Hierarchical Mn2O3 Microspheres In-Situ Coated with Carbon for Supercapacitors with Highly Enhanced Performances
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
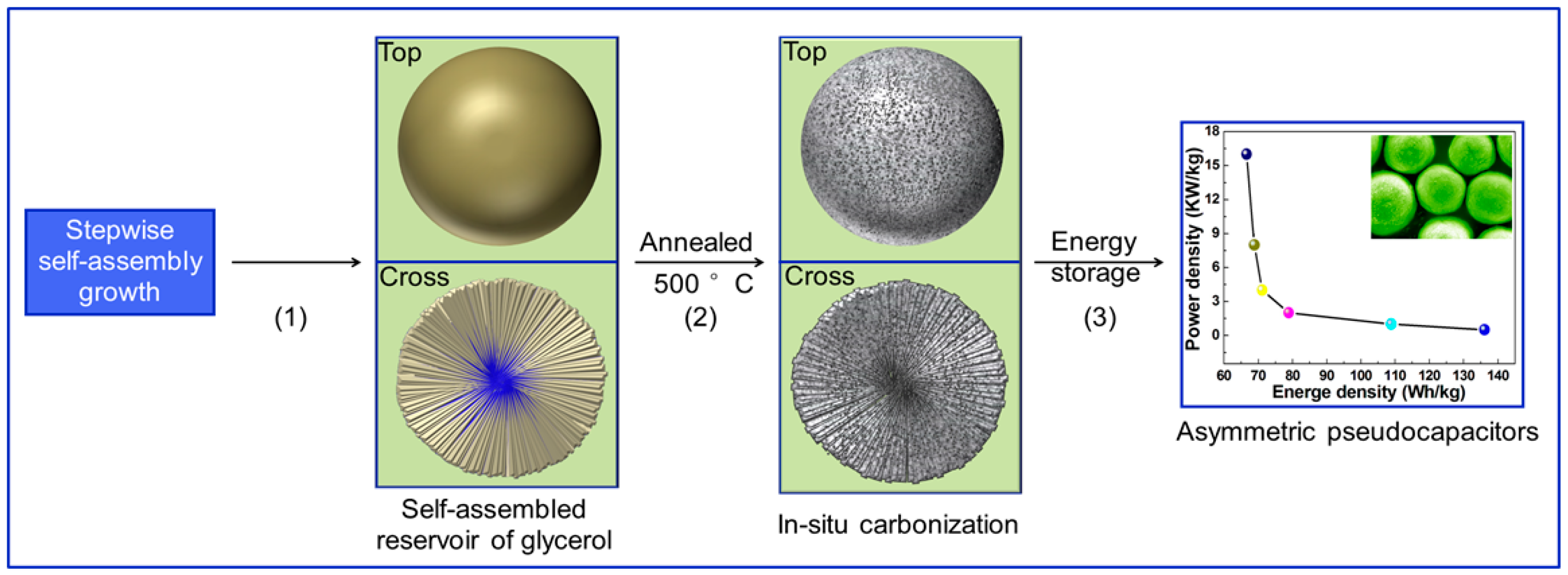
4.1. Preparation of C@Mn2O3Microspheres
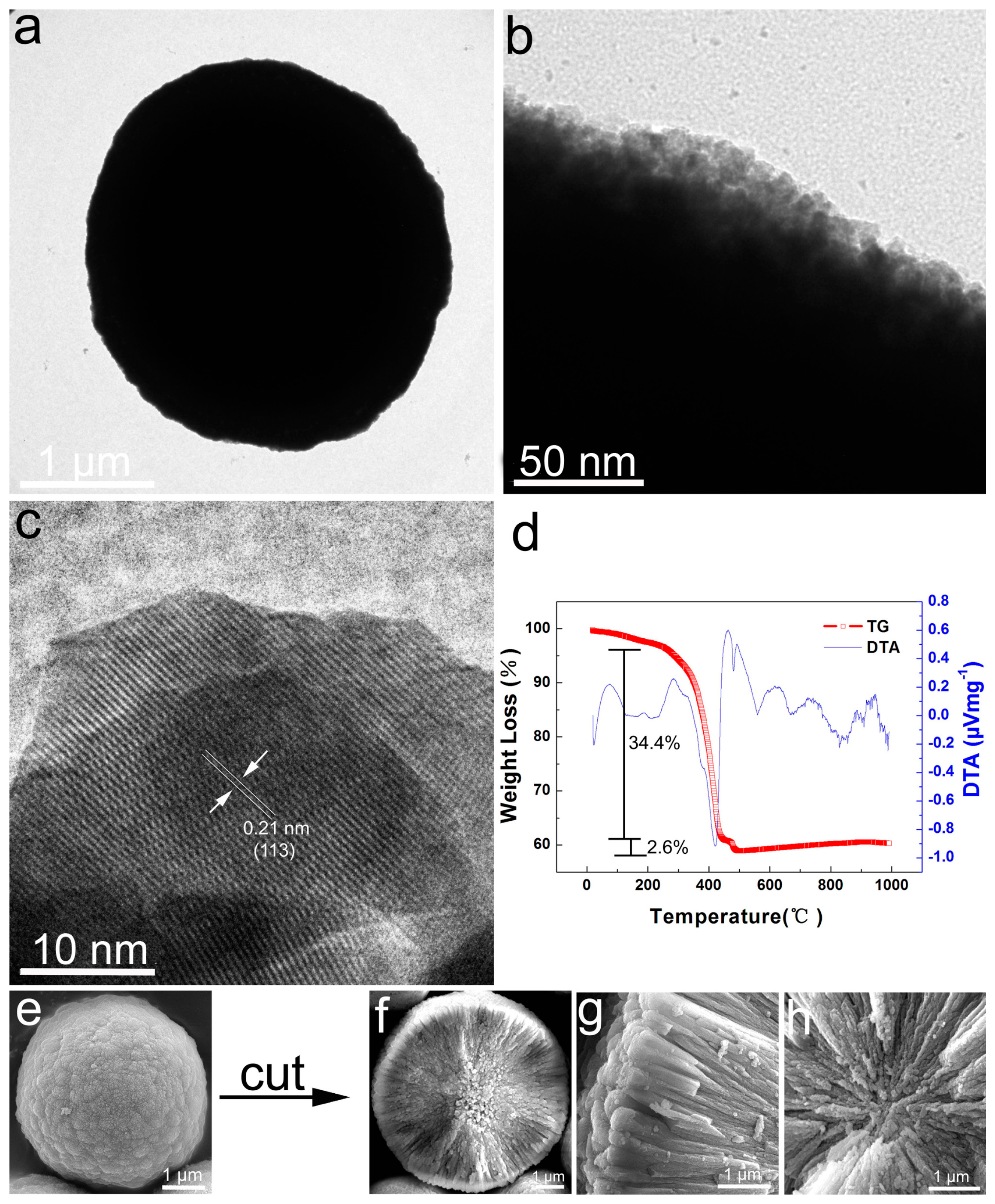
4.2. Dynamic Structure Evolution
4.3. The Reaction Temperature Effect
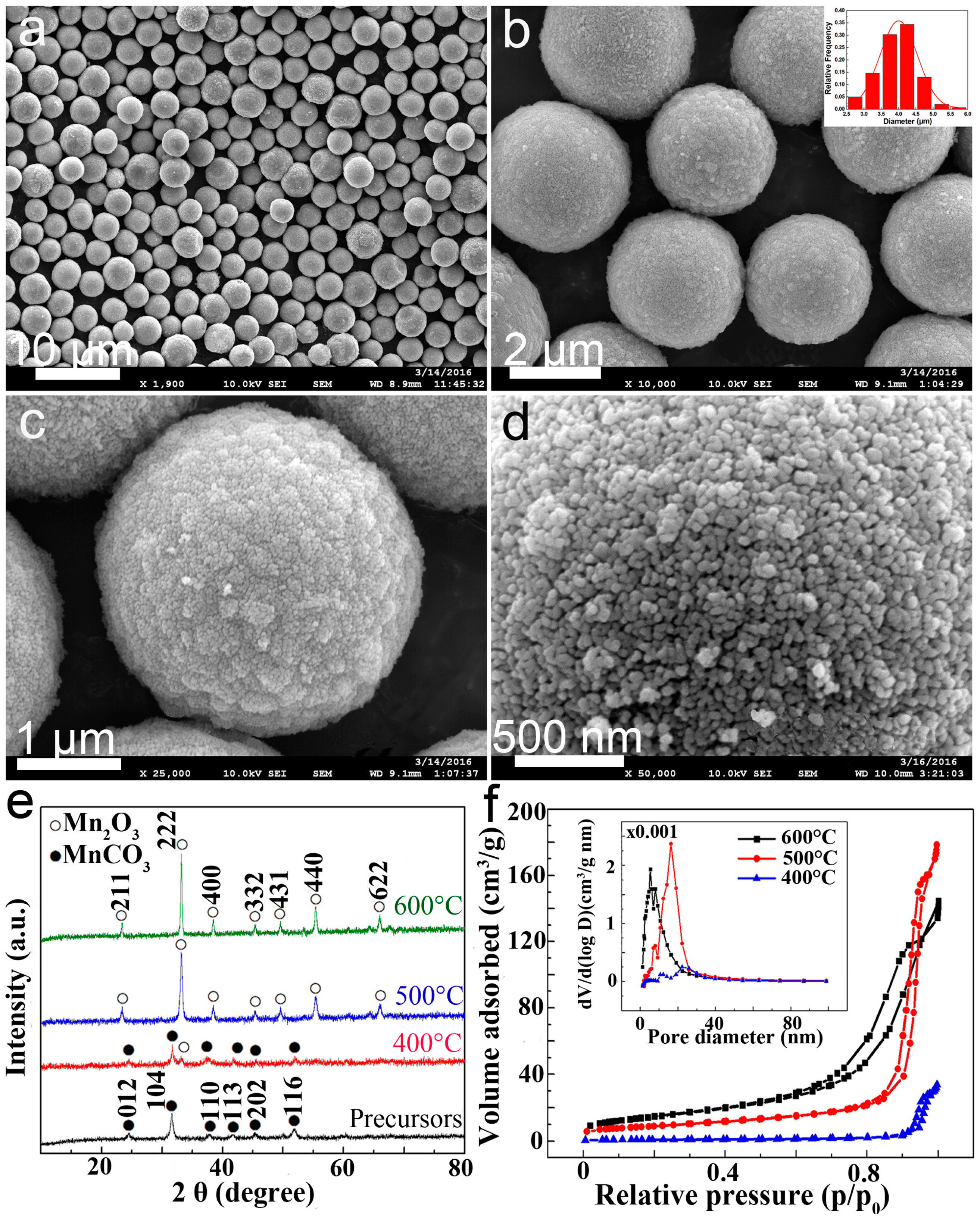
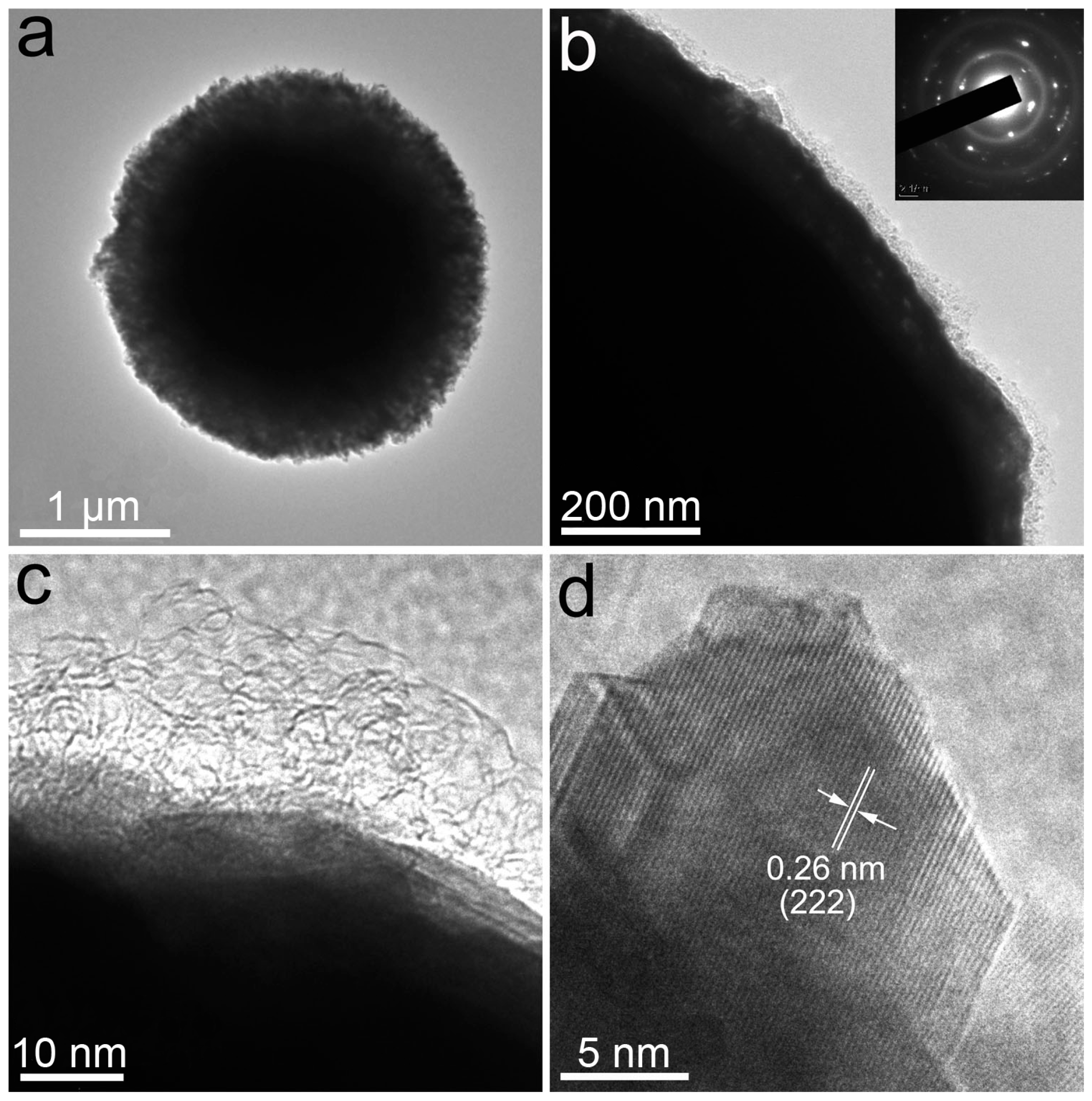
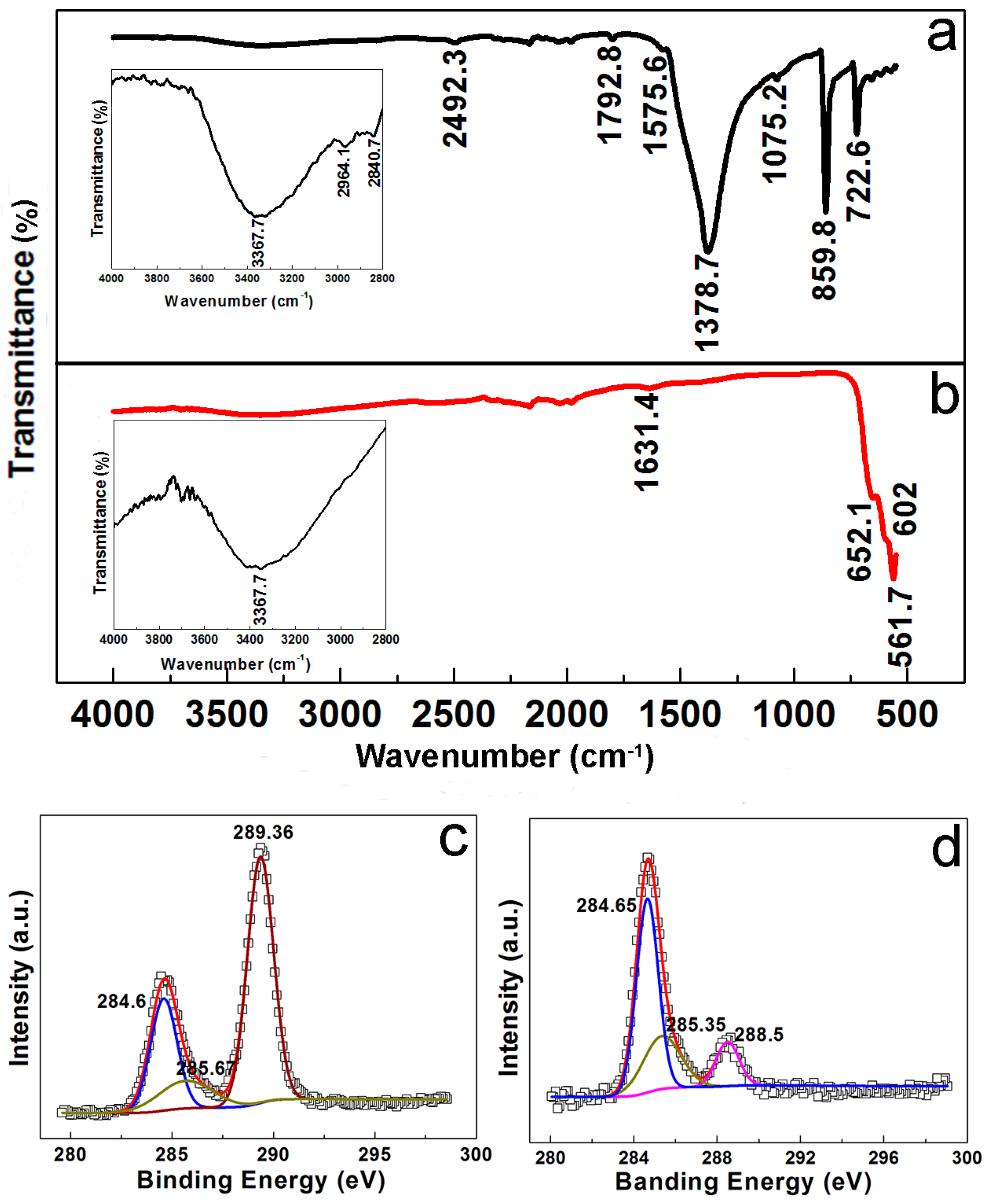
4.4. Materials Characterization
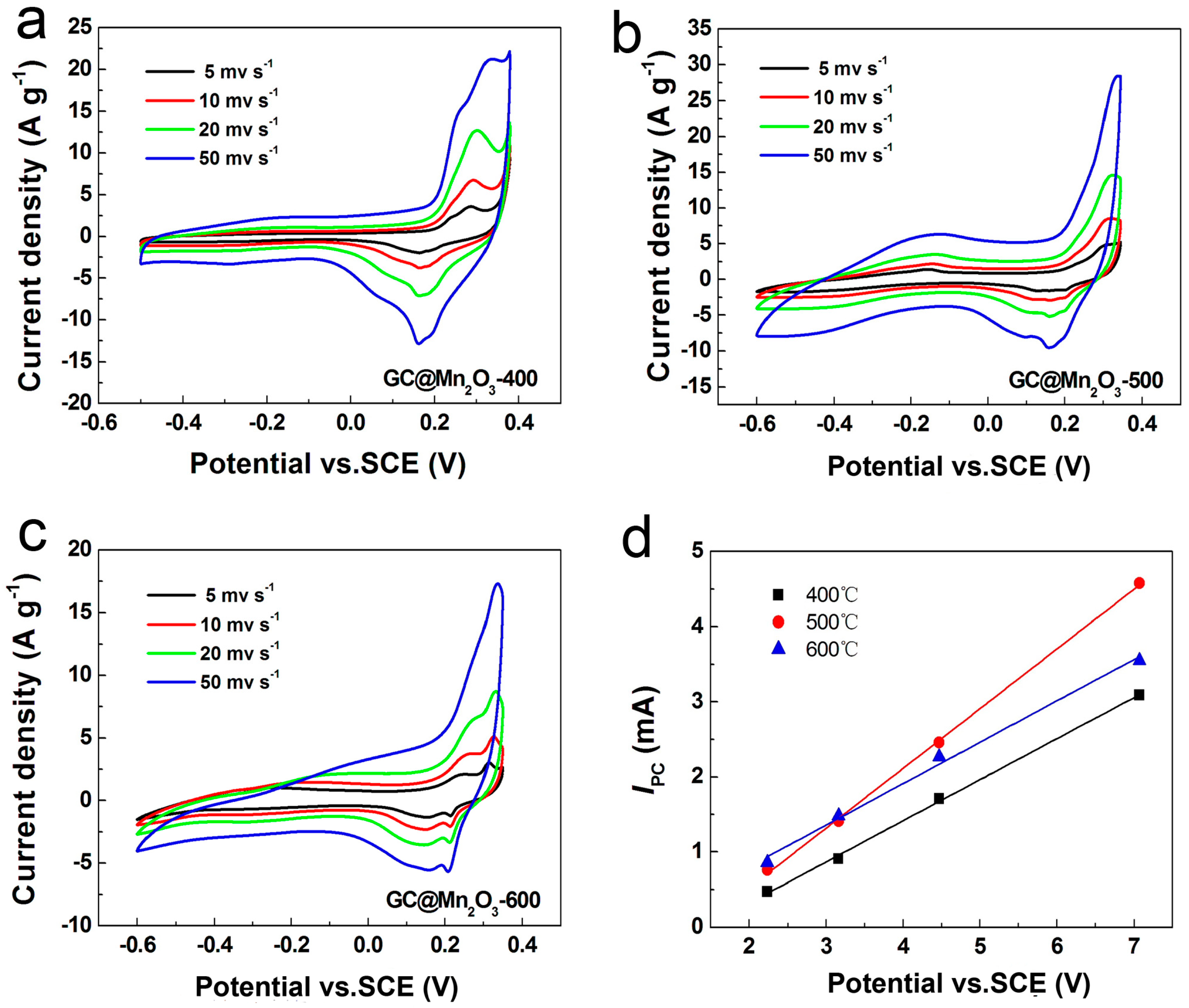
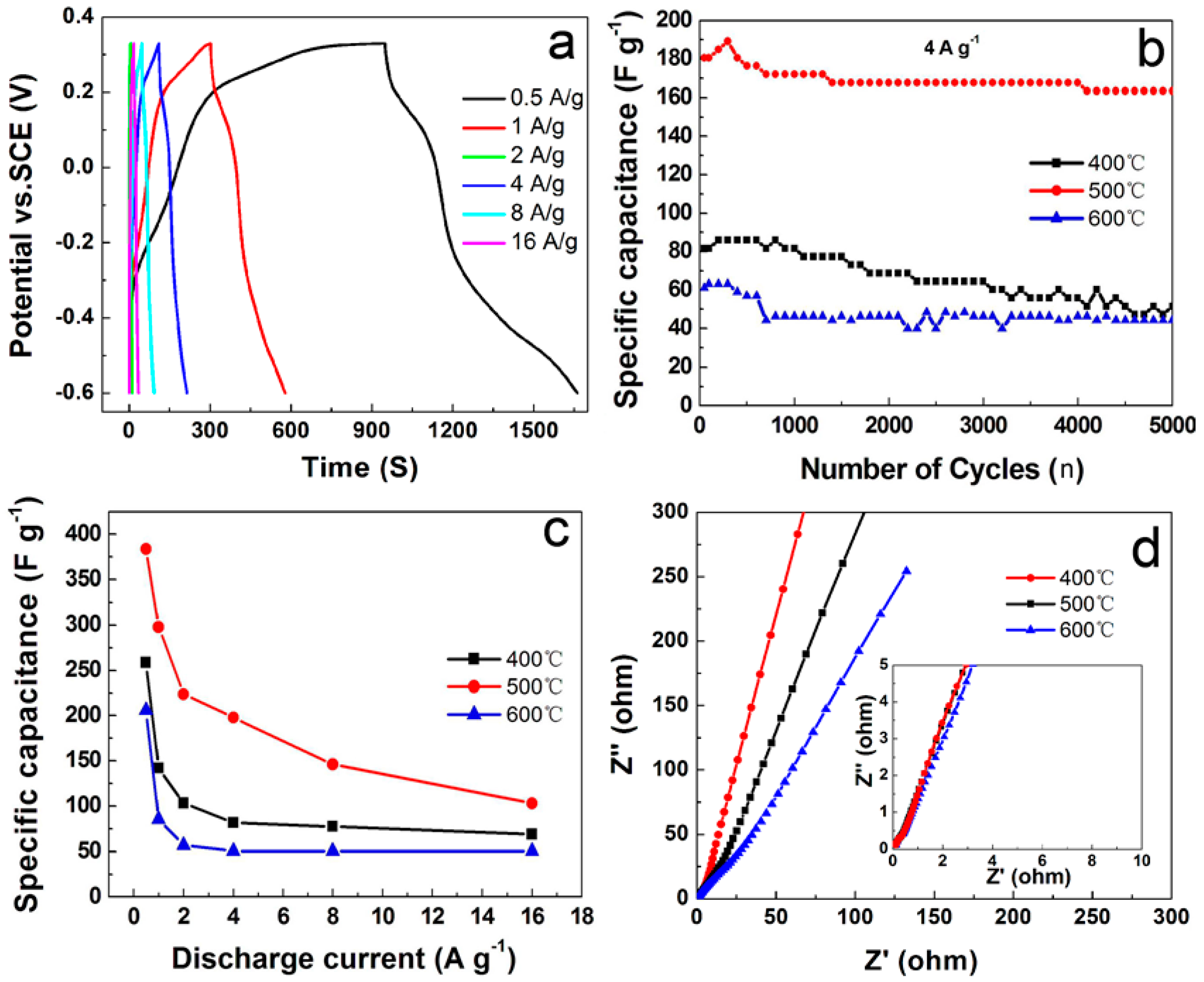
4.5. Electrochemical Properties of C@Mn2O3Microspheres
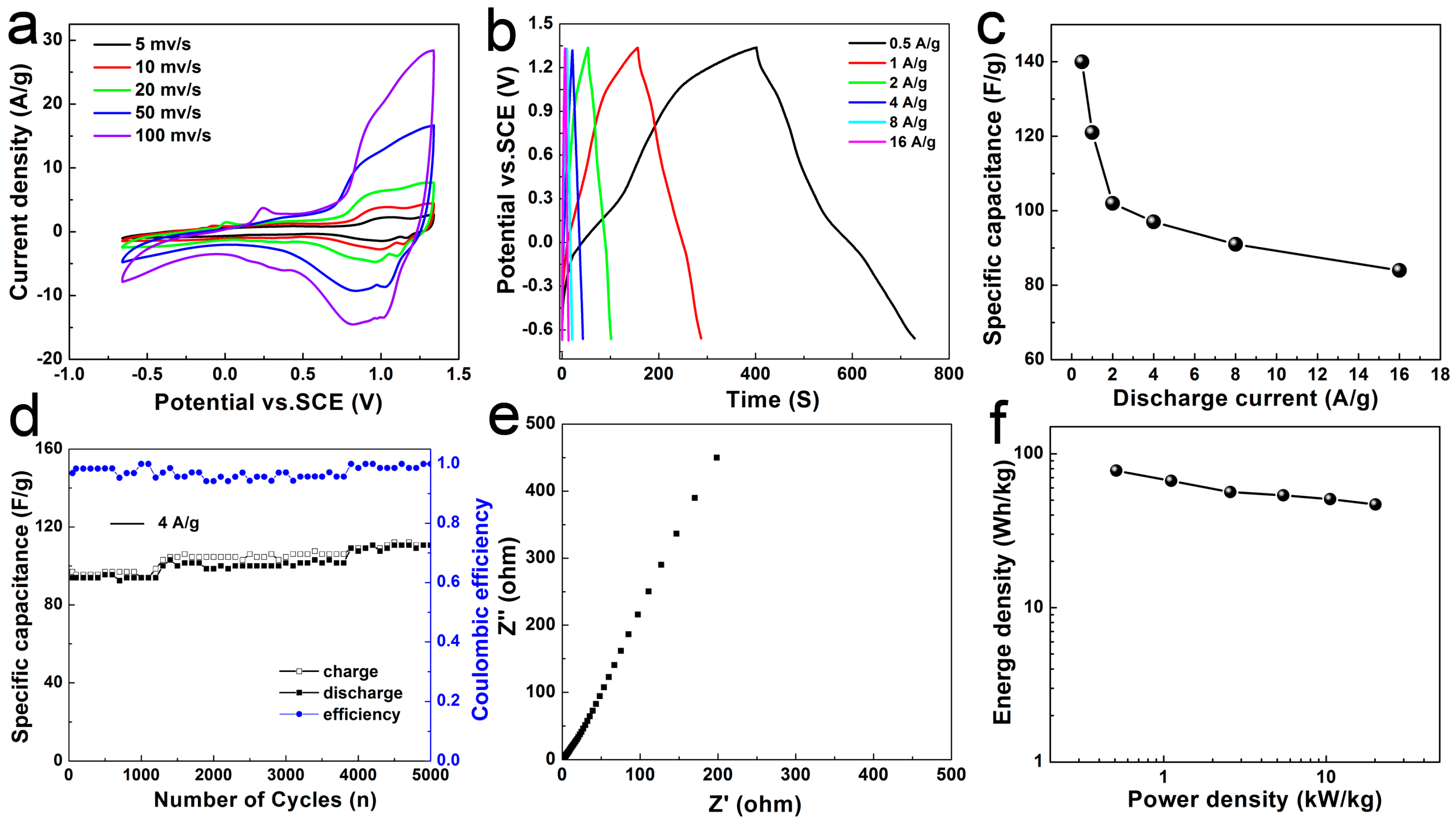
4.6. Asymmetric Supercapacitors
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Z.; Jiao, Z.; Pan, D.; Li, Z.; Wu, M.; Shek, C.H.; Wu, C.M.L.; Lai, J.K.L. Recent Advances in Manganese Oxide Nanocrystals: Fabrication, Characterization, and Microstructure. Chem. Rev. 2012, 112, 3833–3855. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816–1300858. [Google Scholar] [CrossRef]
- Wang, J.G.; Kang, F.; Wei, B. Engineering of MnO2-based nanocomposites for high-performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, H.; Liu, H.; Fu, Z.; Nan, D. Facile synthesis of microsized MnO/C composites with high tap density as high performance anodes for Li-ion batteries. Chem. Eng. J. 2017, 328, 591–598. [Google Scholar] [CrossRef]
- Wang, J.G.; Jin, D.; Zhou, R.; Li, X.; Liu, X.; Shen, C.; Xie, K.; Li, B.; Kang, F.; Wei, B. Highly Flexible Graphene/Mn3O4 Nanocomposite Membrane as Advanced Anodes for Li-Ion Batteries. ACS Nano 2016, 10, 6227–6234. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Jin, D.; Liu, H.; Zhang, C.; Zhou, R.; Shen, C.; Xie, K.; Wei, B. All-manganese-based Li-ion batteries with high rate capability and ultralong cycle life. Nano Energy 2016, 22, 524–532. [Google Scholar] [CrossRef]
- Ji, D.; Zhou, H.; Zhang, J.; Dan, Y.; Yang, H.; Yuan, A. Facile synthesis of a metal-organic framework-derived Mn2O3 nanowire coated three-dimensional graphene network for high-performance free-standing supercapacitor electrodes. J. Mater. Chem. A 2016, 4, 8283–8290. [Google Scholar] [CrossRef]
- Higgins, T.M.; McAteer, D.; Coelho, J.C.M.; Sanchez, B.M.; Gholamvand, Z.; Moriarty, G.; McEvoy, N.; Berner, N.C.; Duesberg, G.S.; Nicolosi, V.; et al. Effect of Percolation on the Capacitance of Supercapacitor Electrodes Prepared from Composites of Manganese Dioxide Nanoplatelets and Carbon Nanotubes. ACS Nano 2014, 8, 9567–9579. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Wei, T.Y.; Chien, H.C.; Lu, S.Y. Manganese Oxide/Carbon Aerogel Composite: An Outstanding Supercapacitor Electrode Material. Adv. Energy Mater. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Li, C.; Yan, C.; Lee, P.S.; Ma, J. High-rate electrochemical capacitors from highly graphitic carbon-tipped manganese oxide/mesoporous carbon/manganese oxide hybrid nanowires. Energy Environ. Sci. 2011, 4, 1813–1819. [Google Scholar] [CrossRef]
- Bongu, C.S.; Karuppiah, S.; Nallathamby, K. Validation of green composite containing nanocrystalline Mn2O3 and biocarbon derived from human hair as a potential anode for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 23981–23989. [Google Scholar] [CrossRef]
- Makgopa, K.; Ejikeme, P.M.; Jafta, C.J.; Raju, K.; Zeiger, M.; Presser, V.; Ozoemena, K.I. A high-rate aqueous symmetric pseudocapacitor based on highly graphitized onion-like carbon/birnessite-type manganese oxide nanohybrids. J. Mater. Chem. A 2015, 3, 3480–3490. [Google Scholar] [CrossRef]
- Klankowski, S.A.; Pandey, G.P.; Malek, G.; Thomas, C.R.; Bernasek, S.L.; Wu, J.; Li, J. Higher-power supercapacitor electrodes based on mesoporous manganese oxide coating on vertically aligned carbon nanofibers. Nanoscale 2015, 7, 8485–8494. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, G.; Wang, R.; Li, X. A novel layered manganese oxide/poly(aniline-co-o-anisidine) nanocomposite and its application for electrochemical supercapacitor. Electrochim. Acta 2010, 55, 5414–5419. [Google Scholar] [CrossRef]
- Kang, J.; Chen, L.; Hou, Y.; Li, C.; Fujita, T.; Lang, X.; Hirata, A.; Chen, M. Electroplated Thick Manganese Oxide Films with Ultrahigh Capacitance. Adv. Energy Mater. 2013, 3, 857–863. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 Nanowire@MnO2 Ultrathin Nanosheet Core/Shell Arrays: A New Class of High-Performance Pseudocapacitive Materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Jin, H.; Chen, B.; Lin, M.; Lu, W.; Tang, W.M.; Xiong, W.; Chan, L.W.H.; Lau, S.P.; Yuan, J. Aqueous Manganese Dioxide Ink for Paper-Based Capacitive Energy Storage Devices. Angew. Chem. Int. Ed. 2015, 54, 6800–6803. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, Y.; Wang, H.; Key, J.; Brett, D.; Ji, S.; Yin, S.; Shen, P.K. A cost effective, highly porous, manganese oxide/carbon supercapacitor material with high rate capability. J. Mater. Chem. A 2016, 4, 5390–5394. [Google Scholar] [CrossRef]
- Li, W.; Shao, J.; Liu, Q.; Liu, X.; Zhou, X.; Hu, J. Facile synthesis of porous Mn2O3 nanocubics for high-rate supercapacitors. Electrochim. Acta 2015, 157, 108–114. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Mahanty, S. Electrochemical energy storage in Mn2O3 porous nanobars derived from morphology-conserved transformation of benzenetricarboxylate-bridged metal-organic framework. Crystengcomm 2016, 18, 450–461. [Google Scholar] [CrossRef]
- Park, K.W. Carboxylated graphene oxide-Mn2O3 nanorod composites for their electrochemical characteristics. J. Mater. Chem. A 2014, 2, 4292–4298. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, Y.; Gong, Y.; Wang, C.; Li, F. One-pot synthesis of MnOOH nanorods on graphene for asymmetric supercapacitors. Electrochim. Acta 2014, 127, 200–207. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, G.; Zeng, W.; Zhu, J.; Gong, F.; Li, F.; Duan, H. Hierarchical Core-Shell Structure of ZnO Nanorod@NiO/MoO2 Composite Nanosheet Arrays for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 13564–13570. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gong, F.; Xiao, Y.; Zhang, A.; Zhao, J.; Fang, S.; Jia, D. ZnO Twin-Spheres Exposed in ±(001) Facets: Stepwise Self-Assembly Growth and Anisotropic Blue Emission. ACS Nano 2013, 7, 10482–10491. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Cao, Y.; Gong, Y.; Zhang, A.; Zhao, J.; Fang, S.; Jia, D.; Li, F. Electrolyte and composition effects on the performances of asymmetric supercapacitors constructed with Mn3O4 nanoparticles-graphene nanocomposites. J. Power Sources 2014, 246, 926–933. [Google Scholar] [CrossRef]
- Li, F.; Ding, Y.; Gao, P.X.; Xin, X.Q.; Wang, Z.L. Single-cystal hexagonal disks and rings of ZnO: Low-temperature, large-scale synthesis and growth mechanism. Angew. Chem. Int. Ed. 2004, 43, 5238–5242. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gong, F.; Wang, C.; Zheng, H.; Li, F. Porous and single crystalline Co3O4 nanospheres for pseudocapacitors with enhanced performance. RSC Adv. 2015, 5, 27266–27272. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Sansiviero, M.T.C.; Araújo, M.H.; Lago, R.M. Modification of vermiculite by polymerization and carbonization of glycerol toproduce highly efficient materials for oil removal. Appl. Clay Sci. 2009, 45, 213–219. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Li, S. Ethanol-directed morphological evolution of hierarchical CeOx architectures as advanced electrochemical capacitors. J. Mater. Chem. A 2015, 3, 13970–13977. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. Facile synthesis route of porous MnCo2O4 and CoMn2O4 nanowires and their excellent electrochemical properties in supercapacitors. J. Mater. Chem. A 2014, 2, 16480–16488. [Google Scholar] [CrossRef]
- Nathan, T.; Cloke, M.; Prabaharan, S.R.S. Electrode Properties of Mn2O3 Nanospheres Synthesized by Combined Sonochemical/Solvothermal Method for Use in Electrochemical Capacitors. J. Nanomater. 2008, 18, 69–79. [Google Scholar]
- Zhao, X.; Zhang, L.L.; Murali, S.; Stoller, M.D.; Zhang, Q.H.; Zhu, Y.W.; Ruoff, R.S. Incorporation of Manganese Dioxide within Ultraporous Activated Graphene for High-Performance Electrochemical Capacitors. ACS Nano 2012, 6, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Rudolph, M.; Mariano, R.G.; Nijem, N.; Ferraris, J.P.; Chabal, Y.J.; Balkus, K.J. Manganese oxide nanorod-graphene/vanadium oxide nanowire-graphene binder-free paper electrodes for metal oxide hybrid supercapacitors. Nano Energy 2013, 2, 966–975. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Pinero, E.; Beguin, F. Optimisation of an asymmetric manganese oxide/activated carbon capacitor working at 2 V in aqueous medium. J. Power Sources 2006, 153, 183–190. [Google Scholar]
- Chen, P.C.; Shen, G.; Shi, Y.; Chen, H.; Zhou, C. Preparation and Characterization of Flexible Asymmetric Supercapacitors Based on Transition-Metal-Oxide Nanowire/Single-Walled Carbon Nanotube Hybrid Thin-Film Electrodes. ACS Nano 2010, 4, 4403–4411. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, F.; Lu, S.; Peng, L.; Zhou, J.; Kong, J.; Jia, D.; Li, F. Hierarchical Mn2O3 Microspheres In-Situ Coated with Carbon for Supercapacitors with Highly Enhanced Performances. Nanomaterials 2017, 7, 409. https://doi.org/10.3390/nano7120409
Gong F, Lu S, Peng L, Zhou J, Kong J, Jia D, Li F. Hierarchical Mn2O3 Microspheres In-Situ Coated with Carbon for Supercapacitors with Highly Enhanced Performances. Nanomaterials. 2017; 7(12):409. https://doi.org/10.3390/nano7120409
Chicago/Turabian StyleGong, Feilong, Shuang Lu, Lifang Peng, Jing Zhou, Jinming Kong, Dianzeng Jia, and Feng Li. 2017. "Hierarchical Mn2O3 Microspheres In-Situ Coated with Carbon for Supercapacitors with Highly Enhanced Performances" Nanomaterials 7, no. 12: 409. https://doi.org/10.3390/nano7120409



