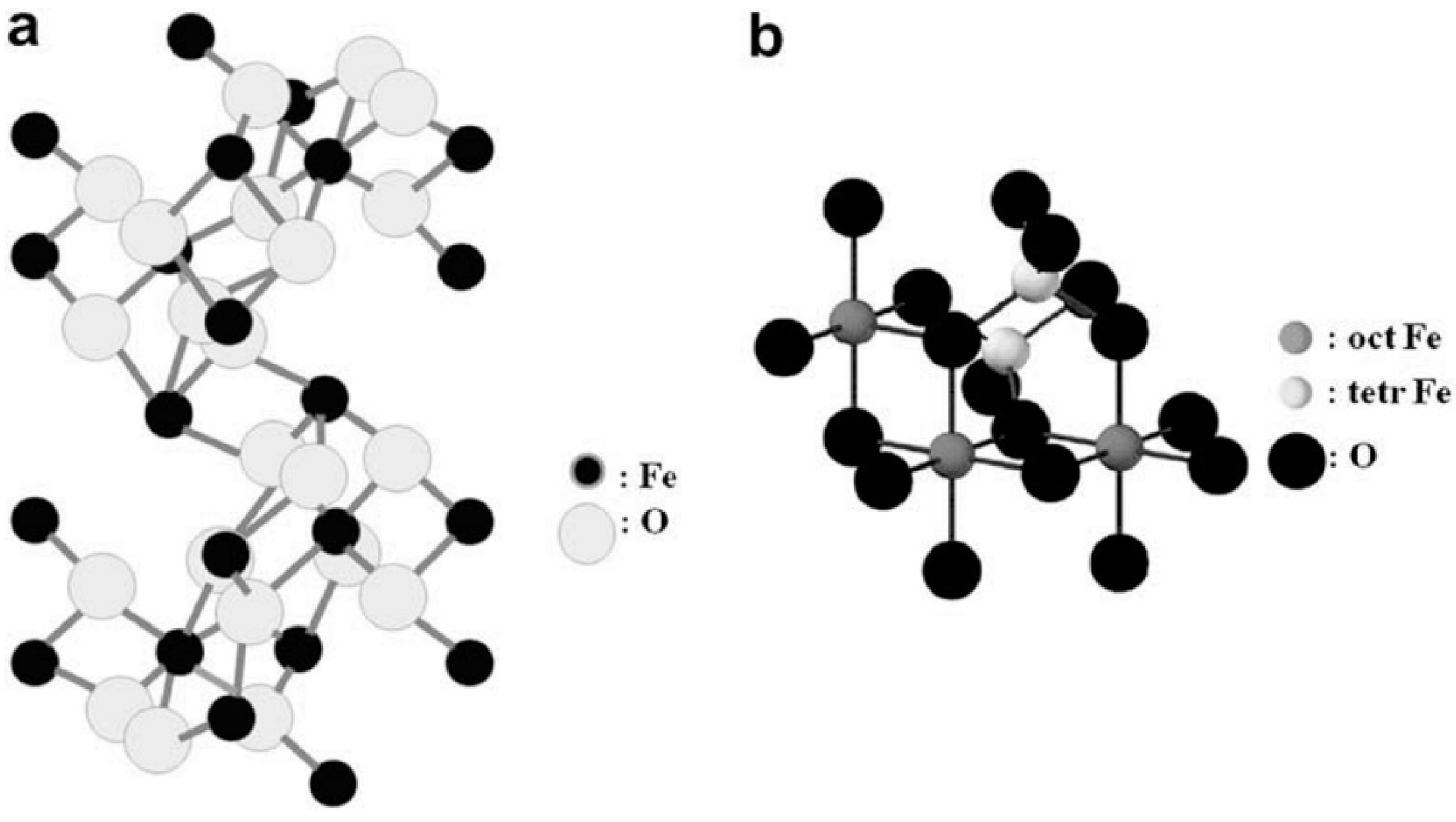
2.3. Magnetite (Fe3O4)
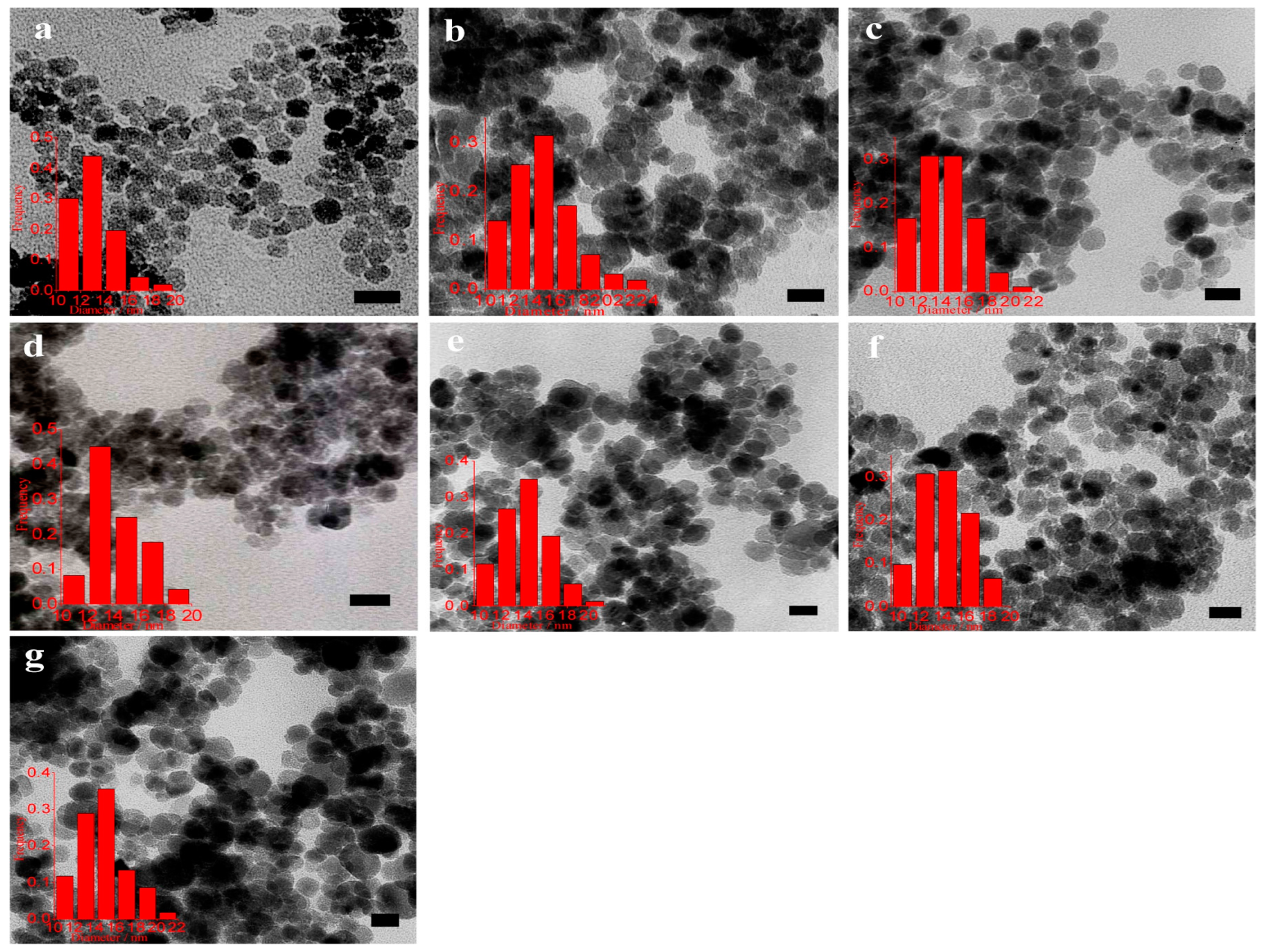
3. Preparation Methods of IONs
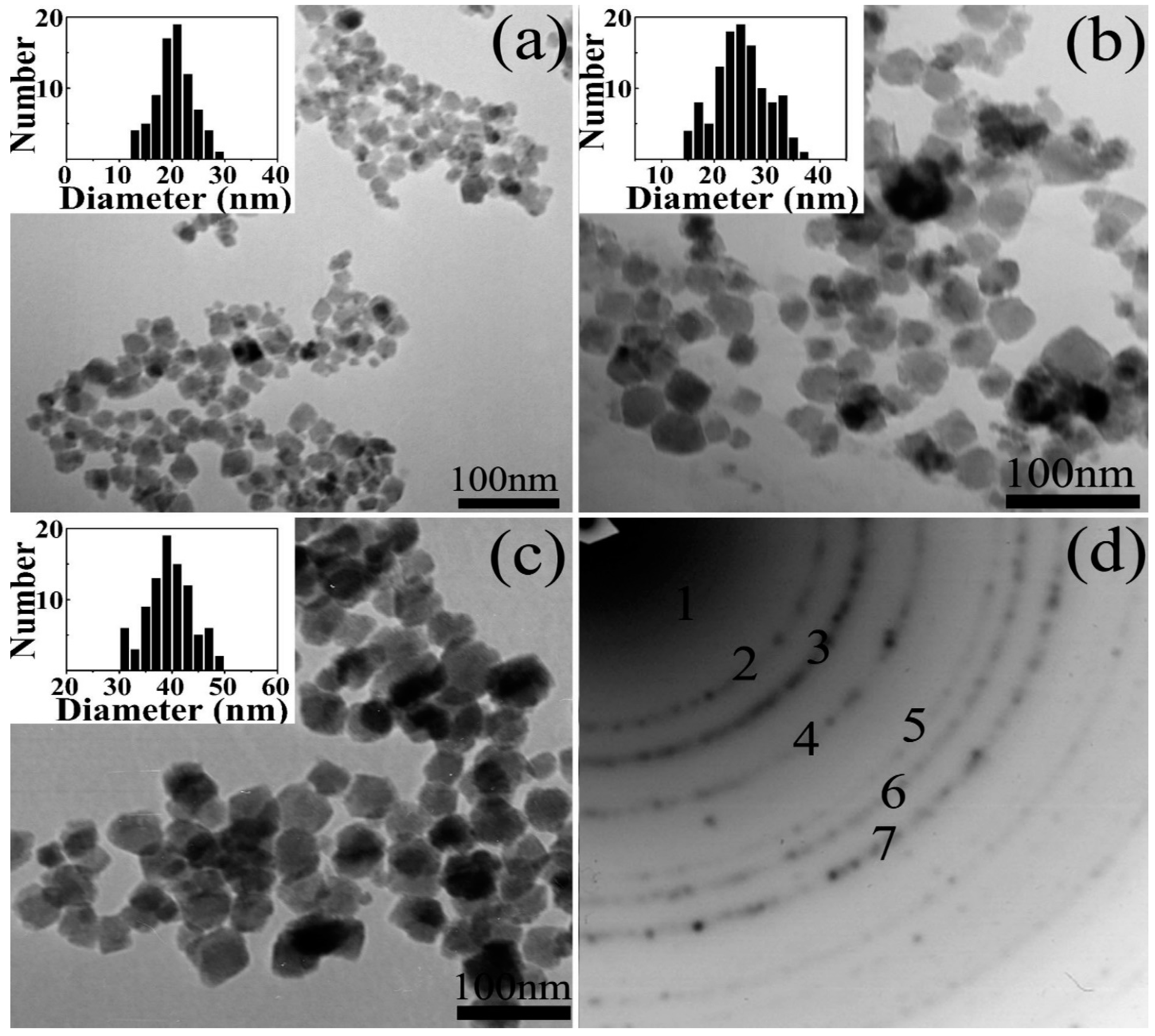
3.1. Hydrothermal Method
3.2. Coprecipitation
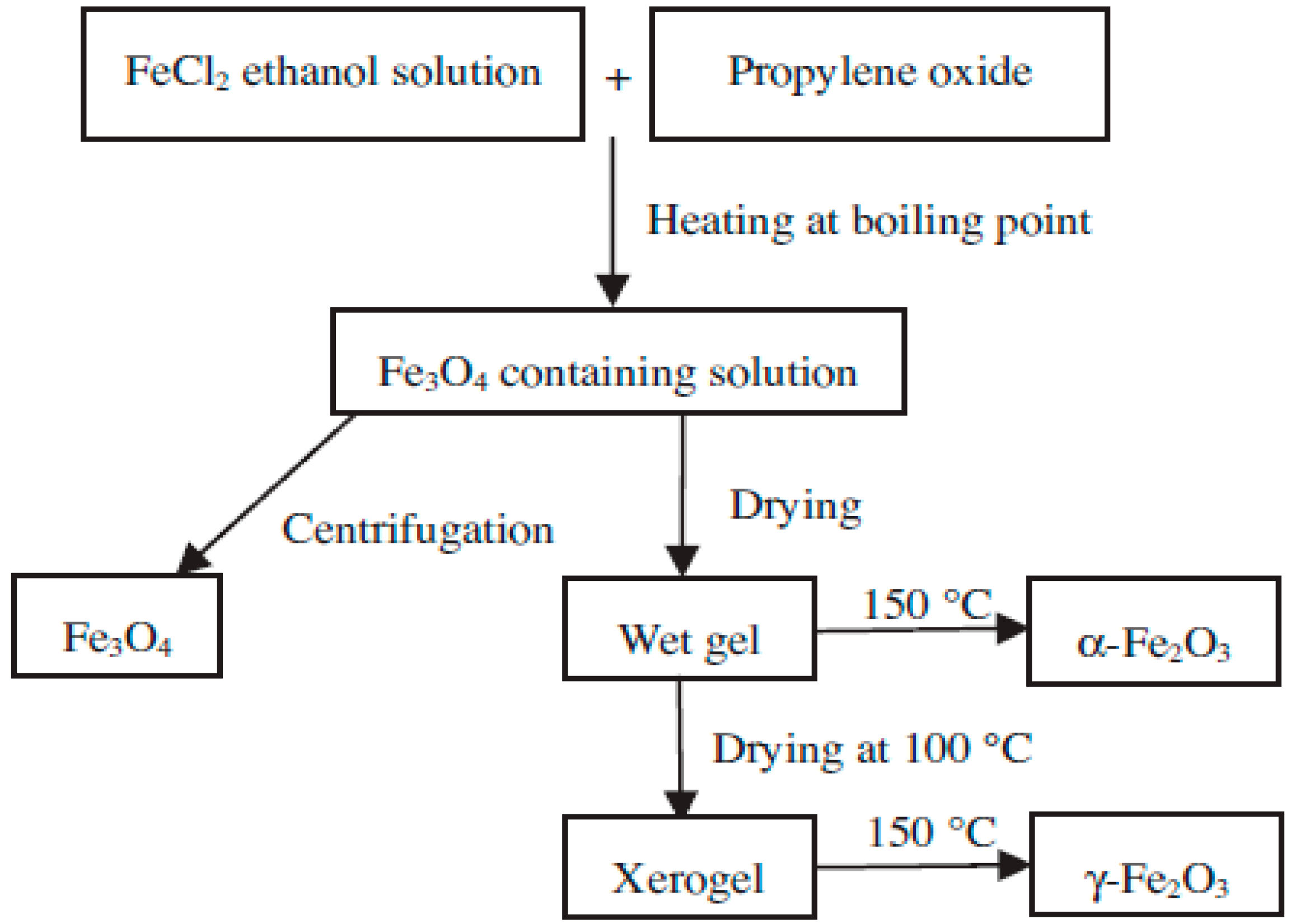
3.3. Sol-Gel
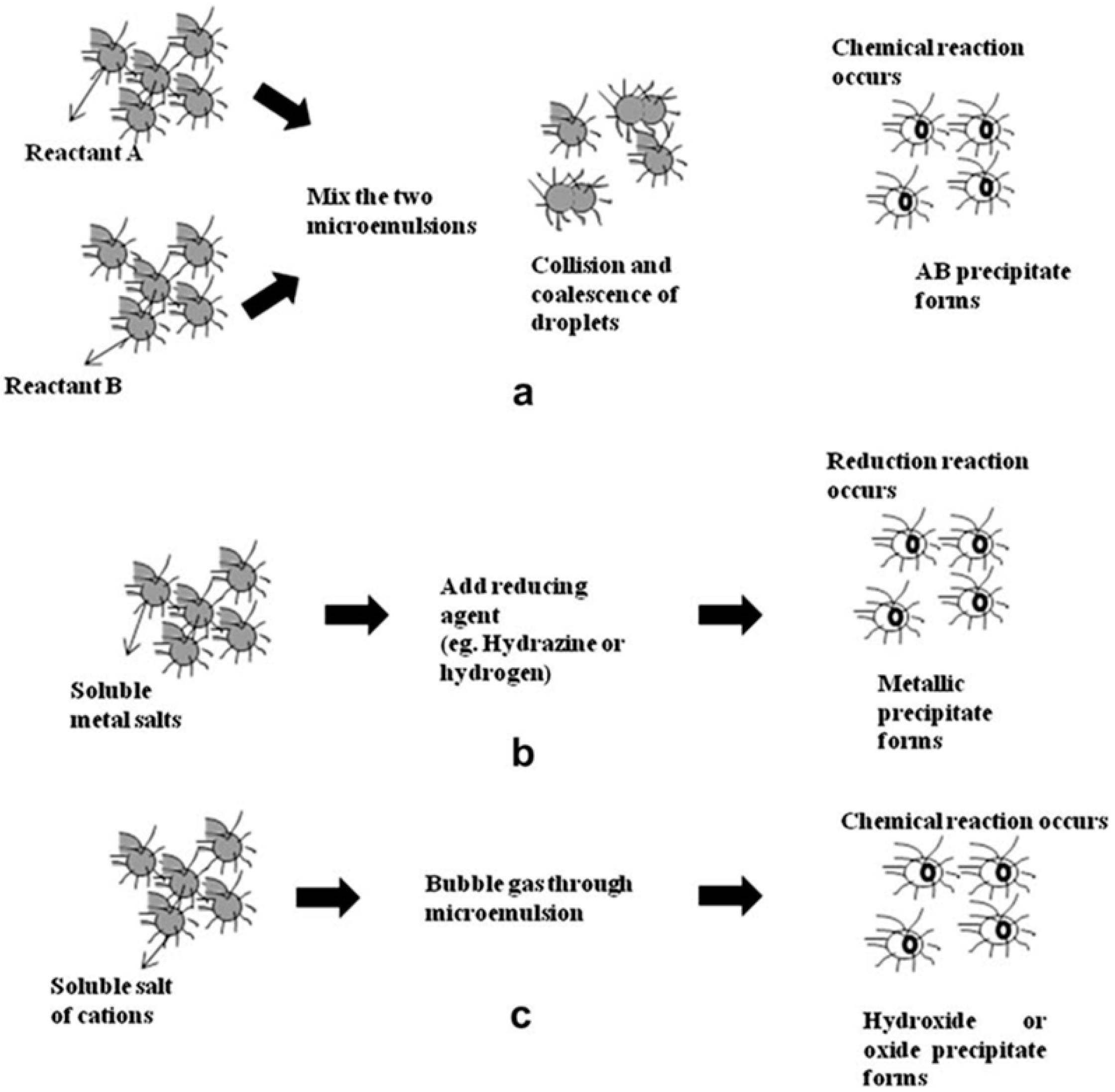
3.4. Microemulsion
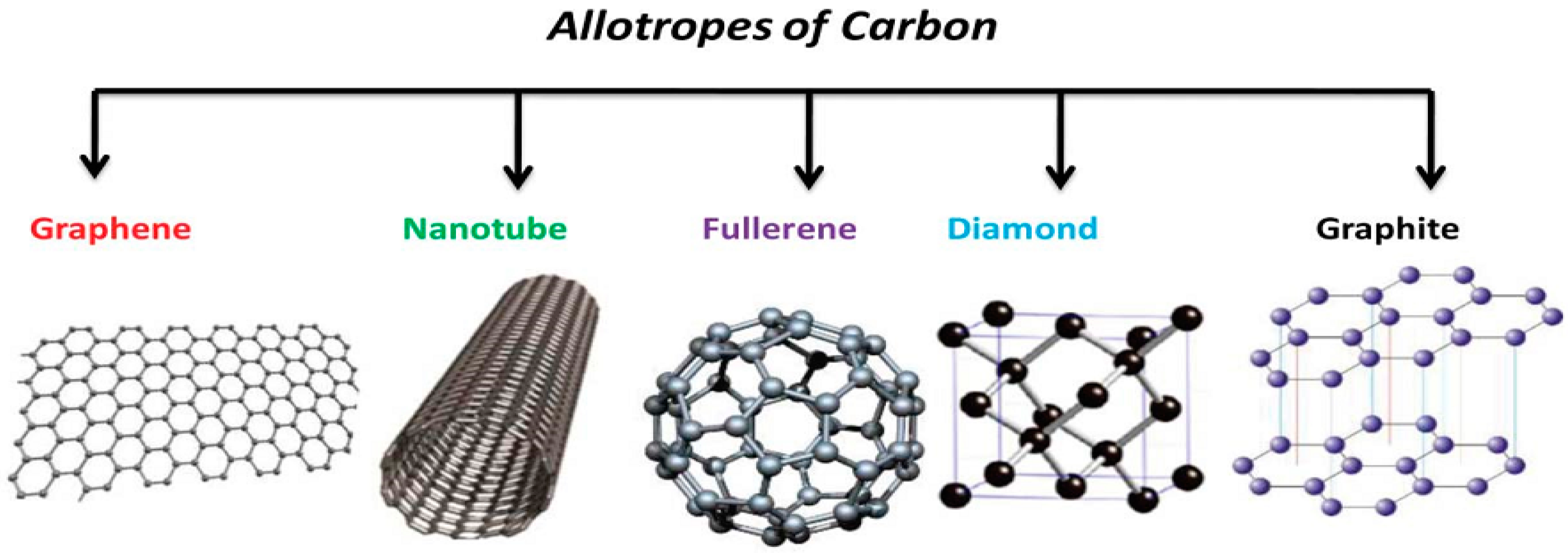
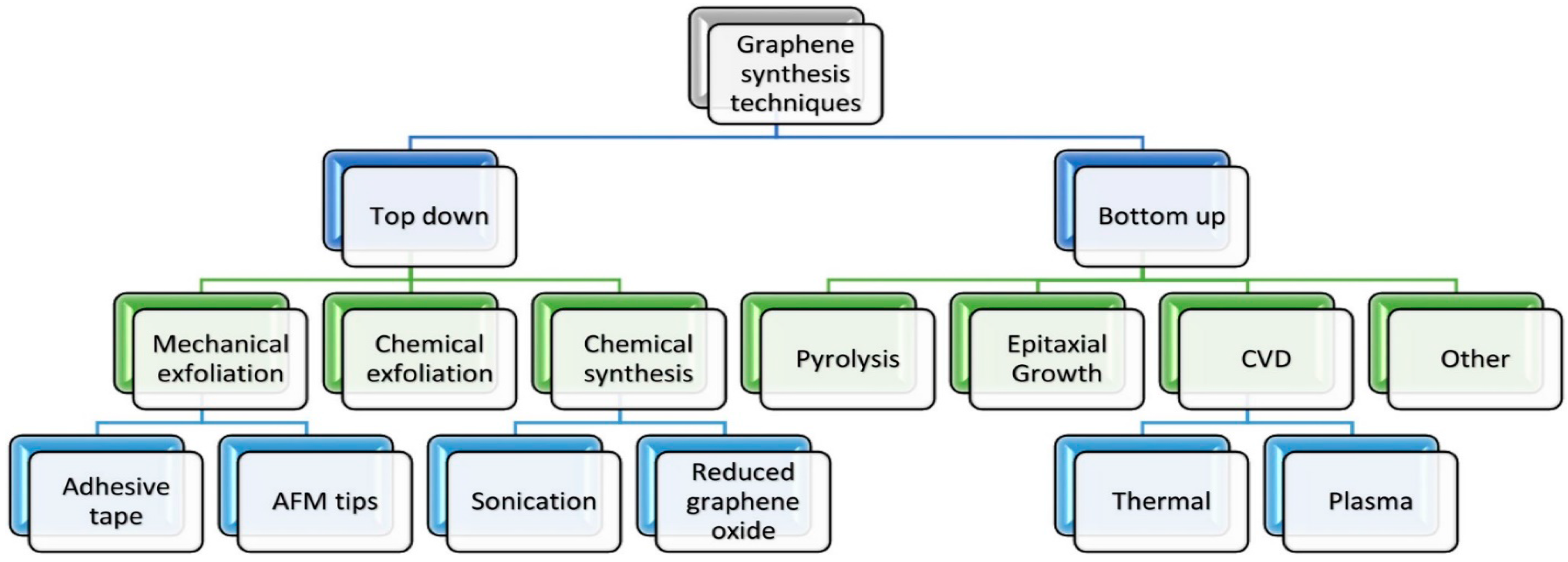
4. Graphene
5. Electrochemical Sensors
6. Application
6.1. Glucose
6.2. Dopamine, Uric and Ascorbic Acid
6.3. Amino Acids
6.4. Deoxyribonucleic Acid
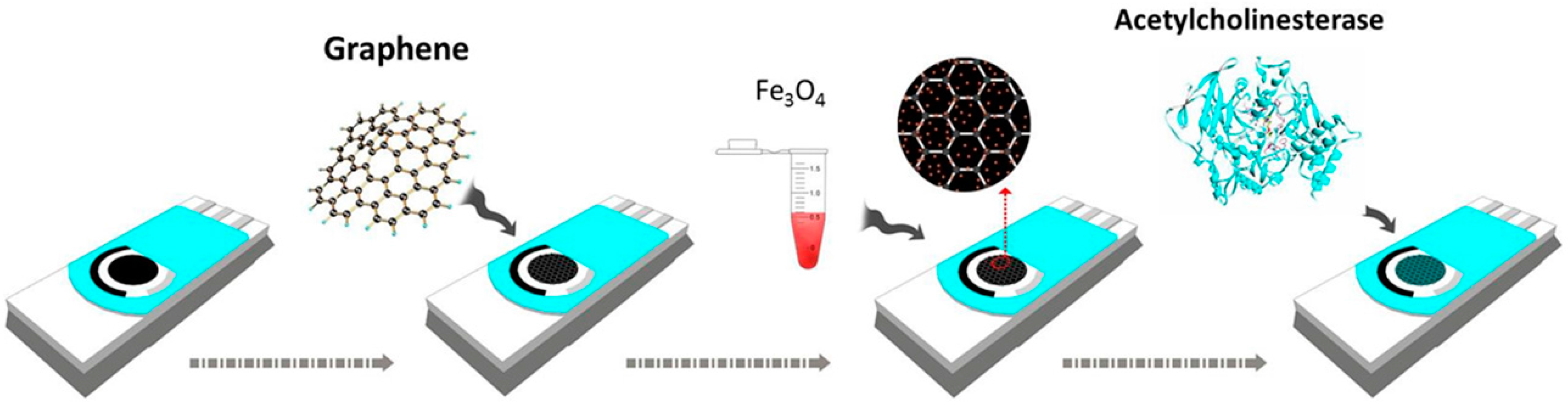
6.5. Pesticides
6.6. Miscellaneous
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.U.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhaohui, W.; Taekyung, Y.; Changzhong, J.; Woo-Sik, K. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. Introduction to the Iron Oxides. In The Iron Oxides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 1–7. [Google Scholar]
- Leonardi, S.G.; Mirzaei, A.; Bonavita, A.; Santangelo, S.; Frontera, P.; Pantò, F.; Antonucci, P.L.; Neri, G. A comparison of the ethanol sensing properties of -iron oxide nanostructures prepared via the sol-gel and electrospinning techniques. Nanotechnology 2016, 27, 075502. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, P.; Sumathi, C.; Wilson, J.; Sekar, C.; Leonardi, S.G.; Neri, G. Fe2O3/Carbon nanotube-based resistive sensors for the selective ammonia gas sensing. Sens. Lett. 2014, 12, 17–23. [Google Scholar] [CrossRef]
- Prieto, G.; Wright, V.; Burger, R.L.; Cooke, C.A.; Zeballos-Velasquez, E.L.; Watanave, A.; Suchomel, M.R.; Suescun, L. The source, processing and use of red pigment based on hematite and cinnabar at Gramalote, an early Initial Period (1500–1200 cal. B.C.) maritime community, north coast of Peru. J. Archaeol. Sci. Rep. 2016, 5, 45–60. [Google Scholar]
- Opuchovic, O.; Kareiva, A. Historical hematite pigment: Synthesis by an aqueous sol–gel method, characterization and application for the colouration of ceramic glazes. Ceram. Int. 2015, 41, 4504–4513. [Google Scholar] [CrossRef]
- Lee, J.-G.; Joshi, B.N.; Lee, J.-H.; Kim, T.-G.; Kim, D.-Y.; Al-Deyab, S.S.; Seong, I.W.; Swihart, M.T.; Yoon, W.Y.; Yoon, S.S. Stable High-Capacity Lithium Ion Battery Anodes Produced by Supersonic Spray Deposition of Hematite Nanoparticles and Self-Healing Reduced Graphene Oxide. Electrochim. Acta 2017, 228, 604–610. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Wu, Z.; Luo, Y.; Qiu, W.; Fan, X.; Long, B.; Huang, M.; Liu, P.; Tong, Y. High power density nitridated hematite (α-Fe2O3) nanorods as anode for high-performance flexible lithium ion batteries. J. Power Sources 2016, 308, 7–17. [Google Scholar] [CrossRef]
- Nathan, D.M.G.T.; Boby, S.J.M. Hydrothermal preparation of hematite nanotubes/reduced graphene oxide nanocomposites as electrode material for high performance supercapacitors. J. Alloys Compd. 2017, 700, 67–74. [Google Scholar] [CrossRef]
- Lee, C.; Jo, E.H.; Kim, S.K.; Choi, J.-H.; Chang, H.; Jang, H.D. Electrochemical performance of crumpled graphene loaded with magnetite and hematite nanoparticles for supercapacitors. Carbon 2017, 115, 331–337. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, X.; Zheng, J. A facile one-step synthesis of Fe2O3/nitrogen-doped reduced graphene oxide nanocomposite for enhanced electrochemical determination of dopamine. J. Alloys Compd. 2017, 709, 581–587. [Google Scholar] [CrossRef]
- Senthil, R.A.; Selvi, A.; Arunachalam, P.; Amudha, L.S.; Madhavan, J.; Al-Mayouf, A.M. A sensitive electrochemical detection of hydroquinone using newly synthesized α-Fe2O3-graphene oxide nanocomposite as an electrode material. J. Mater. Sci. Mater. Electron. 2017, 28, 10081–10091. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, W.; Wu, Y.; Qu, W. Self-assembly and hydrothermal technique syntheized Fe2O3-RGO nanocomposite: The enhancement effect of electrochemical simultaneous detection of honokiol and magnolol. J. Electroceram. 2017, 1–10. [Google Scholar] [CrossRef]
- Campos, E.A.; Pinto, D.V.B.S.; Oliveira, J.I.S.; Mattos, E.D.C.; Dutra, R.D.C.L. Synthesis, Characterization and Applications of Iron Oxide Nanoparticles—A Short Review. J. Aerosp. Technol. Manag. 2015, 7, 267–276. [Google Scholar] [CrossRef]
- Zhang, Z.; Boxall, C.; Kelsall, G.H. Photoelectrophoresis of colloidal iron oxides 1. Hematite (α-Fe2O3). Colloids Surfaces A Physicochem. Eng. Asp. 1993, 73, 145–163. [Google Scholar] [CrossRef]
- Machala, L.; Tuček, J.; Zbořil, R. Polymorphous Transformations of Nanometric Iron(III) Oxide: A Review. Chem. Mater. 2011, 23, 3255–3272. [Google Scholar] [CrossRef]
- Ricardo, G.-C.; Asmaa, Y.A.-B.; Iman, S.; Nora, H.D.L. Vacancy ordering and electronic structure of γ-Fe2O3 (maghemite): A theoretical investigation. J. Phys. Condens. Matter 2010, 22, 255401. [Google Scholar]
- Sun, S.-N.; Wei, C.; Zhu, Z.-Z.; Hou, Y.-L.; Subbu, S.V.; Xu, Z.-C. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar] [CrossRef]
- Fu, C.; Ravindra, N.M. Magnetic iron oxide nanoparticles: Synthesis and applications. Bioinspired Biomim. Nanobiomater. 2012, 1, 229–244. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Figuerola, A.; di Corato, R.; Manna, L.; Pellegrino, T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol. Res. 2010, 62, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Teja, A.S. Continuous hydrothermal crystallization of α–Fe2O3 and Co3O4 nanoparticles. J. Mater. Res. 2011, 18, 415–422. [Google Scholar] [CrossRef]
- Shandilya, M.; Rai, R.; Singh, J. Review: Hydrothermal technology for smart materials. Adv. Appl. Ceram. 2016, 115, 354–376. [Google Scholar] [CrossRef]
- Hui, C.; Shen, C.; Yang, T.; Bao, L.; Tian, J.; Ding, H.; Li, C.; Gao, H.J. Large-scale Fe3O4 nanoparticles soluble in water synthesized by a facile method. J. Phys. Chem. C 2008, 112, 11336–11339. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Hamid, S.B.A. Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2014, 368, 207–229. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ren, W. Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature sol–gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv. Powder Technol. 2013, 24, 93–97. [Google Scholar] [CrossRef]
- Sahu, U.K.; Sahu, M.K.; Mahapatra, S.S.; Patel, R.K. Removal of As(III) from Aqueous Solution Using Fe3O4 Nanoparticles: Process Modeling and Optimization Using Statistical Design. Water Air Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Liang, S.; Li, J.; Wang, F.; Qin, J.; Lai, X.; Jiang, X. Highly sensitive acetone gas sensor based on ultrafine α- Fe2O3nanoparticles. Sens. Actuators B Chem. 2017, 238, 923–927. [Google Scholar] [CrossRef]
- Oulego, P.; Villa-García, M.A.; Laca, A.; Diaz, M. The effect of the synthetic route on the structural, textural, morphological and catalytic properties of iron(III) oxides and oxyhydroxides. Dalton Trans. 2016, 45, 9446–9459. [Google Scholar] [CrossRef] [PubMed]
- Mirza, I.M.; Sarfraz, A.K.; Hasanain, S.K. Effect of surfactant on magnetic and optical properties of α- Fe2O3nanoparticles. Acta Phys. Pol. A 2014, 126, 1280–1287. [Google Scholar] [CrossRef]
- Lu, T.; Wang, J.; Yin, J.; Wang, A.; Wang, X.; Zhang, T. Surfactant effects on the microstructures of Fe3O4 nanoparticles synthesized by microemulsion method. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 675–683. [Google Scholar] [CrossRef]
- Lin, X.-M.; Samia, A.C.S. Synthesis, assembly and physical properties of magnetic nanoparticles. J. Magn. Magn. Mater. 2006, 305, 100–109. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhen, Z.; Zhu, H. Graphene: Fundamental research and potential applications. FlatChem 2017, 4, 20–32. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Movlaee, K.; Beitollahi, H.; Ganjali, M.R.; Norouzi, P. Electrochemical platform for simultaneous determination of levodopa, acetaminophen and tyrosine using a graphene and ferrocene modified carbon paste electrode. Microchim. Acta 2017, 184, 3281–3289. [Google Scholar] [CrossRef]
- Beitollahi, H.; Movlaee, K.; Ganjali, M.R.; Norouzi, P. A sensitive graphene and ethyl 2-(4-ferrocenyl-[1,2,3]triazol-1-yl) acetate modified carbon paste electrode for the concurrent determination of isoproterenol, acetaminophen, tryptophan and theophylline in human biological fluids. J. Electroanal. Chem. 2017, 799, 576–582. [Google Scholar] [CrossRef]
- Gholipour-Ranjbar, H.; Ganjali, M.R.; Norouzi, P.; Naderi, H.R. Synthesis of cross-linked graphene aerogel/Fe2O3 nanocomposite with enhanced supercapacitive performance. Ceram. Int. 2016, 42, 12097–12104. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ni, M.; Ren, X.; Tian, Y.; Li, N.; Su, Y.; Zhang, X. Graphene in Supercapacitor Applications. Curr. Opin. Colloid Interface Sci. 2015, 20, 416–428. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Priftis, D. Polyelectrolyte-graphene Nanocomposites for Biosensing Applications. Curr. Org. Chem. 2015, 19, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Dhand, V.; Rhee, K.Y.; Kim, H.J.; Jung, D.H. A Comprehensive Review of Graphene Nanocomposites: Research Status and Trends. J. Nanomater. 2013, 2013, 14. [Google Scholar] [CrossRef]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef]
- Ulrich, G.; Winfried, V.; Jens, Z. Recent developments in electrochemical sensor application and technology—A review. Meas. Sci. Technol. 2009, 20, 042002. [Google Scholar]
- Jiang, S.; Hua, E.; Liang, M.; Liu, B.; Xie, G. A novel immunosensor for detecting toxoplasma gondii-specific IgM based on goldmag nanoparticles and graphene sheets. Colloids Surfaces B Biointerfaces 2013, 101, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors—Principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Zhang, S.; Du, B.; Li, H.; Xin, X.; Ma, H.; Wu, D.; Yan, L.; Wei, Q. Metal ions-based immunosensor for simultaneous determination of estradiol and diethylstilbestrol. Biosens. Bioelectron. 2014, 52, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Chang, B.Y.; Park, S.M. Electrochemical impedance spectroscopy. Annu. Rev. Anal. Chem. 2010, 3, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Salimi, A.; Firoozi, S. A high performance electrochemical biosensing platform for glucose detection and IgE aptasensing based on Fe3O4 /reduced graphene oxide nanocomposite. Electroanalysis 2014, 26, 129–138. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, F.; Zhang, L.; Liu, L.; Liu, C.; Pu, X. An electrochemical strategy with molecular beacon and hemin/G-quadruplex for the detection of Clostridium perfringens DNA on screen-printed electrodes. RSC Adv. 2014, 4, 57064–57070. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Zhang, M.; Chi, Q. Electrocatalytic Applications of Graphene–Metal Oxide Nanohybrid Materials. In Advanced Catalytic Materials—Photocatalysis and Other Current Trends; Norena, L.E., Wang, J.-A., Eds.; InTech: Rijeka, Croatia, 2016; p. 14. [Google Scholar]
- Yu, Z.; Li, H.; Lu, J.; Zhang, X.; Liu, N.; Zhang, X. Hydrothermal synthesis of Fe2O3/graphene nanocomposite for selective determination of ascorbic acid in the presence of uric acid. Electrochim. Acta 2015, 158, 264–270. [Google Scholar] [CrossRef]
- Sethuraman, V.; Muthuraja, P.; Raj, J.A.; Manisankar, P. A highly sensitive electrochemical biosensor for catechol using conducting polymer reduced graphene oxide–metal oxide enzyme modified electrode. Biosens. Bioelectron. 2016, 84, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Ahmed, M.S.; Jeon, S. Determination of dopamine by dual doped graphene-Fe2O3 in presence of ascorbic acid. J. Electrochem. Soc. 2015, 162, B363–B369. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Wang, G.; Deng, D.; Qu, L. A novel electrochemical sensor based on Fe2O3 nanoparticles/N-doped graphene for electrocatalytic oxidation of L-cysteine. J. Solid State Electrochem. 2015, 19, 3613–3620. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, C.; Huang, M.; Song, N.; Hu, W. Highly enhanced electrochemical responses of rutin by nanostructured Fe2O3/RGO composites. Ionics 2015, 21, 1427–1434. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Krishnamoorthy, K.; Sekar, C.; Wilson, J.; Kim, S.J. A promising electrochemical sensing platform based on ternary composite of polyaniline-Fe2O3-reduced graphene oxide for sensitive hydroquinone determination. Chem. Eng. J. 2015, 259, 594–602. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, Q.; Li, L.; Chen, J.; Wu, K. Synergetic signal amplification of graphene-Fe2O3 hybrid and hexadecyltrimethylammonium bromide as an ultrasensitive detection platform for bisphenol A. Electrochim. Acta 2014, 115, 434–439. [Google Scholar] [CrossRef]
- Du, M.; Yang, T.; Guo, X.; Zhong, L.; Jiao, K. Electrochemical synthesis of Fe2O3 on graphene matrix for indicator-free impedimetric aptasensing. Talanta 2013, 105, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zheng, D.; Tanaka, H.; Zhan, F.; Yuan, X.; Gao, F.; Wang, Q. An electrochemical sensor for gallic acid based on Fe2O3/electro-reduced graphene oxide composite: Estimation for the antioxidant capacity index of wines. Mater. Sci. Eng. C 2015, 57, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Gou, Y.; Ma, Y.; Zheng, X.; Bai, R.; Abdelmoaty, A.A.A.; Hu, F. Investigate electrochemical immunosensor of cortisol based on gold nanoparticles/magnetic functionalized reduced graphene oxide. Biosens. Bioelectron. 2017, 88, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Gou, X.; Bai, R.; Abdelmoaty, A.A.A.; Ma, Y.; Zheng, X.; Hu, F. Direct electrochemistry and electrocatalysis of lobetyolin via magnetic functionalized reduced graphene oxide film fabricated electrochemical sensor. Mater. Sci. Eng. C 2017, 74, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xue, G.; Zhang, J.; Wang, G.; Ye, B.C.; Sun, S.; Tian, L.; Li, Y. Simultaneous voltammetric determination of dopamine and uric acid using carbon-encapsulated hollow Fe3O4 nanoparticles anchored to an electrode modified with nanosheets of reduced graphene oxide. Microchim. Acta 2017, 184, 843–853. [Google Scholar] [CrossRef]
- Shi, X.; Lu, J.; Yin, H.; Qiao, X.; Xu, Z. A Biomimetic Sensor with Signal Enhancement of Ferriferrous Oxide-Reduced Graphene Oxide Nanocomposites for Ultratrace Levels Quantification of Methamidophos or Omethoate in Vegetables. Food Anal. Methods 2017, 10, 910–920. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Bishop, G.W.; Bhakta, S.; El-Sawy, A.; Suib, S.L.; Rusling, J.F. Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins. Biosens. Bioelectron. 2017, 91, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Pakapongpan, S.; Poo-arporn, R.P. Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater. Sci. Eng. C 2017, 76, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Movlaee, K.; Norouzi, P.; Beitollahi, H.; Rezapour, M.; Larijani, B. Highly selective differential pulse voltammetric determination of uric acid using modified glassy carbon electrode. Int. J. Electrochem. Sci. 2017, 12, 3241–3251. [Google Scholar] [CrossRef]
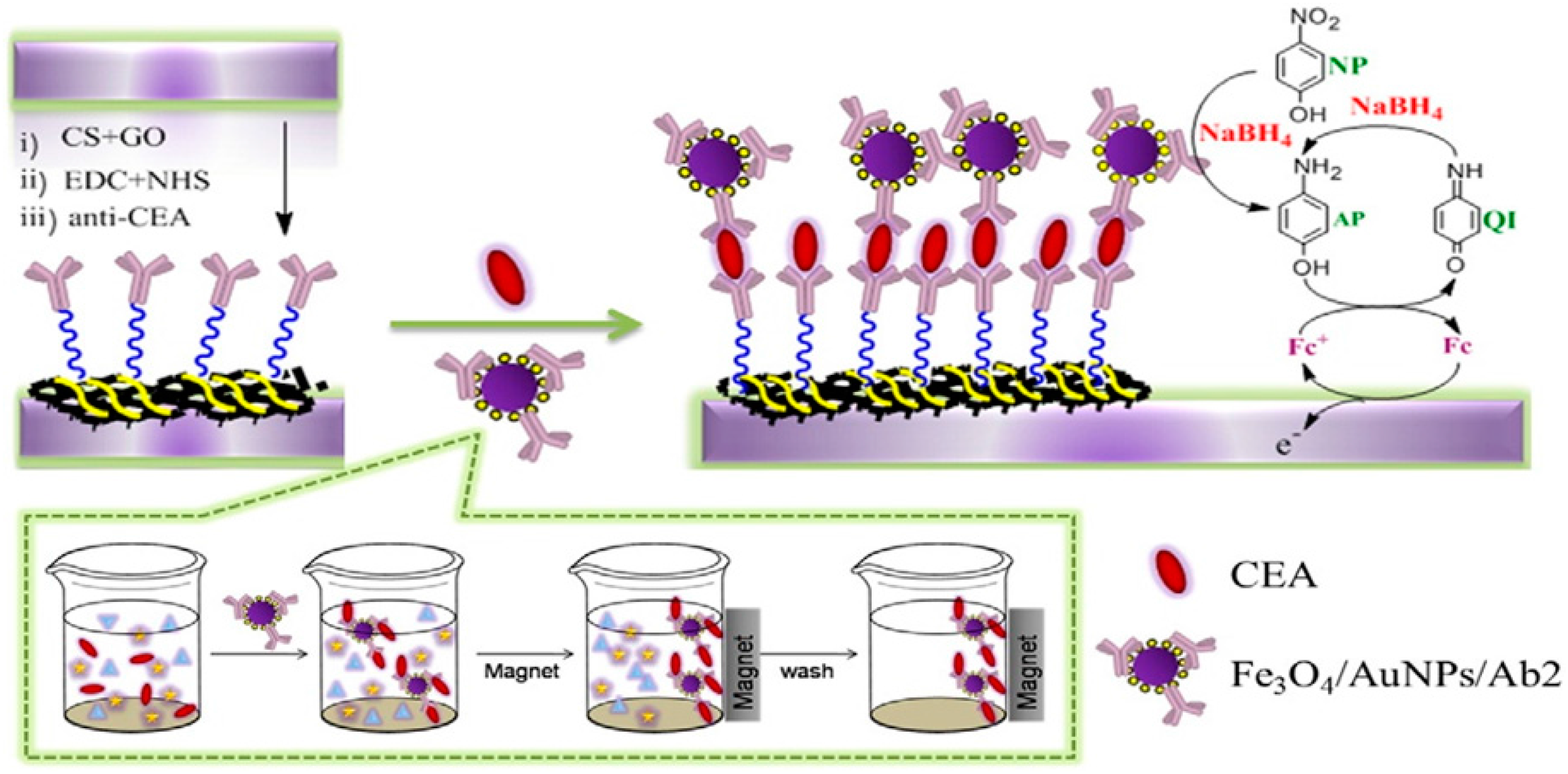
- Li, Y.; Zhang, Y.; Li, F.; Li, M.; Chen, L.; Dong, Y.; Wei, Q. Sandwich-type amperometric immunosensor using functionalized magnetic graphene loaded gold and silver core-shell nanocomposites for the detection of Carcinoembryonic antigen. J. Electroanal. Chem. 2017, 795, 1–9. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Mehrgardi, M.A. Amplified detection of leukemia cancer cells using an aptamer-conjugated gold-coated magnetic nanoparticles on a nitrogen-doped graphene modified electrode. Bioelectrochemistry 2017, 114, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Hou, W.; Fu, J.; Guo, Y.; Sun, X.; Wang, X.; Zhao, J. A nanostructured electrochemical aptasensor for highly sensitive detection of chlorpyrifos. Sens. Actuators B Chem. 2017, 243, 1164–1170. [Google Scholar] [CrossRef]
- Movlaee, K.; Ganjali, M.R.; Aghazadeh, M.; Beitollahi, H.; Hosseini, M.; Shahabi, S.; Norouzi, P. Graphene Nanocomposite Modified Glassy Carbon Electrode: As a Sensing Platform for Simultaneous Determination of Methyldopa and Uric Acid. Int. J. Electrochem. Sci. 2017, 12, 305–315. [Google Scholar] [CrossRef]
- He, B.; Yan, S. Electrochemical determination of sulfonamide based on glassy carbon electrode modified by Fe3O4/functionalized graphene. Int. J. Electrochem. Sci. 2017, 12, 3001–3011. [Google Scholar] [CrossRef]
- Derikvand, H.; Azadbakht, A. An Impedimetric Sensor Comprising Magnetic Nanoparticles–Graphene Oxide and Carbon Nanotube for the Electrocatalytic Oxidation of Salicylic Acid. J. Inorg. Organomet. Polym. Mater. 2017, 27, 901–911. [Google Scholar] [CrossRef]
- Chou, J.C.; Chen, J.S.; Liao, Y.H.; Lai, C.H.; Yan, S.J.; Huang, M.S.; Wu, T.Y. Fabrication and Characteristic Analysis for Enzymatic Glucose Biosensor Modified by Graphene Oxide and Magnetic Beads Based on Microfluidic Framework. IEEE Sens. J. 2017, 17, 1741–1748. [Google Scholar] [CrossRef]
- Chen, X.; Yan, H.; Shi, Z.; Feng, Y.; Li, J.; Lin, Q.; Wang, X.; Sun, W. A novel biosensor based on electro-co-deposition of sodium alginate-Fe3O4-graphene composite on the carbon ionic liquid electrode for the direct electrochemistry and electrocatalysis of myoglobin. Polym. Bull. 2017, 74, 75–90. [Google Scholar] [CrossRef]
- Chen, M.; Hou, C.; Huo, D.; Fa, H.; Zhao, Y.; Shen, C. A sensitive electrochemical DNA biosensor based on three-dimensional nitrogen-doped graphene and Fe3O4 nanoparticles. Sens. Actuators B Chem. 2017, 239, 421–429. [Google Scholar] [CrossRef]
- Bagheri, H.; Pajooheshpour, N.; Jamali, B.; Amidi, S.; Hajian, A.; Khoshsafar, H. A novel electrochemical platform for sensitive and simultaneous determination of dopamine, uric acid and ascorbic acid based on Fe3O4-SnO2-Gr ternary nanocomposite. Microchem. J. 2017, 131, 120–129. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Magnetic nanoparticles embedded with graphene quantum dots and multiwalled carbon nanotubes as a sensing platform for electrochemical detection of progesterone. Sens. Actuators B Chem. 2017, 238, 346–356. [Google Scholar] [CrossRef]
- Zhan, X.; Hu, G.; Wagberg, T.; Zhan, S.; Xu, H.; Zhou, P. Electrochemical aptasensor for tetracycline using a screen-printed carbon electrode modified with an alginate film containing reduced graphene oxide and magnetite (Fe3O4) nanoparticles. Microchim. Acta 2016, 183, 723–729. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hou, C.; Liu, M. Ultrasensitive electrochemical sensing of dopamine using reduced graphene oxide sheets decorated with p-toluenesulfonate-doped polypyrrole/Fe3O4 nanospheres. Microchim. Acta 2016, 183, 1145–1152. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Li, G.; Liu, Q.; Wei, T.; Li, B.; Jiang, C.; Sun, Y. Electrochemical detection of L-cysteine using a glassy carbon electrode modified with a two-dimensional composite prepared from platinum and Fe3O4 nanoparticles on reduced graphene oxide. Microchim. Acta 2016, 183, 3221–3228. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, G.; Chen, D.; Wang, Z.; Liu, G. A sensitive acetylcholinesterase biosensor based on screen printed electrode modified with Fe3O4 nanoparticle and graphene for chlorpyrifos determination. Int. J. Electrochem. Sci. 2016, 11, 10906–10918. [Google Scholar] [CrossRef]
- Soleymani, J.; Hasanzadeh, M.; Shadjou, N.; Jafari, M.K.; Gharamaleki, J.V.; Yadollahi, M.; Jouyban, A. A new kinetic-mechanistic approach to elucidate electrooxidation of doxorubicin hydrochloride in unprocessed human fluids using magnetic graphene based nanocomposite modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 61, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Hoseini, S.J.; Azadpour, M.; Heidari, V.; Bahrami, M.; Maddahfar, M. Electrocatalytic oxidation behavior of NADH at Pt/Fe3O4/reduced-graphene oxide nanohybrids modified glassy carbon electrode and its determination. Mater. Sci. Eng. C 2016, 67, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Rani, G.J.; Babu, K.J.; kumar, G.G.; Rajan, M.A.J. Watsonia meriana flower like Fe3O4/reduced graphene oxide nanocomposite for the highly sensitive and selective electrochemical sensing of dopamine. J. Alloys Compd. 2016, 688, 500–512. [Google Scholar] [CrossRef]
- Peng, D.; Liang, R.P.; Huang, H.; Qiu, J.D. Electrochemical immunosensor for carcinoembryonic antigen based on signal amplification strategy of graphene and Fe3O4 Au NPs. J. Electroanal. Chem. 2016, 761, 112–117. [Google Scholar] [CrossRef]
- Nejad, F.G.; Beitollahi, H.; Shaker, S. Magnetic core–shell Fe3O4@SiO2/graphene nanocomposite modified carbon paste electrode for voltammetric determination of ascorbic acid in the presence of L-cysteine. Anal. Bioanal. Electrochem. 2016, 8, 318–328. [Google Scholar]
- He, B.; Du, G. Electrochemical detection of nitrofuranzone and its metabolite using glassy carbon electrode modified by Fe3O4 functionalized graphene. Int. J. Electrochem. Sci. 2016, 11, 8546–8560. [Google Scholar] [CrossRef]
- Han, S.; Du, T.; Lai, L.; Jiang, X.; Cheng, C.; Jiang, H.; Wang, X. Highly sensitive biosensor based on the synergistic effect of Fe3O4-Co3O4 bimetallic oxides and graphene. RSC Adv. 2016, 6, 82033–82039. [Google Scholar] [CrossRef]
- Fei, J.; Dou, W.; Zhao, G. Amperometric immunoassay for the detection of Salmonella pullorum using a screen—Printed carbon electrode modified with gold nanoparticle-coated reduced graphene oxide and immunomagnetic beads. Microchim. Acta 2016, 183, 757–764. [Google Scholar] [CrossRef]
- Arvand, M.; Abbasnejad, S.; Ghodsi, N. Graphene quantum dots decorated with Fe3O4nanoparticles/functionalized multiwalled carbon nanotubes as a new sensing platform for electrochemical determination of l-DOPA in agricultural products. Anal. Methods 2016, 8, 5861–5868. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, X.Y.; Zhang, H.; Liu, M.; Wang, Q.; Zhang, X.; Shen, Y.; Yang, F. Fabrication of a Modified Electrode Based on Fe3O4-Graphene Oxide Hybrid Composite: Applying to Simultaneous Determination of Adenine and Guanine in DNA. Electroanalysis 2015, 27, 2201–2208. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hou, C.; Liu, M. Magnetic Fe3O4@MOFs decorated graphene nanocomposites as novel electrochemical sensor for ultrasensitive detection of dopamine. RSC Adv. 2015, 5, 98260–98268. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Wang, X.; Pang, X.; Wu, D.; Du, B.; Wei, Q. Novel signal amplification strategy for ultrasensitive sandwich-type electrochemical immunosensor employing Pd-Fe3O4-GS as the matrix and SiO2 as the label. Biosens. Bioelectron. 2015, 74, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Zhang, H.; Huo, J.; Lin, X. An electrochemical sensor based on iron(ii,iii)@graphene oxide@molecularly imprinted polymer nanoparticles for interleukin-8 detection in saliva. Anal. Methods 2015, 7, 7784–7791. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Li, L.; Duan, H.; Luo, C. Electrochemical sensor based on magnetic graphene oxide@gold nanoparticles-molecular imprinted polymers for determination of dibutyl phthalate. Talanta 2015, 131, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Erogul, S.; Bas, S.Z.; Ozmen, M.; Yildiz, S. A new electrochemical sensor based on Fe3O4 functionalized graphene oxide-gold nanoparticle composite film for simultaneous determination of catechol and hydroquinone. Electrochim. Acta 2015, 186, 302–313. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, Y.T.; Tsai, R.Y.; Chen, M.C.; Chen, S.L.; Xiao, M.C.; Chen, C.L.; Hua, M.Y. A sensitive and selective magnetic graphene composite-modified polycrystalline-silicon nanowire field-effect transistor for bladder cancer diagnosis. Biosens. Bioelectron. 2015, 66, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Benvidi, A.; Jahanbani, S.; Akbari, A.; Zare, H.R. Simultaneous determination of hydrazine and hydroxylamine on a magnetic bar carbon paste electrode modified with reduced graphene oxide/Fe3O4 nanoparticles and a heterogeneous mediator. J. Electroanal. Chem. 2015, 758, 68–77. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Hashemi, P.; Ghanei, M. Simultaneous and sensitive determination of melatonin and dopamine with Fe3O4 nanoparticle-decorated reduced graphene oxide modified electrode. RSC Adv. 2015, 5, 21659–21669. [Google Scholar] [CrossRef]
- Yang, X.; Wu, F.; Chen, D.Z.; Lin, H.W. An electrochemical immunosensor for rapid determination of clenbuterol by using magnetic nanocomposites to modify screen printed carbon electrode based on competitive immunoassay mode. Sens. Actuators B Chem. 2014, 192, 529–535. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Ma, H.; Li, Y.; Cao, W.; Du, B.; Wei, Q. Magnetic electrode-based label-free electrochemical impedance spectroscopy immunosensor for sensitive detection of human malignant melanoma markers using gold nanoparticles functionalized magnetic graphene sheets as signal amplifier. RSC Adv. 2014, 4, 59106–59113. [Google Scholar] [CrossRef]
- Peik-See, T.; Pandikumar, A.; Nay-Ming, H.; Hong-Ngee, L.; Sulaiman, Y. Simultaneous electrochemical detection of dopamine and ascorbic acid using an iron oxide/reduced graphene oxide modified glassy carbon electrode. Sensors (Switz.) 2014, 14, 15227–15243. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Cai, W.; Du, H.; Ye, J. Fe3O4 microspheres and graphene oxide encapsulated with chitosan: A new platform for sensitive determination of hydroquinone and catechol. Electroanalysis 2014, 26, 216–222. [Google Scholar] [CrossRef]
- He, G.; Yang, X.; Hu, Y.; Hu, Y.; Zhang, F. A sensitive and selective amperometric immunosensor for chloramphenicol detection based on magnetic nanocomposites modify screen-printed carbon electrode as a disposable platform. Int. J. Electrochem. Sci. 2014, 9, 6962–6974. [Google Scholar]
- Hasanzadeh, M.; Pournaghi-Azar, M.H.; Shadjou, N.; Jouyban, A. A new mechanistic approach to elucidate furosemide electrooxidation on magnetic nanoparticles loaded on graphene oxide modified glassy carbon electrode. RSC Adv. 2014, 4, 6580–6590. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Ji, L.; Shi, Z.; Liu, Y. Electrocatalytic oxidation and determination of synthetic food dyes at iron/nickel oxide nanoparticles-graphene based sensor. Sens. Lett. 2014, 12, 75–83. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Qi, Q.; Ding, J.; Gao, X.; Zhang, Y.; Sun, Y. Electrocatalytic oxidation and detection of N-acetylcysteine based on magnetite/reduced graphene oxide composite-modified glassy carbon electrode. Electrochim. Acta 2013, 111, 31–40. [Google Scholar] [CrossRef]
- Luo, S.X.; Wu, Y.H.; Gou, H. Electrocatalysis and sensitive determination of Sudan I at Fe3O4/graphene modified glassy carbon electrodes. Appl. Mech. Mater. 2013, 401–403, 775–778. [Google Scholar]
- Han, J.; Zhuo, Y.; Chai, Y.; Yu, Y.; Liao, N.; Yuan, R. Electrochemical immunoassay for thyroxine detection using cascade catalysis as signal amplified enhancer and multi-functionalized magnetic graphene sphere as signal tag. Anal. Chim. Acta 2013, 790, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhou, Y.; Ma, Q.; Ai, S.; Chen, Q.; Zhu, L. Electrocatalytic oxidation behavior of guanosine at graphene, chitosan and Fe3O4 nanoparticles modified glassy carbon electrode and its determination. Talanta 2010, 82, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wu, H.; Wu, B.; Wang, Z.; Cao, H.; Fu, C.; Jia, N. Magnetic Fe3O4-Reduced Graphene Oxide Nanocomposites-Based Electrochemical Biosensing. Nano-Micro Lett. 2014, 6, 258–267. [Google Scholar] [CrossRef]
- Naghib, S.M.; Rahmanian, M.; Keivan, M.A.; Asiaei, S.; Vahidi, O. Novel magnetic nanocomposites comprising reduced graphene oxide/Fe3O4/gelatin utilized in ultrasensitive non- enzymatic biosensing. Int. J. Electrochem. Sci. 2016, 11, 10256–10269. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.; Atanasov, P. Glucose monitoring: State of the art and future possibilities. Med. Eng. Phys. 1996, 18, 273–288. [Google Scholar] [CrossRef]
- Yang, H.W.; Hua, M.Y.; Chen, S.L.; Tsai, R.Y. Reusable sensor based on high magnetization carboxyl-modified graphene oxide with intrinsic hydrogen peroxide catalytic activity for hydrogen peroxide and glucose detection. Biosens. Bioelectron. 2013, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yan, Y.; Yang, X.; Qian, J.; Cai, J.; Wang, K. Fe3O4-functionalized graphene nanoribbons: Preparation, characterization and improved electrochemical activity. J. Electroanal. Chem. 2013, 704, 86–89. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Zhang, Y.; Wang, P.; Wei, Q.; Du, B. Sensitive electrochemical sensor for simultaneous determination of dopamine, ascorbic acid and uric acid enhanced by amino-group functionalized mesoporous Fe3O4@Graphene sheets. Electrochim. Acta 2014, 116, 244–249. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Liu, Z.; Guo, Y.; Li, C.; Dong, C.; Shuang, S. An ultra-sensitive sensor based on β-cyclodextrin modified magnetic graphene oxide for detection of tryptophan. J. Electroanal. Chem. 2016, 781, 363–370. [Google Scholar] [CrossRef]
- Omidinia, E.; Shadjou, N.; Hasanzadeh, M. (Fe3O4)-graphene oxide as a novel magnetic nanomaterial for non-enzymatic determination of phenylalanine. Mater. Sci. Eng. C 2013, 33, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Karimzadeh, A.; Shadjou, N.; Mokhtarzadeh, A.; Bageri, L.; Sadeghi, S.; Mahboob, S. Graphene quantum dots decorated with magnetic nanoparticles: Synthesis, electrodeposition, characterization and application as an electrochemical sensor towards determination of some amino acids at physiological pH. Mater. Sci. Eng. C 2016, 68, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, L.; Zheng, X. Indicator-free electrochemical genosensing originated from the self-signal of poly-xanthurenic acid enhanced by Fe3O4/reduced graphene oxide. J. Solid State Electrochem. 2014, 18, 2367–2373. [Google Scholar] [CrossRef]
- Jahanbani, S.; Benvidi, A. A novel electrochemical DNA biosensor based on a modified magnetic bar carbon paste electrode with Fe3O4 NPs-reduced graphene oxide/PANHS nanocomposite. Mater. Sci. Eng. C 2016, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Jing, Y.F.; Wang, Z.L.; Zhan, H.J.; Shen, Q. Highly sensitive electrochemical biosensing of methyl parathion pesticide based on acetylcholinesterase immobilized onto graphene-Fe3O4 Nanocomposite. Sens. Lett. 2013, 11, 531–538. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Mao, K.; Li, Y.; Du, B.; Zhang, Y.; Wei, Q. Electrochemical immunosensor for α-fetoprotein detection using ferroferric oxide and horseradish peroxidase as signal amplification labels. Anal. Biochem. 2014, 465, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Si, Y.; Sun, Z.; Li, S.; Lin, Y. Recyclable enzyme mimic of cubic Fe3O4 nanoparticles loaded on graphene oxide-dispersed carbon nanotubes with enhanced peroxidase-like catalysis and electrocatalysis. J. Mater. Chem. B 2014, 2, 4442–4448. [Google Scholar] [CrossRef]
- Wu, D.; Guo, Z.; Liu, Y.; Guo, A.; Lou, W.; Fan, D.; Wei, Q. Sandwich-type electrochemical immunosensor using dumbbell-like nanoparticles for the determination of gastric cancer biomarker CA72–4. Talanta 2015, 134, 305–309. [Google Scholar] [CrossRef] [PubMed]


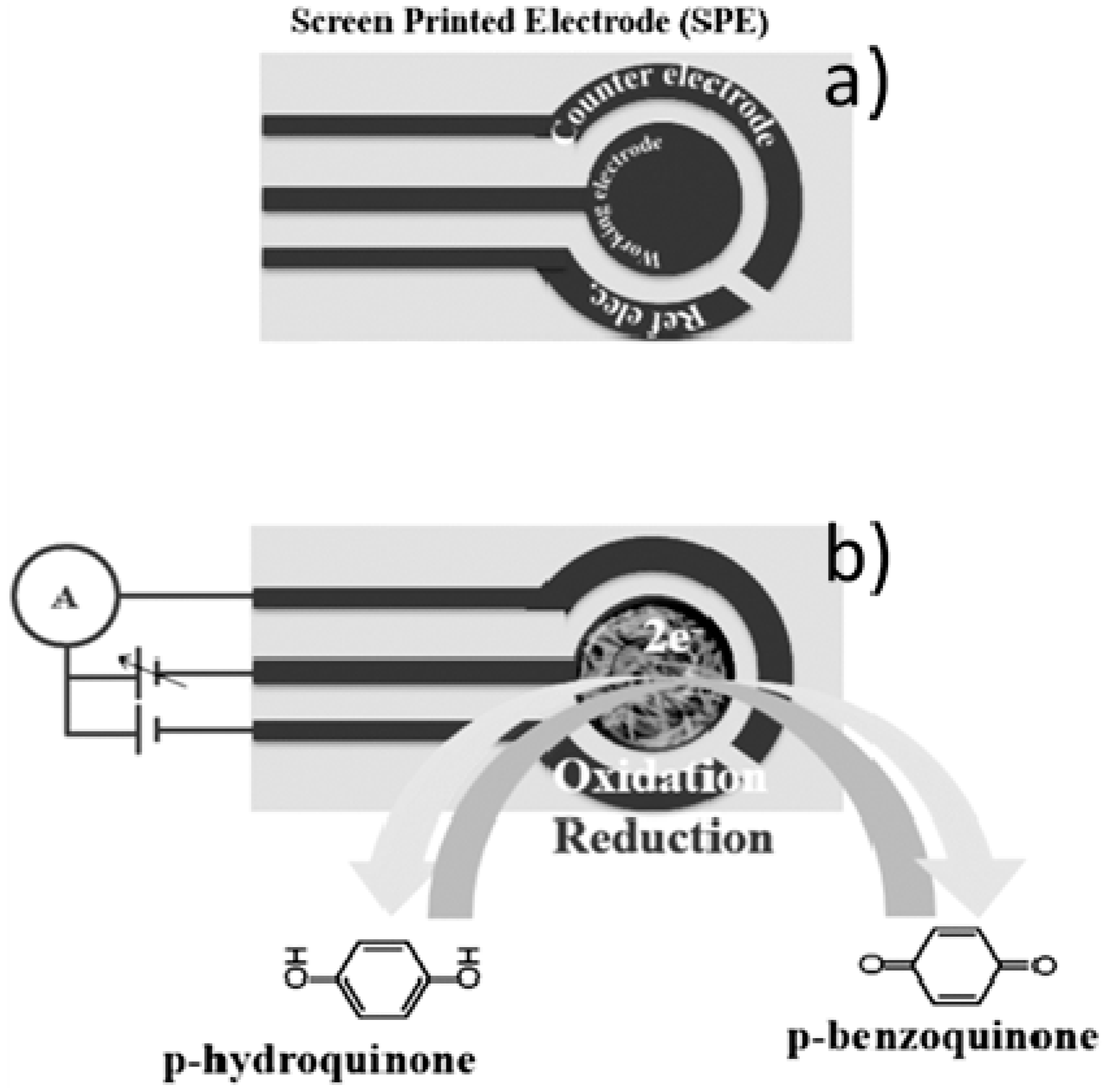
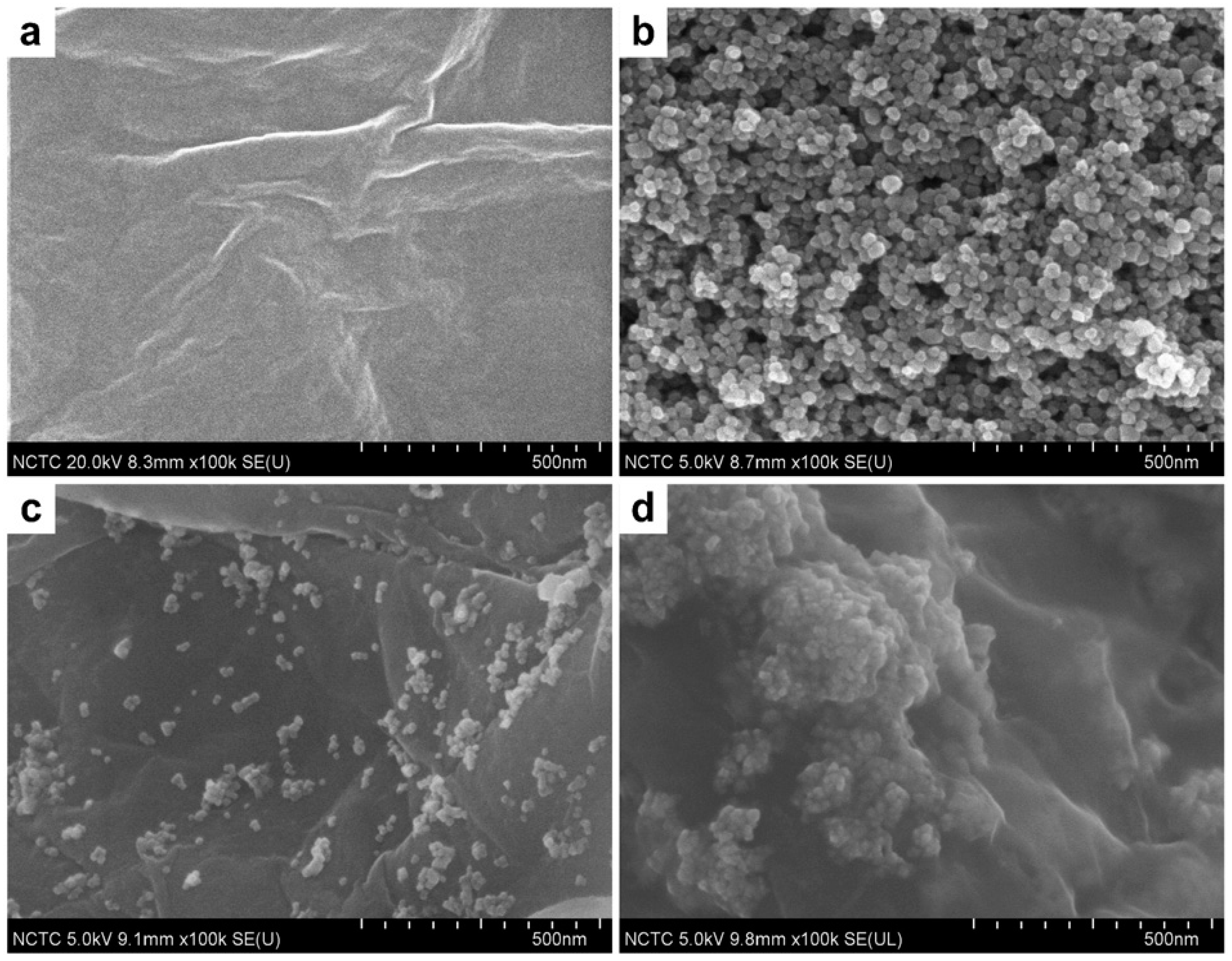
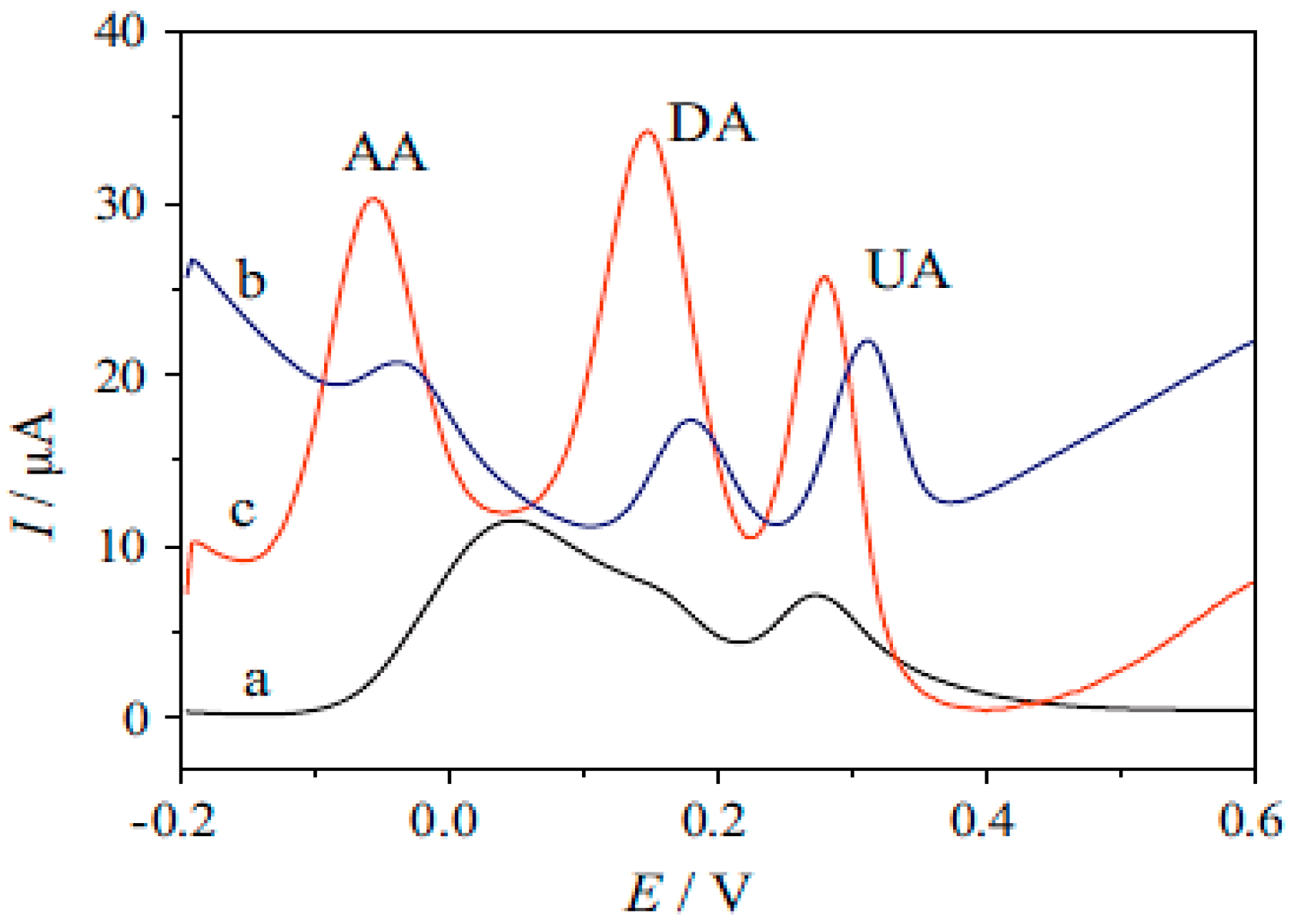
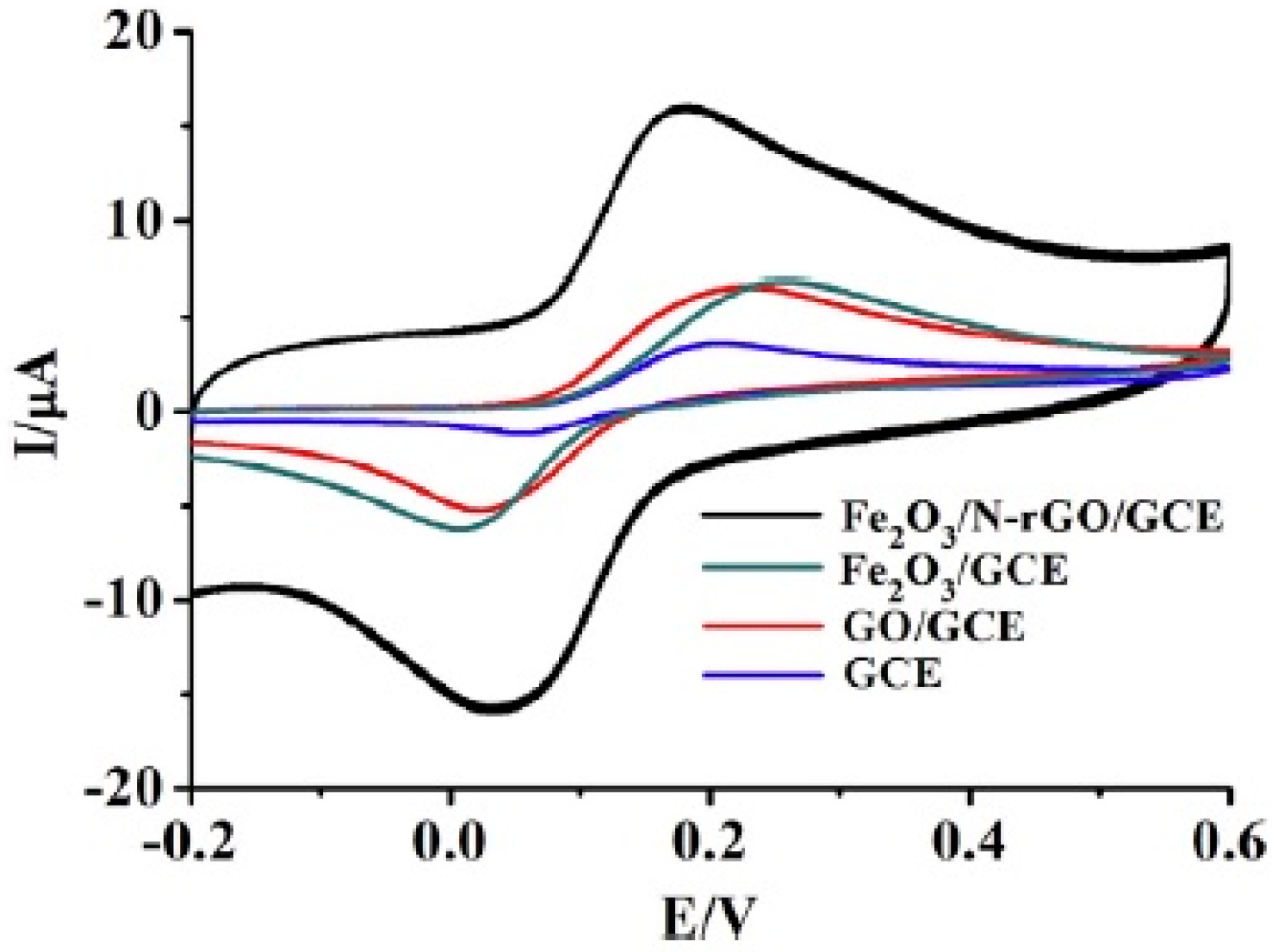
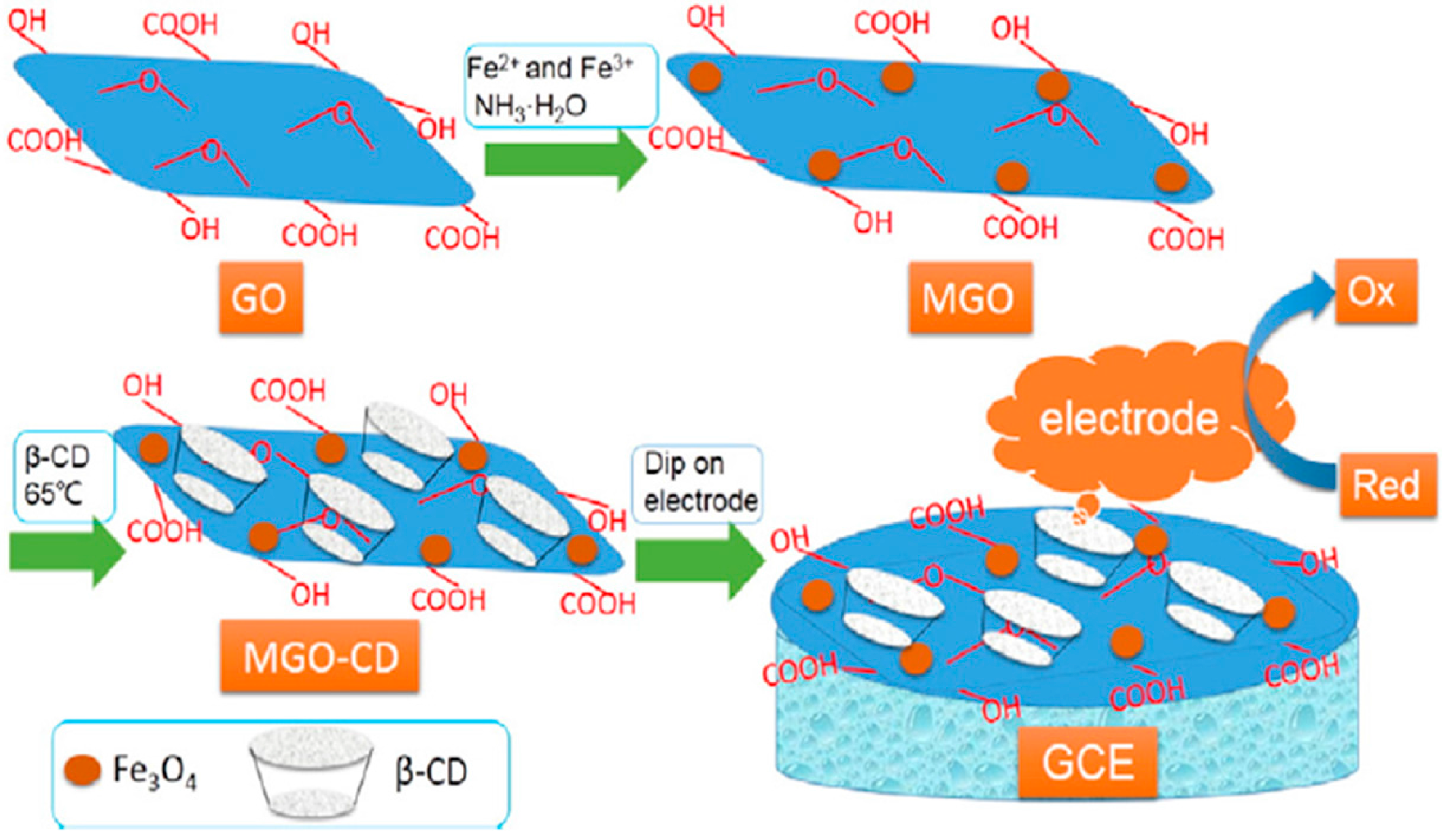
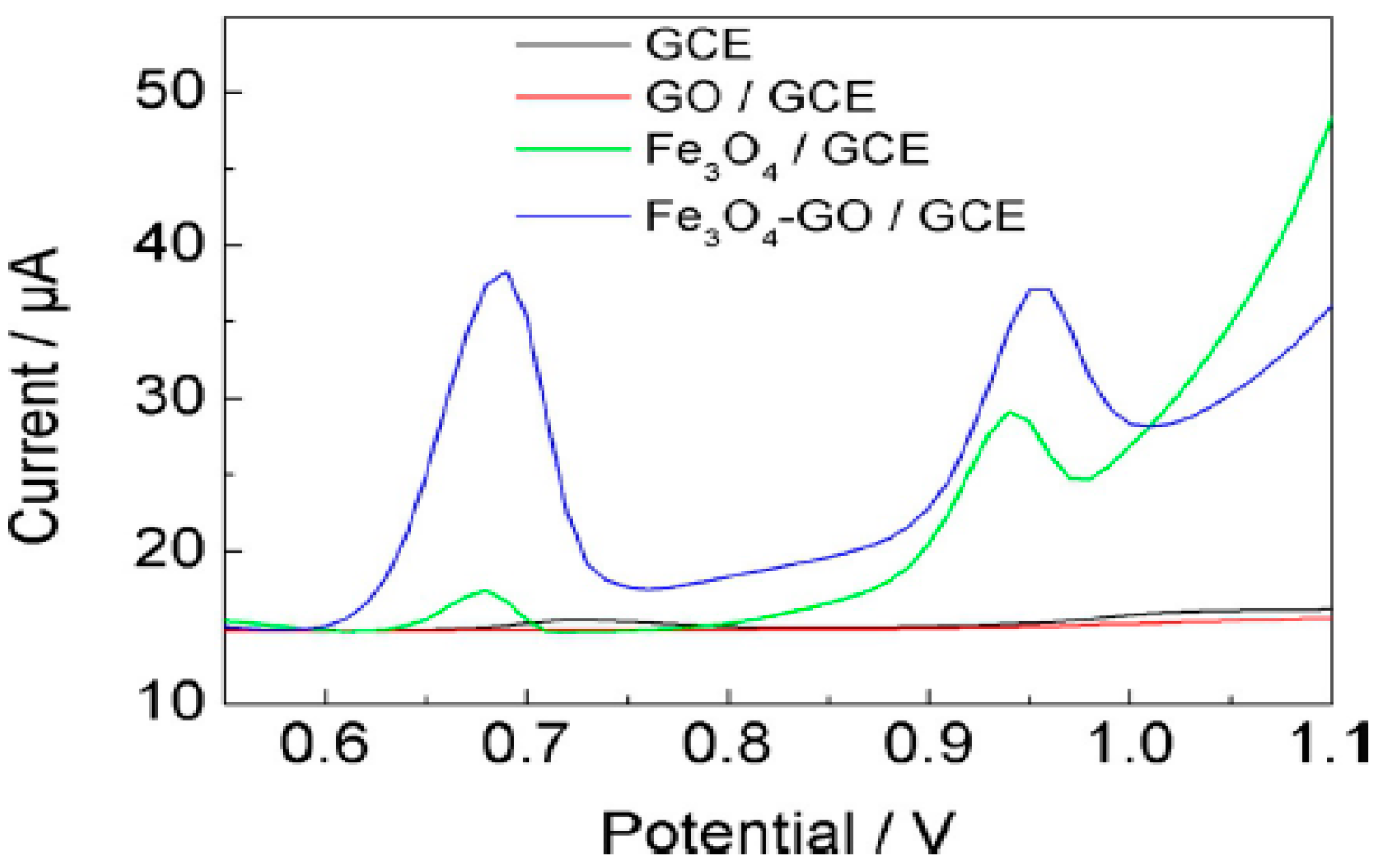
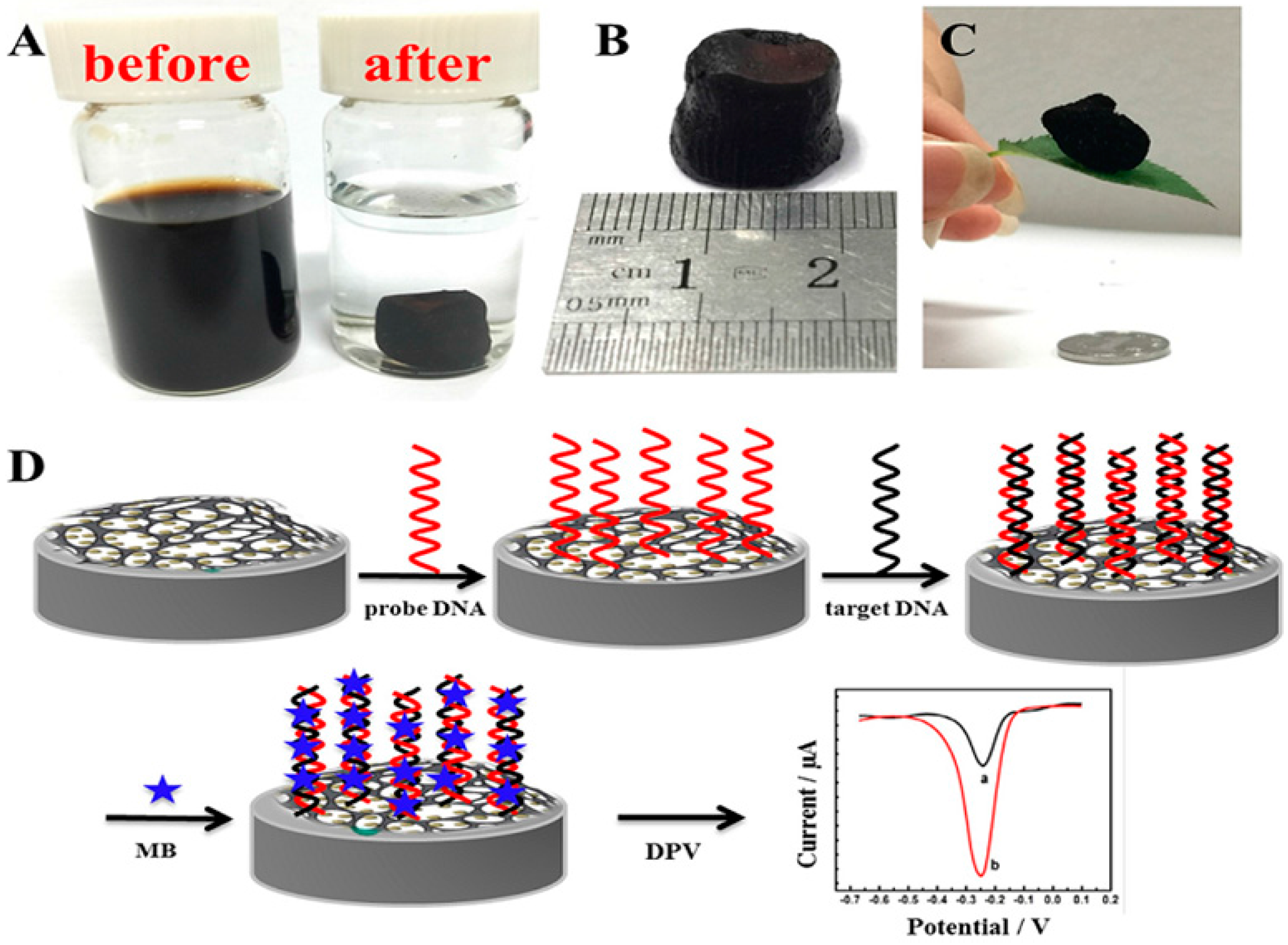
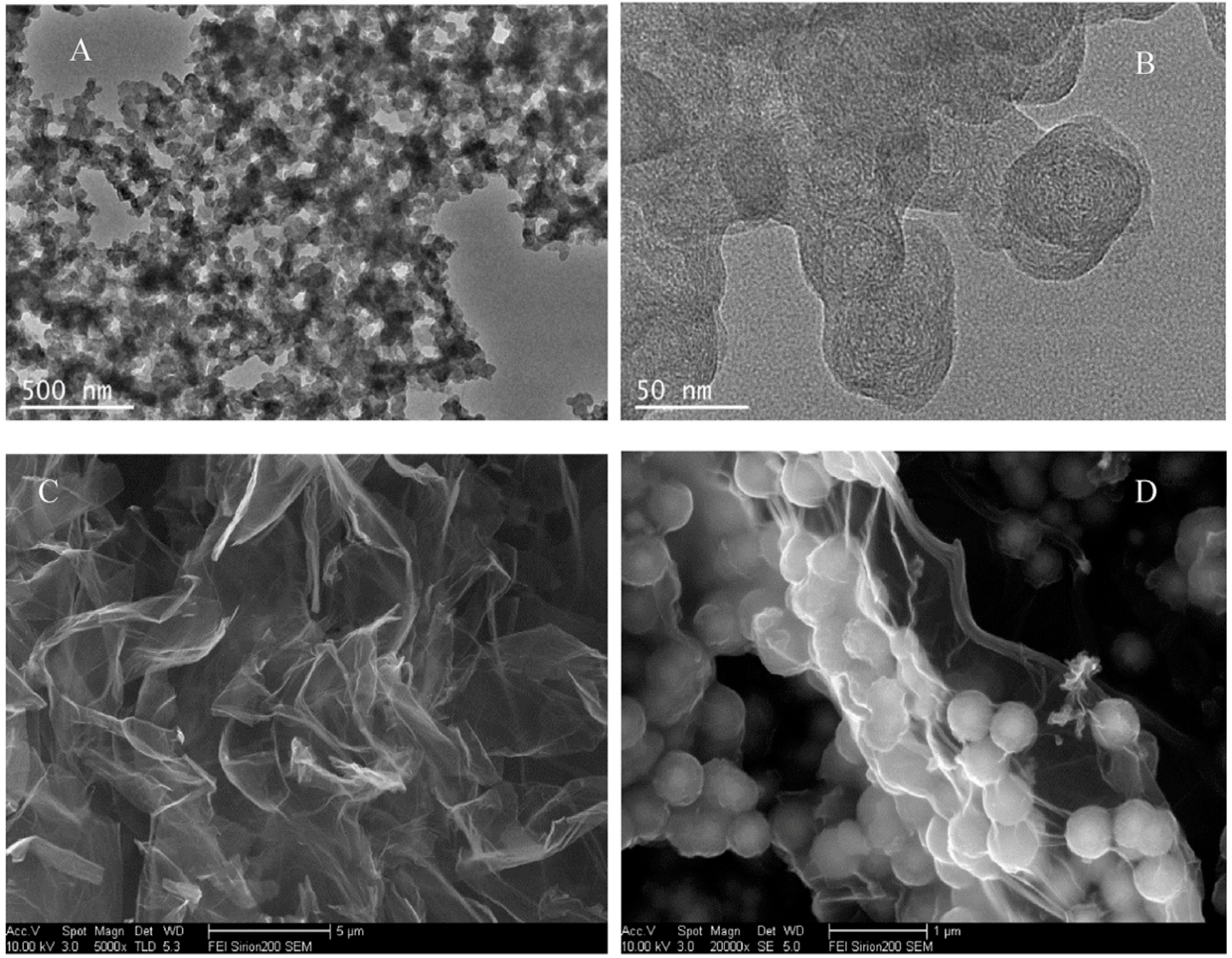


| Method | Effective Parameters | Advantages | Disadvantages |
|---|---|---|---|
| Hydrothermal | Pressure temperature reaction time type and concentration of precursors | environmental friendly versatile reactants have elevated reactivity morphology of products can be altered easily producing IONs with good crystallization | need to costly autoclave safety issues usually polydisperse samples are obtained |
| Sol-gel | rates of condensation and hydrolysis composition of media type and concentration of reactants temperature pH | homogenous and high adhesion products low temperature processing Desired shape and length can obtain | product contains sol–gel matrix components metal alkoxides are almost expensive safety measurements should be considered since large amount of alcohol is released in calcination step |
| Coprecipitation | concentration of cations counterions pH ionic strength temperature | simplest, cheapest and environmental friendly since the surface of produced IONs using this method contains a great number of hydrophilic ligands, they can be well dispersed in polar solutions, e.g., water | limited by the boiling point of water usually produced iron oxide under these situations shows high polydispersity as well as low crystallinity |
| Microemulsion | water to surfactant ratio amount of reactants (particularly surfactant) temperature pH surfactant film flexibility | uniform properties narrow pore size distribution | surfactants are difficult to remove difficulty in scale-up procedures |
| Iron Oxide | Composite | Analyte(s) | Linear Dynamic Range (LDR) | Limit of Detection (LOD) | Stability | Real Sample(s) | Reference |
|---|---|---|---|---|---|---|---|
| Fe2O3 | Fe2O3/N-rGO 1 | Dopamine | 0.5 μM to 0.34 mM | 0.49 μM | 82% of its initial current response after twenty days | Dopamine hydrochloride injection | [13] |
| Fe2O3-rGO | Honokiol | 1.5 × 10−8~3.3 × 10−5 M | 9.64 × 10−9 M | - | Traditional Chinese medicine | [15] | |
| Magnolol | 7.5 × 10−8~2.6 × 10−5 M | 1.05 × 10−8 M | |||||
| Fe2O3/rGO | AA 2 | 0.57–3.97 mM | 0.543 μM | 85.3% of the initial currents after 2 weeks | - | [64] | |
| PEDOT 3-rGO-Fe2O3 | Catechol | 4 × 10−8 to 6.20 × 10−5 M | 7 × 10−9 M | More than 75 days | Green tea | [65] | |
| NSG-Fe2O3 4 | Dopamine | 0.3–210 μM | 0.035 μM | 91% of initial current after 7 days | Urine sample | [66] | |
| Fe2O3/N-rGO | l-cysteine | 0.2–400 μM | 0.1 μM | 90.6% of initial current after 7 days | Syrup | [67] | |
| Fe2O3/rGO | Rutin | 1.5 × 10−8 to 1.8 × 10−5 M | 9.8 × 10−9 M | - | Tablets | [68] | |
| PANi 5-Fe2O3-rGO | Hydroquinone | 1.0 × 10−7–5.5 × 10−4 M | 6.0 × 10−8 M | 95.17% of the initial currents after 3 weeks | Tab water | [69] | |
| GS-Fe2O3-CTAB 6 | Bisphenol A | 5.0 × 10−9–1.0 × 10−6 M | 2.5 nM | - | Water samples | [70] | |
| Fe2O3/rGO | Lysozyme | 0.5 ng·mL−1–5 μg·mL−1 | 0.16 ng·mL−1 | 95.52% of initial current after 10 days | - | [71] | |
| Fe2O3-rGO/CS 7 | Gallic acid | 1.0 × 10−6 to 1.0 × 10−4 M | 1.5 × 10−7 M | More than one week | Red and white wines | [72] | |
| Fe3O4 | AuNPs/MrGO 8 | Cortisol | 0.1 to 1000 ng/mL | 0.05 ng/mL | 90.16% of initial current after 20 days | Human serum | [73] |
| Fe3O4-rGO/nafion | Lobetyolin | 1.0 × 10−7–1.0 × 10−4 M | 4.3 × 10−8 M | 93.62% of initial current after 14 days | Radix Codonopsis | [74] | |
| H-Fe3O4@C/GNS 9 | Dopamine | 0.1 to 150 μM | 0.053 μM | More than 15 days | Rat brain tissue and urine | [75] | |
| Uric acid | 1.0 to 100 μM | 0.41 μM | |||||
| (Fe3O4/rGO) and MIL@MIP 10 | Methamidophos and Omethoate | 1.0 × 10−7–1.0 × 10−12 M and 1.0×10−7–1.0×10−13 M | 2.67 × 10−13 M and 2.05 × 10−14 M | 94.5% of initial current after 15 days | Cucumber and kidney bean samples | [76] | |
| Fe3O4-GO | PSA and PSMA 11 | 1.25–1000 pg/mL and 9.7–5000 pg/mL | 1.25 pg/mL and 9.7 pg/mL | 80% of initial current after 4 days | Prostate cancer patient serum samples | [77] 12 | |
| Fe3O4-rGO | Glucose | 0.05 to 1 mM | 0.1 μM | 95.6% of initial current after one month | - | [78] | |
| Fe3O4-SiO2/GO | Uric acid | 0.5 to 250.0 μM | 0.07 μM | - | Urine sample | [79] | |
| GS-Fe3O4/Au@Ag 13 | CEA 14 | 0.1 pg/mL to 100 ng/mL | 0.0697 pg/mL | More than 2 weeks | Human serum samples | [80] | |
| AuM/N-rGO 15 | Leukemia cancer cells | 10 to 1 × 106 cell mL−1 | 10 cell mL−1 | - | Human blood plasma | [81] | |
| CB/Fe3O4-GO 16 | Chlorpyrifos | 0.1–105 ng/mL | 0.033 ng/mL | 91.2% of initial current after 20 days | Leafy vegetable | [82] | |
| Fe3O4@SiO2/GO | Methyldopa (MD) | 0.1–400.0 µM | 86.0 nM | - | MD tablet and urine samples | [83] | |
| Fe3O4-rGO | Sulfonamide | 5 × 10−7~1.1 × 10−4 M | 5.0 × 10−8 M | [84] | |||
| Fe3O4-GO/carbon nanotube | Salicylic acid | 5.00 to 155 µM | 900 nM | - | Water sample | [85] | |
| magnetic bead-GO/IGZO 17 | Glucose | 3–7 mM | - | - | - | [86] | |
| Nafion/Mb-SA-Fe3O4-GR/CILE 18 | Trichloroacetic acid | 1.4 to 119.4 mM | 0.174 mM | - | - | [87] | |
| 3D NG-Fe3O4 | DNA | 1.0 × 10−14 to 1.0 × 10−6 M | 3.63 × 10−15 M | 90% of initial current after 2 weeks | Serum samples | [88] | |
| Fe3O4-SnO2-Gr | AA | 0.1 to 23.00 μM | 62.0 nM | More than 4 weeks | Biological fluids—pharmaceutical samples | [89] | |
| DA | 0.02 to 2.8 μM | 7.1 nM | |||||
| UA | 0.015 to 2.40 μM | 5.0 nM | |||||
| Fe3O4@GQD/f-MWCNTs | Progesterone | 0.01–0.5 and 0.5–3.0 μM | 2.18 nM | 85% of initial current after 6 weeks | Serum samples—pharmaceutical products | [90] | |
| Alginate/Fe3O4-rGO | Tetracycline | 1 nM to 5 μM | 0.6 nM | 95.86% of initial current after 2 weeks | Food, environmental and clinical samples | [91] | |
| TSA-doped PPy/Fe3O4/rGO | Dopamine | 7.0–2.0 μM | 2.33 nM | More than 10 days | Urine and serum samples | [92] | |
| Pt-Fe3O4/rGO | Cysteine | 0.10 to 1.0 mM | 10 μM | More than 2 weeks | - | [93] | |
| Fe3O4-rGO | Chlorpyrifos | 0.05 to 100 μg/L | 0.02 μg/L | - | Vegetable samples | [94] | |
| PS/Fe3O4-GO-SO3H 19 | Doxorubicin | 4.3×10−8 to 3.5×10−6 M | 4.9 nM, 14 nM and 4.3 nM | - | Plasma, cerebrospinal fluid, urine | [95] | |
| 8.6×10−7 to 13×10−6 M | |||||||
| 2.6×10−8 to 3.5×10−6 M | |||||||
| Pt/Fe3O4/rGO | NADH 20 | 0.03–1.5 nM | 5 nM | - | - | [96] | |
| Fe3O4-rGO | Dopamine | 0.010 and 0.270 μM | 5 nM | 93.5% of initial current after 30 days | Urine sample | [97] | |
| GO/CS-Fc 21 | CEA 22 | 0.001–30 ng·mL−1 | 0.39 pg | - | Human serum | [98] | |
| ILFSGo 23 | Ascorbic acid | 1.0 × 10−6 to 9.0×10−4 M | 2.3 × 10−7 M | - | - | [99] | |
| Fe3O4-rGO | Nitrofuranzone | 1.0 × 10−5 to 1.09×10−4 M | 2.92 × 10−7 M | - | - | [100] | |
| Semicarbazide | 1.0 × 10−6 to 1.09×10−4 M | 6.17 × 10−7 M | |||||
| Fe3O4-Co3O4/rGO | Dopamine | 5 × 10−7 to 1.55×10−3 M | 1.3 × 10−7 M | More than 2 weeks | Human serum samples | [101] | |
| Uric acid | 1.5 × 10−6 to 1.6 × 10−3 M | 1.8 × 10−7 M | |||||
| rGO/AuNP/Ab2/S/IMB 24 | Salmonella pullorum | 102 to 106 CFU·mL−1 | 89 CFU·mL−1 | - | Chicken liver | [102] | |
| GQD-Fe3O4/CNT 25 | L-DOPA | 3.0 to 400 μM | 14.3 nM | - | Seeds and fava bean | [103] | |
| Fe3O4-rGO | Adenine | 0.05–25 μM | 4 nM | More than 20 days | Fish, urine samples and vitamin B4 tablet | [104] | |
| Guanine | 0.05–25 μM | 3 nM | |||||
| Fe3O4 @ZIF-8 26/RGO | Dopamine | 2.0 × 10−9 to 1.0 × 10−5 M | 6.67 × 10−10 M | More than 10 days | Urine and serum samples | [105] | |
| Pd–Fe3O4-GS | immunoglobulin G | 5 × 10−6 to 5 ng/mL | 3.2 fg/mL | More than one month | Human serum samples | [106] | |
| Fe3O4-GO/MIP 27 | interleukin-8 | 0.1 to 10 pM | 0.04 pM | 92.9% of initial current after 1 month | Saliva | [107] | |
| Fe3O4-GO@AuNPs-MIP | Dibutyl phthalate | 2.5 × 10−9 to 5.0 × 10−6 M | 8×10−10 M | 96.3% of initial current after 4 weeks | Drink samples | [108] | |
| AuNPs/Fe3O4-APTES 28-GO | Catechol | 2–145 μM | 0.8 μM | 90% of initial current after 5 days | Tap water | [109] | |
| Hydroquinone | 3–137 μM | 1.1 μM | |||||
| MGLA 29 | APOA2 protein 30 | 0.19 to 1.95 μg·mL−1 | 6.7 pg·mL−1 | About 80% decrease after one week | Human urine | [110] | |
| DPSPP 31/rGO/Fe3O4 | Hydrazine | 120.0–600.0 nM | 40.0 nM | - | Water samples | [111] | |
| Hydroxylamine | 10–155.0 μM | 3.4 μM | |||||
| rGO/Fe3O4 | Melatonin | 0.02–5.80 μM | 8.40 × 10−6 M | - | Pharmaceutical and biological fluids | [112] | |
| Dopamine | 0.02–5.80 μM | 6.50 × 10−6 M | |||||
| GS-Nf 32/Au-Fe3O4 | Clenbuterol | 0.5 ng·mL−1 to 200 ng·mL−1 | 0.22 ng/mL | 92% of initial current after 4 weeks | Pork sample | [113] | |
| GS-Au-Fe3O4 | 146 antigen (CD146) | 5 pg·mL−1 to 500 ng·mL−1 | 2.5 pg·mL−1 | More than 2 months | Human serum samples | [114] | |
| rGO/Fe3O4 | Ascorbic acid | 1–9 mM | 0.42 μM | - | - | [115] | |
| Dopamine | 0.5–100 μM | 0.12 μM | |||||
| CS-Fe3O4-GO | Hydroquinone | 1.5 to 150 μM | 20 nM | 95% of initial current after 2 weeks | Tap water | [116] | |
| Catechol | 1 to 410 μM | 250 nM | |||||
| GS-Nf/Au-Fe3O4 | chloramphenicol | 2.0 ng/mL to 200.0 ng/mL | 0.82 ng/mL | 93.2% of initial current after one month | Milk sample | [117] | |
| Fe3O4-GO-SO3H | Furosemide | 20–100 μM (serum) | 0.1 μM | 92.5% of initial current after 20 days | Human serum and urine | [118] | |
| 18–720 μM (Urine) | 0.11 μM | ||||||
| Iron/nickel oxide nanoparticles-graphene | Orange II | 5.0 Nm–3.0 μM | 2.0 nM (for all) | - | Different kinds of food samples | [119] | |
| Allura red and Amaranth | 5.0 nM–10.0 μM and 5.0 nM–5.0 μM | ||||||
| rGO/Fe3O4 | N-acetylcysteine | 0.10–10.0 mM | 11.1 mM | 93.8% of initial current after 2 weeks | - | [120] | |
| rGO/Fe3O4 | Sudan I | 0.008 μM to 6 μM | 0.5 nM | - | Food samples | [121] | |
| Multi-functionalized magnetic graphene sphere | Thyroxine | 0.05 pg·mL−1 to 5 ng·mL−1 | 15 fg·mL−1 | 85.3% of initial current after 20 days | - | [122] | |
| Gr-chitosan/Fe3O4 | Guanosine | 2.0 × 10−6 to 3.5 × 10−4 M | 7.5 × 10−7 M | 90.75% of initial current after 15 days | Urine samples and traditional Chinese medicines | [123] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Movlaee, K.; Ganjali, M.R.; Norouzi, P.; Neri, G. Iron-Based Nanomaterials/Graphene Composites for Advanced Electrochemical Sensors. Nanomaterials 2017, 7, 406. https://doi.org/10.3390/nano7120406
Movlaee K, Ganjali MR, Norouzi P, Neri G. Iron-Based Nanomaterials/Graphene Composites for Advanced Electrochemical Sensors. Nanomaterials. 2017; 7(12):406. https://doi.org/10.3390/nano7120406
Chicago/Turabian StyleMovlaee, Kaveh, Mohmmad Reza Ganjali, Parviz Norouzi, and Giovanni Neri. 2017. "Iron-Based Nanomaterials/Graphene Composites for Advanced Electrochemical Sensors" Nanomaterials 7, no. 12: 406. https://doi.org/10.3390/nano7120406





