The Preparation and Microstructure of Nanocrystal 3C-SiC/ZrO2 Bilayer Films
Abstract
:1. Introduction
2. Experiments
2.1. Sample Fabrication
- (1)
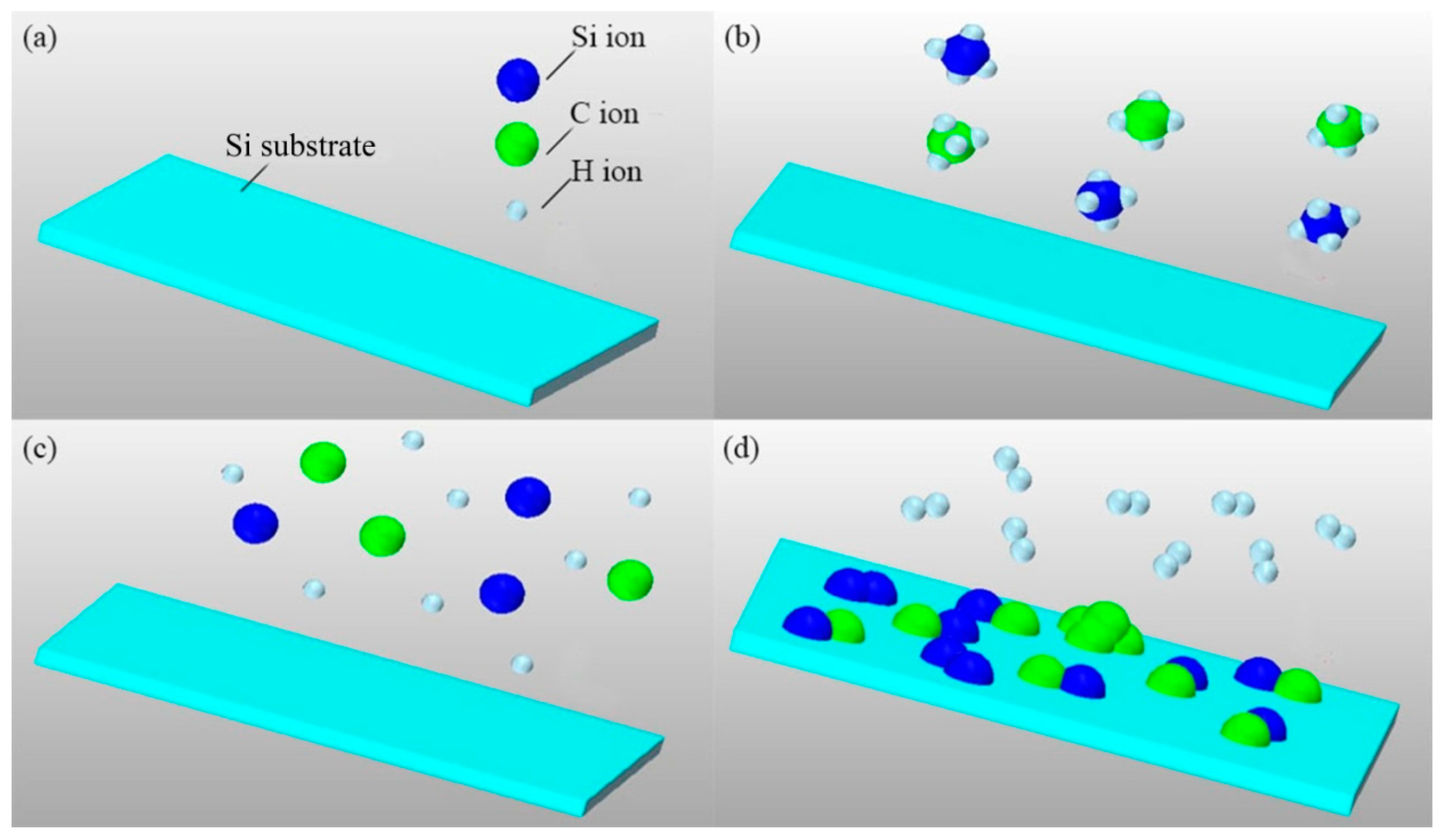
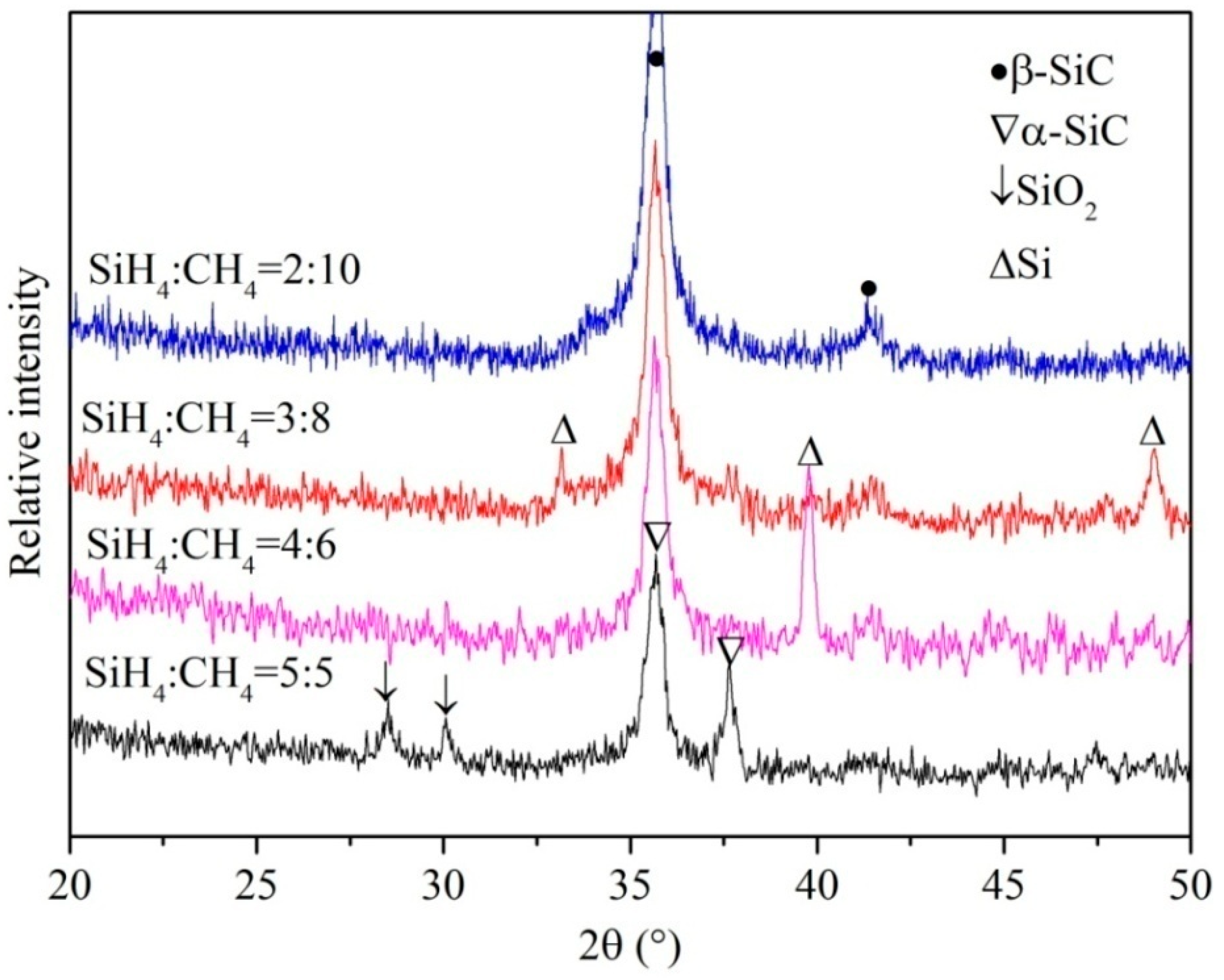
- Step one: The precursor gases of methane (CH4) and silicane (SiH4) were used to deposit a-SiC: H (amorphous SiC after hydrogenation) thin films by using Plasma Enhanced Chemical Vapor Deposition (PECVD) System (PECVD 350 series, Beijing Tai Ke Nuo Company, Beijing, China) with a radio frequency (RF) generator of 13.56 MHz and PLC (Programmable Logic Control) control mode. The hydrogen (H2) was used to smooth the surface of deposited films. Firstly, only CH4 gas was used to offer carbon ions to react with single crystal Si substrate with [100] direction in order to form a-SiC: transition layer. Then, a-SiC: H thin films under the conditions of a different ratio of CH4 to SiH4 and different RF powers were deposited on the a-SiC: transition layer at 400 °C for 30 min. The pressure in the deposition chamber was set to 0.2 Torr. After being deposited, the samples were annealed for 1.5 h at 1000 °C in a tube furnace with the protection of 20 sccm (standard-state cubic centimeter per minute) argon (99.999% purity) flow rate. A method was used to decrease oxygen content during the annealing process, which was that before annealing the samples were previously loaded into a corundum can, which was sealed using Al2O3 adhesion agent in the glove box protected by high pure argon gas. The oxygen content was less than 12 ppm in the glove box, so the samples were only exposed at an environment with very low oxygen content. The experimental parameters of deposition and annealing used to prepare the nanocrystal 3C-SiC thin film were listed in Table 1.
- (2)
- Step two: A Zr layer with a thickness of about 500 nm was first deposited on a single crystal Si substrate with [100] direction using magnetron sputtering system, and then annealed for 1.5 h at 1000 °C in a tube furnace with a low-speed flow argon protection. The target material used in magnetron sputtering is a circular α-Zr target produced by JAH Technology Company (Beijing, China) with the diameter of 50.8 mm, a purity of 99.9%, and a thickness of 5 mm. The magnetron sputtering system is a Desktop Pro series product of the Denton company (Denton, TX, USA), which is equipped with RF/DC (Direct Current) sputtering power and can drive two target guns. It is based on a PLC control system and can reach 5% of the coating uniformity. Because of the low gas tightness of tube furnace and low-speed flow argon, at high temperature the deposited Zr layer could be easily oxidized to form zirconium oxides at rare oxygen conditions, especially for the amorphous zirconium. Therefore, it did not need to be exposed to the atmosphere to form zirconium oxide film at high temperature annealing. The experimental parameters of deposition and annealing used to prepare the nano-polycrystalline ZrO2 thin film were listed in Table 2.
- (3)
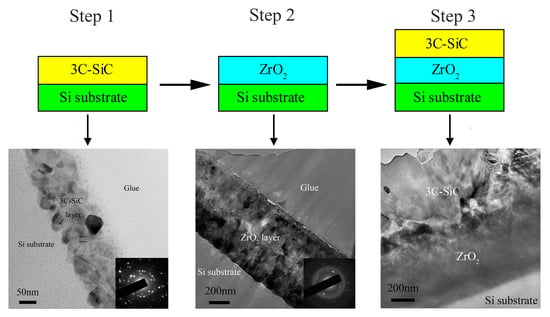
- Step three: For the preparation of the 3C-SiC/ZrO2 bilayer films, two different annealing schemes were used in the present work including: (i) Firstly, ZrO2 thin film was prepared on the single crystal Si substrate as mentioned in step two. Then, 3C-SiC thin film was prepared on ZrO2 layer as mentioned in step one. Thus, the whole preparation process has undergone two separate annealing processes. In order to conveniently express this in the next content, the T-3C-SiC/ZrO2 was used to represent the prepared thin film; (ii) the Zr layer was deposited on a single crystal Si substrate firstly, and then SiC layer was deposited on the as-deposited Zr layer surface. Finally, the deposited amorphous SiC/Zr bilayer films were annealed for 1.5 h at 1000 °C in a tube furnace with a low-speed flow argon protection. The O-3C-SiC/ZrO2 was used to represent the abovementioned thin film. After being annealed, amorphous SiC/Zr bilayer films were changed to nanocrystal 3C-SiC/ZrO2 bilayer films. The deposited amorphous Zr layer was oxidized to ZrO2 layer even at the coverage of SiC layer. This preparation process only underwent one annealing process. The microstructure and chemical composition of the interface between 3C-SiC and ZrO2 layers prepared by the above two methods were characterized and analyzed.
2.2. Microstructural Analysis
3. Results and Discussion
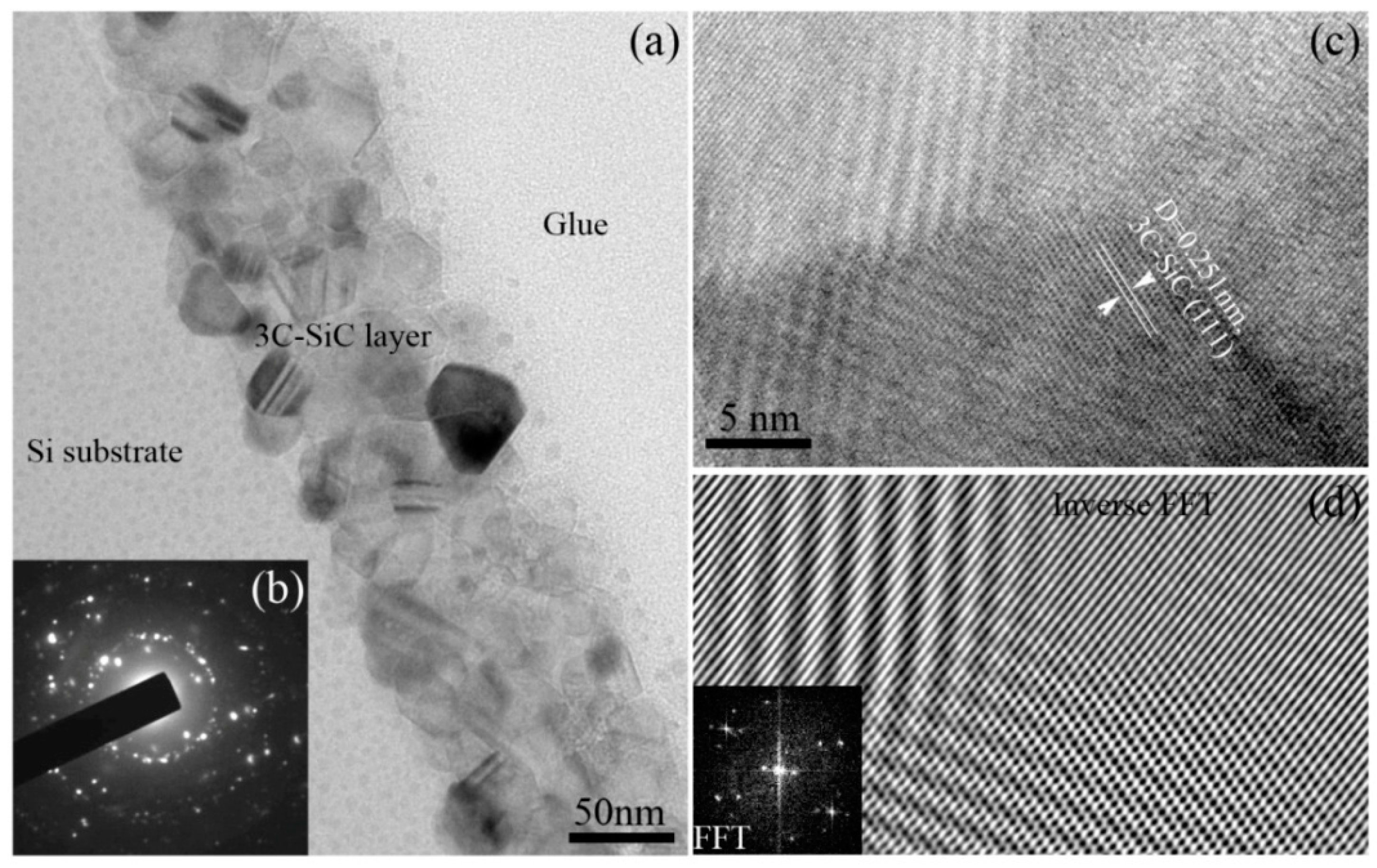
3.1. Microstructure Analysis of 3C-SiC Thin Film
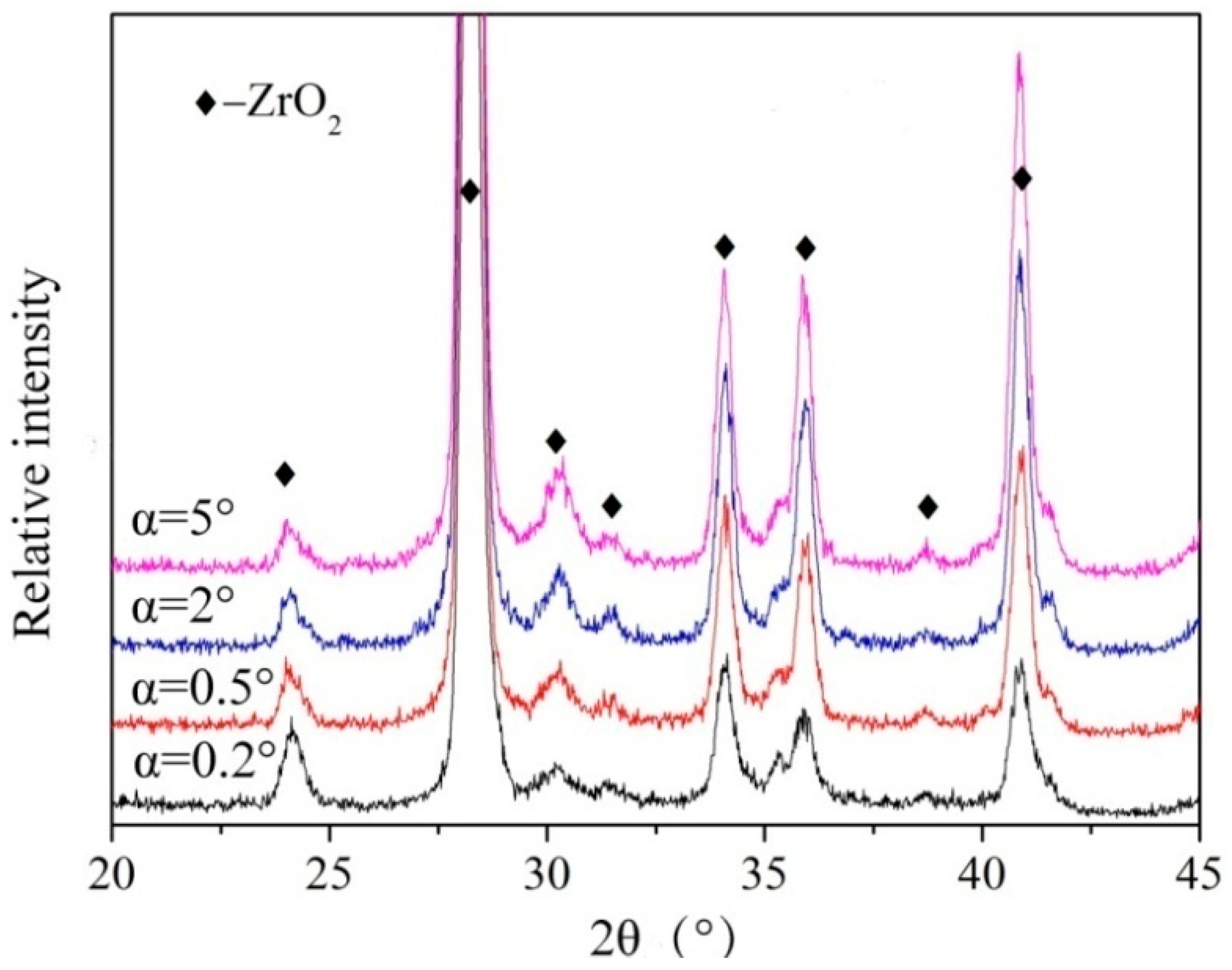
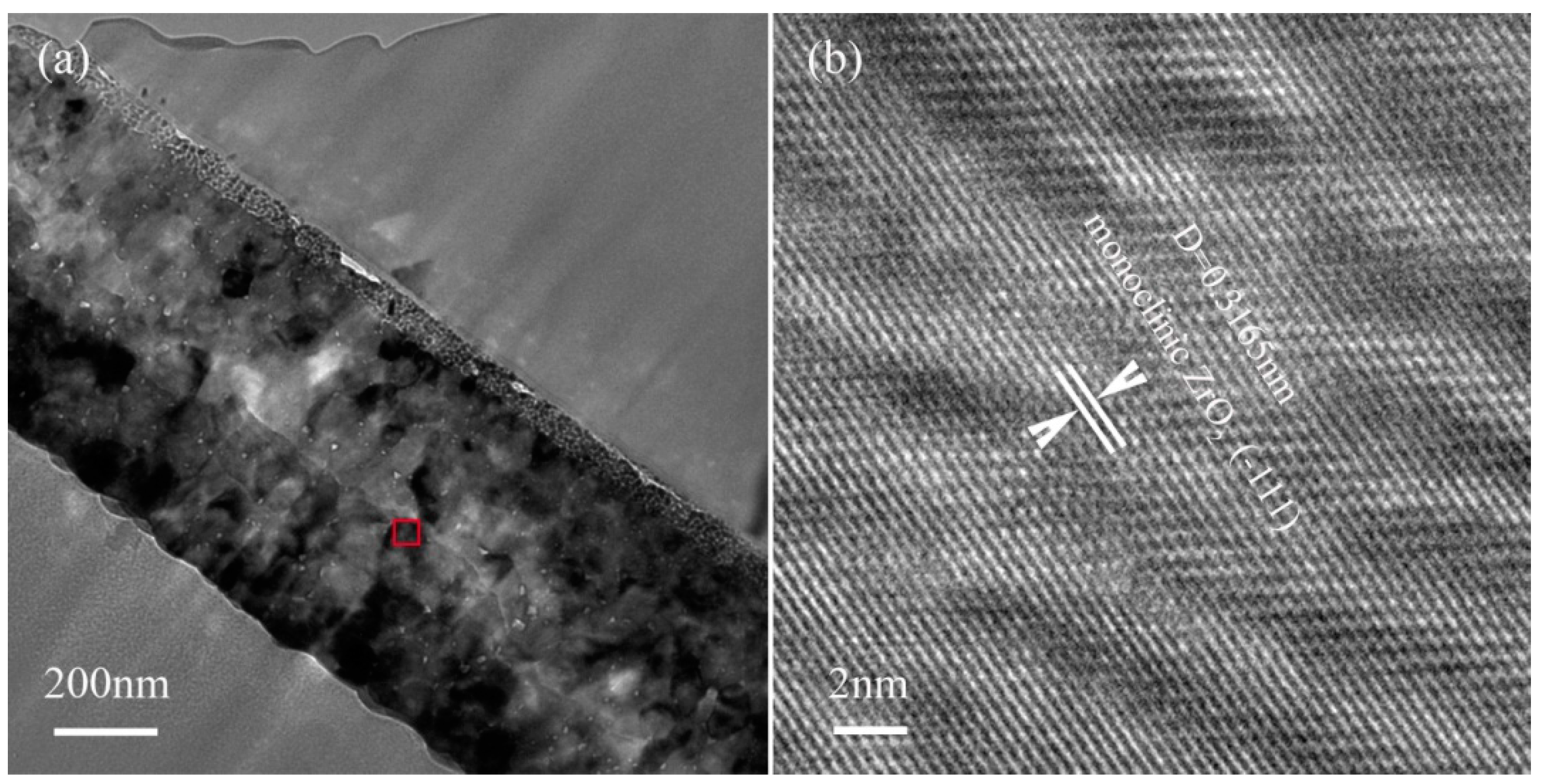
3.2. Microstructure Analysis of ZrO2 Thin Film
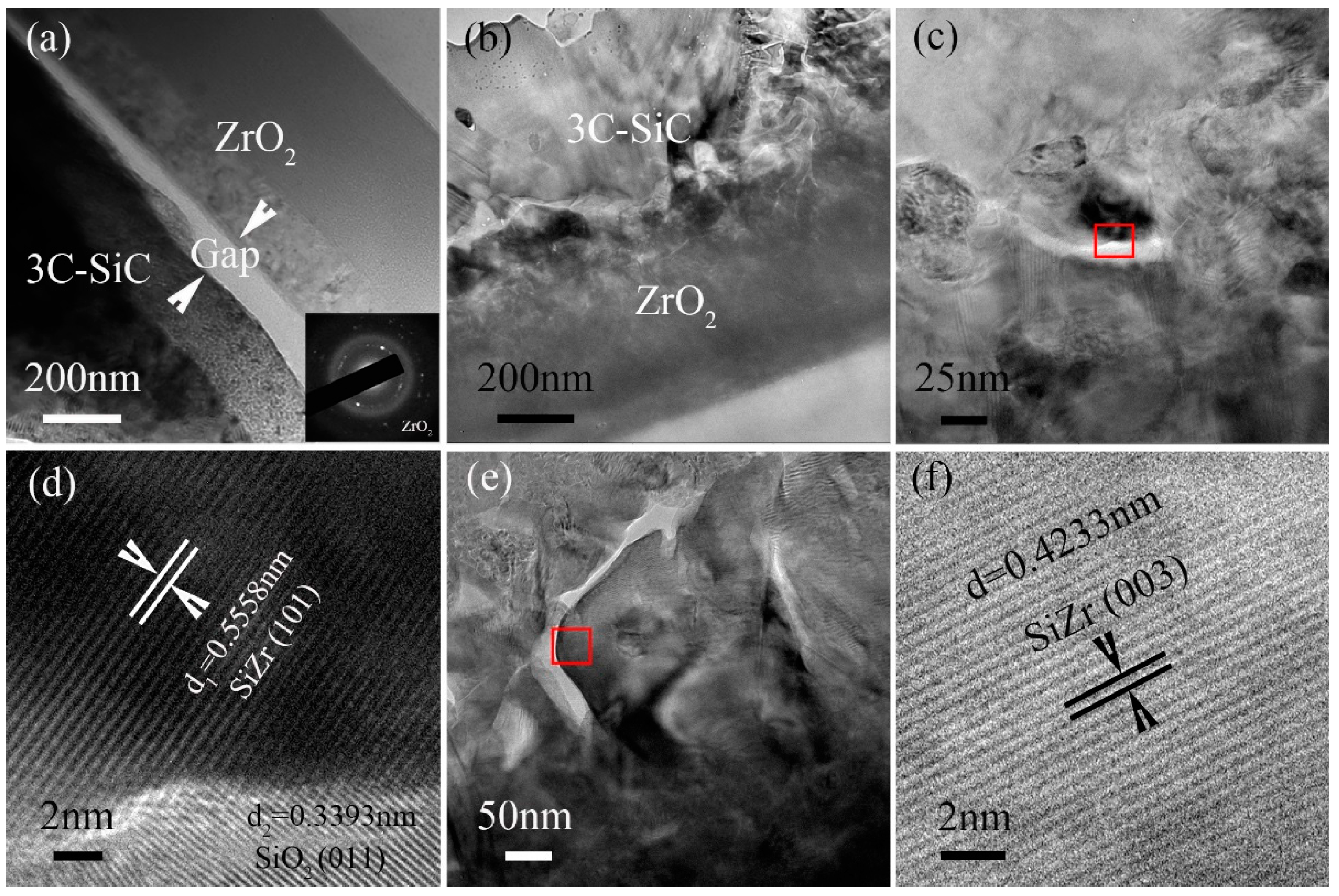
3.3. Microstructure Analysis of 3C-SiC/ZrO2 Bilayer Films
3.4. Corrosion Results
4. Conclusions
- (1)
- The nanocrystal 3C-SiC thin film with a width of approximately 100 nm was synthesized by PECVD and annealing. The average grain size was less than 30 nm. The optimal preparation parameters were an annealing time of 1.5 h, an annealing temperature of 1000 °C and the ratio of SiH4:CH4 = 2:10.
- (2)
- An amorphous Zr layer with a width of about 600 nm was first deposited on a single crystal Si substrate by magnetron sputtering and then oxidized to form a ZrO2 layer with a crystal structure during the annealing process.
- (3)
- The interface characteristics of the nanocrystal 3C-SiC/ZrO2 bilayer films prepared by two different processes were obviously different. There was a gap and a weak binding force between 3C-SiC film and ZrO2 film under the preparation technique that first synthesized the crystal ZrO2 film and then prepared the crystal 3C-SiC film on the surface of the crystal ZrO2 film. However, the strong binding force and the compounds such as SiZr and SiO2 were formed at the interface of 3C-SiC/ZrO2 bilayer films under the preparation technique that first deposited the amorphous Zr layer, then deposited the amorphous SiC layer, and finally annealed at the argon protection with low-speed flow.
- (4)
- The corrosion resistance of 3C-SiC/ZrO2 bilayer films is good after corrosion for 30 h at 100 °C in a water bath and under normal pressure.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Ni, N.; Hudson, D.; Wei, J.; Wang, P.; Lozano-Perez, S.; Smith, G.D.W.; Sykes, J.M.; Yardley, S.S.; Moore, K.L.; Lyon, S.; et al. How the crystallography and nanoscale chemistry of the metal/oxide interface develops during the aqueous oxidation of zirconium cladding alloys. Acta Mater. 2012, 60, 7132–7149. [Google Scholar] [CrossRef]
- Couet, A.; Motta, A.T.; Ambard, A. The coupled current charge compensation model for zirconium alloy. Corros. Sci. 2015, 100, 73–84. [Google Scholar] [CrossRef]
- Nikulin, S.A.; Rozhnov, A.B.; Koteneva, M.V.; Belov, V.A.; Komissarov, A.A. Effect of Corrosion Damages on the Mechanical Properties of Zirconium Alloy Cladding Tubes. Russ. Metall. 2012, 10, 906–910. [Google Scholar] [CrossRef]
- Mazères, B.; Desgranges, C.; Toffolon-Masclet, C.; Monceau, D. Experimental study and numerical simulation of high temperature (1100–1250 °C) oxidation of prior-oxidized zirconium alloy. Corros. Sci. 2016, 103, 10–19. [Google Scholar] [CrossRef]
- Araki, H.; Suzuki, H.; Yang, W.; Sato, S.; Noda, T. Effect of high temperature heat treatment in vacuum on microstructure and bending properties of SiCf/SiC composites prepared by CVI. J. Nucl. Mater. 1998, 258–263, 1540–1545. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Pint, B.A.; Terrani, K.A.; Field, K.G.; Yang, Y.; Snead, L.L. Development and property evaluation of nuclear grade wrought FeCrAl fuel cladding for light water reactors. J. Nucl. Mater. 2015, 467, 703–716. [Google Scholar] [CrossRef]
- Duan, Z.G.; Yang, H.L.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.S.; Shen, J.J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Parish, C.M.; Koyanagi, T.; Kondo, S.; Katoh, Y. Irradiation-induced β to α SiC transformation at low temperature. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Allen, T.R. The measurement of silver diffusivity in zirconium carbide to study the release behavior of 110 m Ag in the ZrC TRISO-coated nuclear fuel particle. J. Nucl. Mater. 2016, 470, 76–83. [Google Scholar] [CrossRef]
- Honstein, G.; Chatillon, C.; Baillet, F. Mass spectrometric and thermodynamic analyses of (SiC-SiO2) powders vaporization behavior. J. Chem. Thermodyn. 2013, 59, 144–157. [Google Scholar] [CrossRef]
- Wang, F.L.; Zhang, L.Y.; Zhang, Y.F. SiC Nanowires Synthesized by Rapidly Heating a Mixture of SiO and Arc-Discharge Plasma Pretreated Carbon Black. Nanoscale Res. Lett. 2008, 4, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Du, S.; Zhang, H.; Li, F.; Wang, J.; Zhao, W.; Liang, F.; Huang, Z.; Zhang, S. Preparation and characterization of ZrB2-SiC composite powders from zircon via microwave-assisted boro/carbothermal reduction. Ceram. Int. 2015, 41, 14419–14426. [Google Scholar] [CrossRef]
- Liang, M.; Li, F.; Ma, X.; Kang, Z.; Huang, X.; Wang, X.G.; Zhang, G.J. Syntheses of ZrC-SiC nanopowder via sol-gel method. Ceram. Int. 2016, 42, 1345–1351. [Google Scholar] [CrossRef]
- Dragomir, M.; Valant, M.; Fanetti, M.; Mozharivskyj, Y. A facile chemical method for the synthesis of 3C-SiC nanoflakes. RSC Adv. 2016, 6, 21795–21801. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, R.R.; Chen, X.H.; Zhu, J.; Wang, N.L. Synthesis of SiC nanorods using floating catalyst. Solid State Commun. 2001, 118, 595–598. [Google Scholar] [CrossRef]
- Tu, R.; Zheng, D.; Sun, Q.; Han, M.; Zhang, S.; Hu, Z.; Goto, T.; Zhang, L. Ultra-Fast Fabrication of <110>-Oriented β-SiC Wafers by Halide CVD. J. Am. Ceram. Soc. 2016, 99, 84–88. [Google Scholar] [CrossRef]
- Xun, S.; Haitao1, L.; Junsheng, L.; Haifeng, C. Effects of CVD SiBCN interphases on mechanical and dielectric properties of SiCf/SiC composites fabricated via a PIP process. Ceram. Int. 2016, 42, 82–89. [Google Scholar]
- Liu, S.; Li, H.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X. Synthesis of β-SiC nanowires via a facile CVD method and their photoluminescence properties. RSC Adv. 2016, 6, 24267–24272. [Google Scholar] [CrossRef]
- Cheng, L.; Xu, Y.; Zhang, L.; Luan, X. Oxidation and defect control of CVD SiC coating on three-dimensional C/SiC composites. Carbon 2002, 40, 2229–2234. [Google Scholar] [CrossRef]
- Hong, R.D.; Chen, X.P.; Huang, Q.; Xie, Y.N.; Wu, S.X. High Temperature Annealing Amorphous Hydrogenated SiC Films for the Application as Window Layers in Si-Based Solar Cell. Appl. Mech. Mater. 2013, 401–403, 631–634. [Google Scholar] [CrossRef]
- Wang, M.; Diao, X.G.; Huang, A.P.; Chu, P.K. Influence of substrate bias on the composition of SiC thin films fabricated by PECVD and underlying mechanism. Surf. Coat. Technol. 2006, 201, 6777–6780. [Google Scholar] [CrossRef]
- Coscia, U.; Ambrosone, G.; Minarini, C.; Parisi, V.; Schutzmann, S.; Tebano, A.; Restello, S.; Rigato, V. Laser annealing of hydrogenated amorphous silicon carbon films. Thin Solid Films 2004, 453, 7–12. [Google Scholar] [CrossRef]
- Semenov, A.V.; Puzikov, V.M.; Dobrotvorskaya, M.V.; Fedorov, A.G.; Lopin, A.V. Nanocrystalline SiC films prepared by direct deposition of carbon and silicon ions. Thin Solid Films 2008, 516, 2899–2903. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Carreno, M.N.P. Post thermal annealing crystallization and reactive ion etching of SiC films produced by PECVD. J. Non-Cryst. Solids 2006, 352, 1392–1397. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, C. Large-area SiC membrane produced by plasma enhanced chemical vapor deposition at relatively high temperature. J. Vac. Sci. Technol. A 2015, 33, 05E114. [Google Scholar] [CrossRef]
- Valdez, J.A.; Chi, Z.; Sickafus, K.E. Light ion irradiation-induced phase transformation in the monoclinic polymorph of zirconia. J. Nucl. Mater. 2008, 381, 259–266. [Google Scholar] [CrossRef]
- The Parameters of Chemical Bond. Available online: http://wenku.baidu.com/link?url=QZ5Vo3xJFs9WoDsTWZxLiXwtJ0lK9YPEd4jHLIEl42p9NS1nYR8HgkqJ0ZAs59mN71UwtIKd3Jx_qhT4XjpQsMntEpC0g7E4fSUP5SnHim (accessed on 21 September 2016).
- Mingzhe, R.; Dingxin, L.; Mei, L.; Weizong, W. Research status and new progress of non-equilibrium plasma simulation. Trans. China Electrotech. Soc. 2014, 6, 271–282. (In Chinese) [Google Scholar]
- Karch, K.; Bechstedt, F.; Pavone, P.; Strauch, D. Pressure-dependent properties of SiC polytypes. Phys. Rev. B 1996, 53, 13400–13413. [Google Scholar] [CrossRef]
- Durandurdu, M. Pressure-induced phase transition of SiC. J. Phys. Condens. Matter 2004, 16, 4411–4417. [Google Scholar] [CrossRef]
- Lu, Y.-P.; He, D.-W.; Zhu, J.; Yang, X.D. First-principles study of pressure-induced phase transition in silicon carbide. Physica B 2008, 403, 3543–3546. [Google Scholar] [CrossRef]
- Eker, S.; Durandurdu, M. Phase transformation of 6H-SiC at high pressure: An ab initio constant-pressure study. EPL-Europhys. Lett. 2008, 84. [Google Scholar] [CrossRef]
- Eker, S.; Durandurdu, M. Pressure-induced phase transformation of 4H-SiC: An ab initio constant-pressure study. EPL-Europhys. Lett. 2009, 87. [Google Scholar] [CrossRef]
- Chang, K.J.; Cohen, M.L. Ab initio pseudopotential study of structural and high-pressure properties of SiC. Phys. Rev. B Condens. Matter 1987, 35, 8196–8201. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.S.; Lambrecht, W.R.L. Unified path for high-pressure transitions of SiC polytypes to the rocksalt structure. Phys. Rev. B. 2003, 68. [Google Scholar] [CrossRef]
- Durandurdu, M. Ab initio simulations of the structural phase transformation of 2H-SiC at high pressure. Phys. Rev. B 2007, 75. [Google Scholar] [CrossRef]
- Lee, W.H.; Yao, X.H. First principle investigation of phase transition and thermodynamic properties of SiC. Comput. Mater. Sci. 2015, 106, 76–82. [Google Scholar] [CrossRef]
- Cao, X.X.; Yao, M.E.; Peng, J.C. The corruption of Zr(Fe, Cr) alloys in 400 °C superheated steam. Acta Metall. Sin. 2011, 47, 882–886. (In Chinese) [Google Scholar]


| Code | SiH4 (sccm) | CH4 (sccm) | Deposition Pressure (Torr) | RF Power (W) | Si Substrate Temperature (°C) | Annealing Temperature (°C) |
|---|---|---|---|---|---|---|
| #1 | 2 | 10 | 0.2 | 100 | 400 | 1000 |
| #2 | 3 | 8 | 0.2 | 100 | 400 | 1000 |
| #3 | 4 | 6 | 0.2 | 100 | 400 | 1000 |
| #4 | 5 | 5 | 0.2 | 100 | 400 | 1000 |
| Target | Base Pressure (Torr) | Working Pressure (Torr) | DC Power (W) | Ar (sccm) | Deposited Time (Min) | Annealing Temperature (°C) | Annealing Time (h) |
|---|---|---|---|---|---|---|---|
| Zr | 1.6 × 10−5 | 5 × 10−3 | 150 | 10 | 90 | 1000 | 1.5 |
| Bond Type | Energy (kJ/mol) | Length (Pm) |
|---|---|---|
| Si–H | 318 | 148 |
| Si–C | 318 | 185 |
| Si–Si | 222 | 233 |
| C–H | 411 | 109 |
| C–C | 346 | 154 |
| H–H | 432 | 74 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Ran, G.; Zhou, W.; Qu, Y.; Yan, X.; Cheng, Q.; Li, N. The Preparation and Microstructure of Nanocrystal 3C-SiC/ZrO2 Bilayer Films. Nanomaterials 2017, 7, 408. https://doi.org/10.3390/nano7120408
Ye C, Ran G, Zhou W, Qu Y, Yan X, Cheng Q, Li N. The Preparation and Microstructure of Nanocrystal 3C-SiC/ZrO2 Bilayer Films. Nanomaterials. 2017; 7(12):408. https://doi.org/10.3390/nano7120408
Chicago/Turabian StyleYe, Chao, Guang Ran, Wei Zhou, Yazhou Qu, Xin Yan, Qijin Cheng, and Ning Li. 2017. "The Preparation and Microstructure of Nanocrystal 3C-SiC/ZrO2 Bilayer Films" Nanomaterials 7, no. 12: 408. https://doi.org/10.3390/nano7120408