The Effect of Eu Doping on Microstructure, Morphology and Methanal-Sensing Performance of Highly Ordered SnO2 Nanorods Array
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses of Eu-Doped SnO2 Layered Nanoarrays
2.3. Characterization of Eu-Doped SnO2 Layered Nanoarrays
2.4. Sensor Fabrication and Gas-Sensing Performance Tests
3. Results and Discussion
3.1. Characterization of Eu-Doped SnO2 Nanoarray
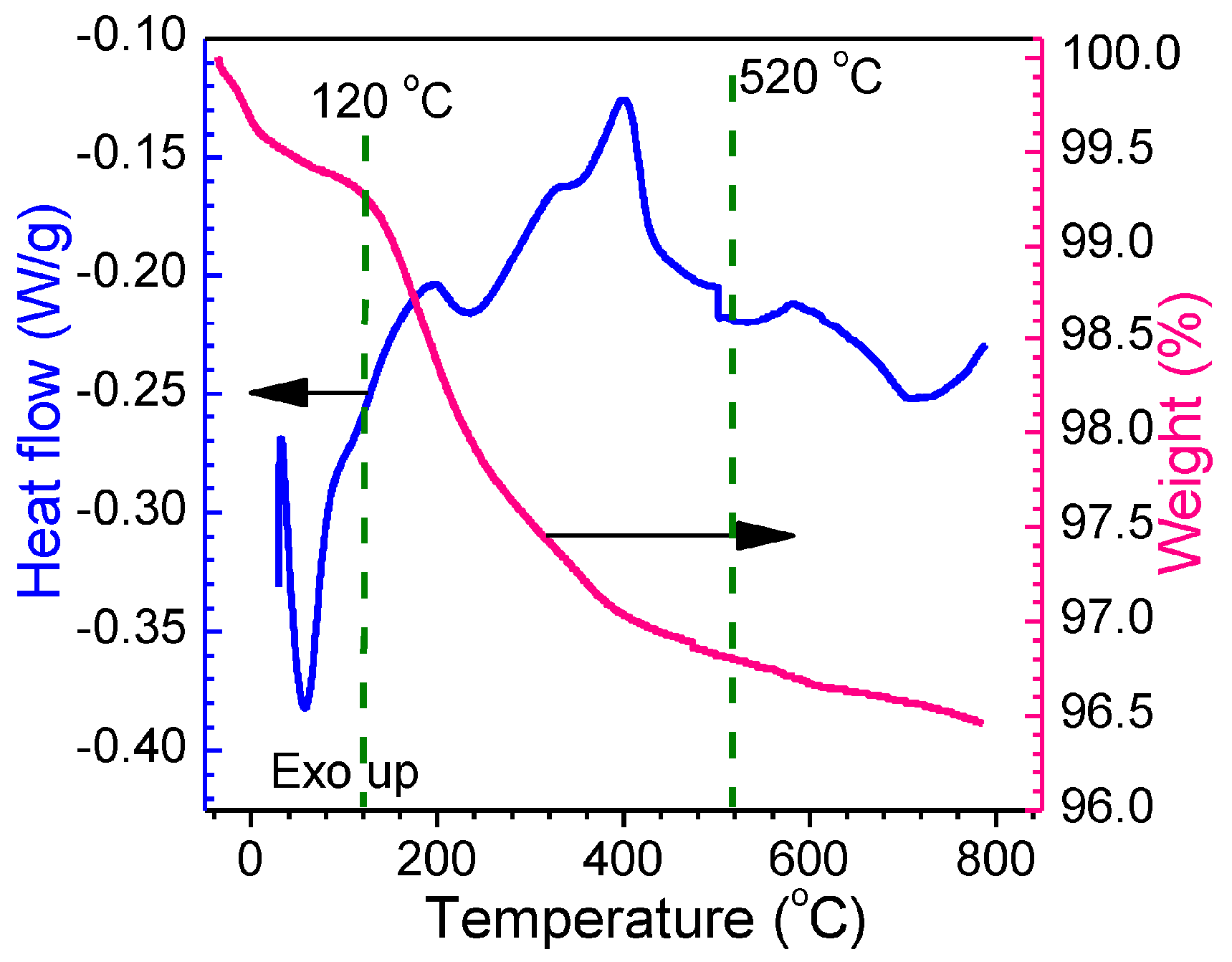
3.1.1. TGA-DSC Thermoanalysis
3.1.2. SEM and EDS
3.1.3. XRD
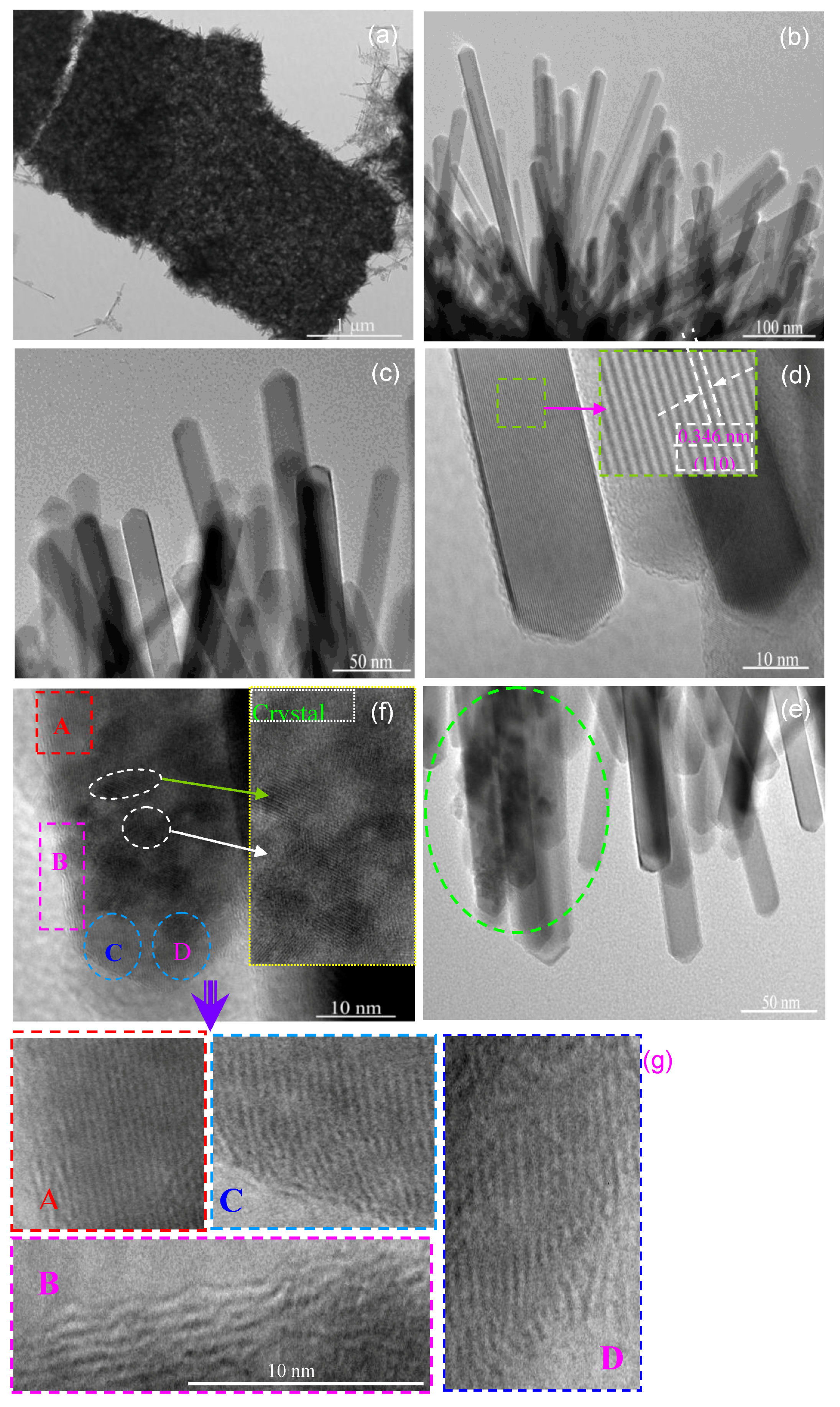
3.1.4. TEM and HRTEM
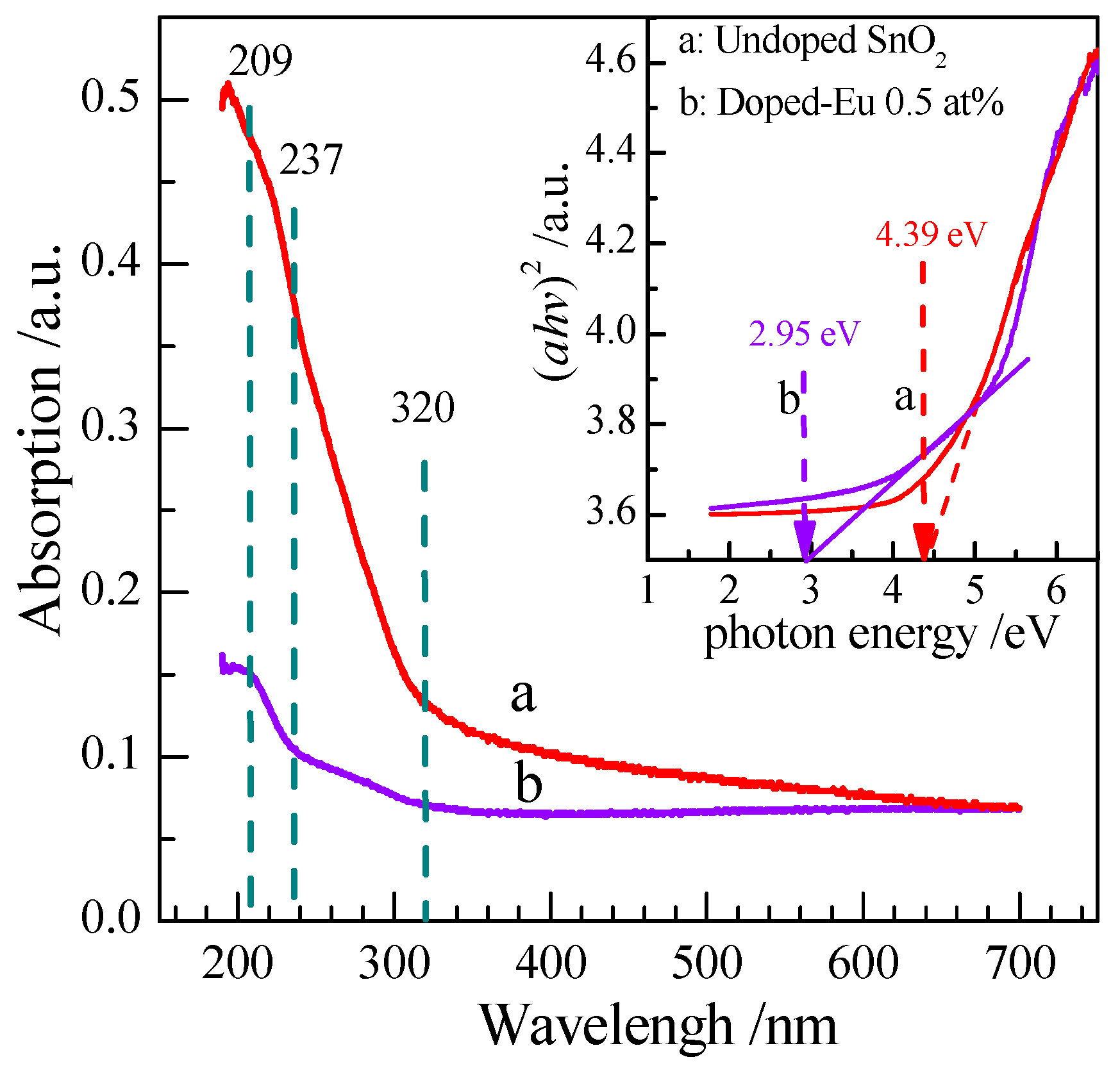
3.1.5. UV-Vis Spectrum
3.1.6. X-ray Photoelectron Spectroscopy (XPS)
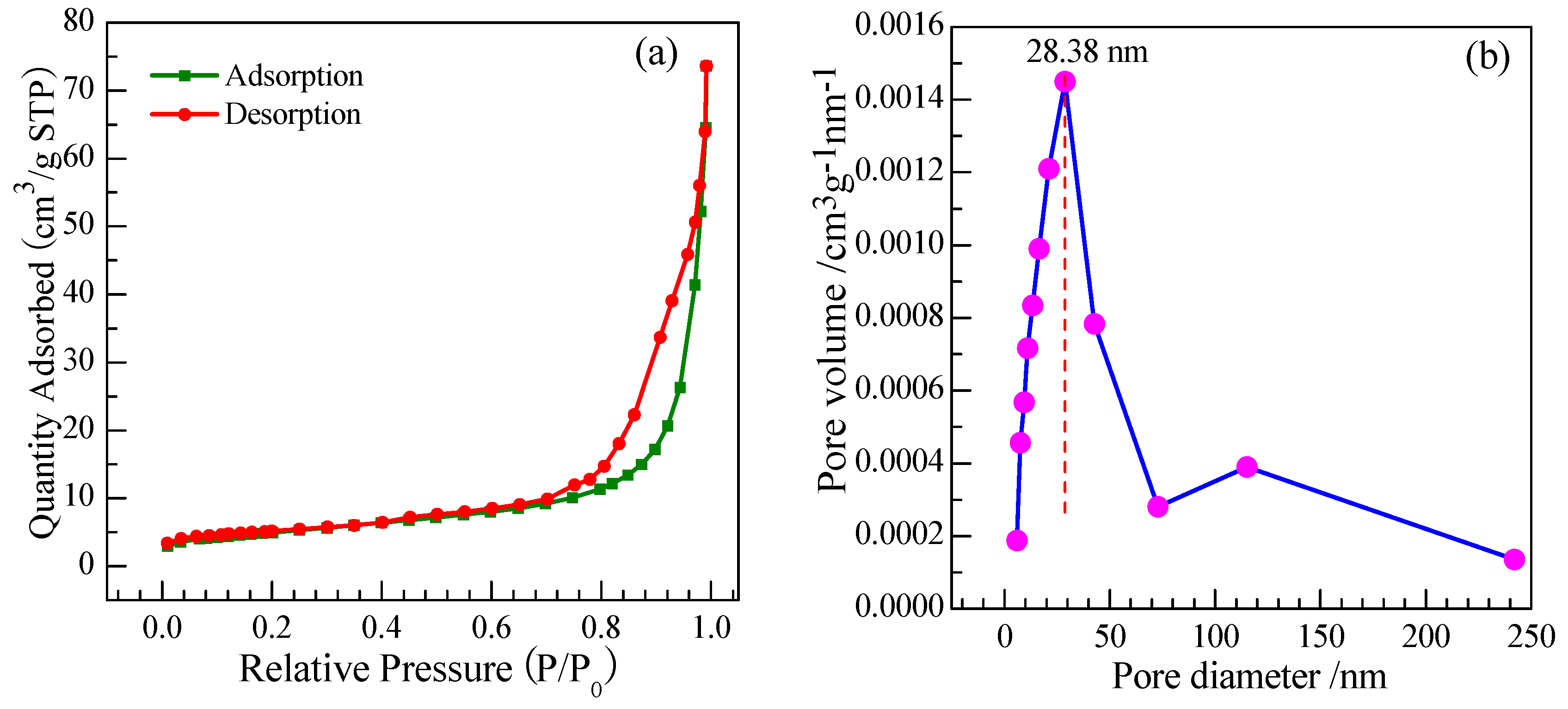
3.1.7. Brunauer-Emmett-Teller (BET)
3.2. Methanal-Sensing Performance of Eu-Doped SnO2 Nanorarray
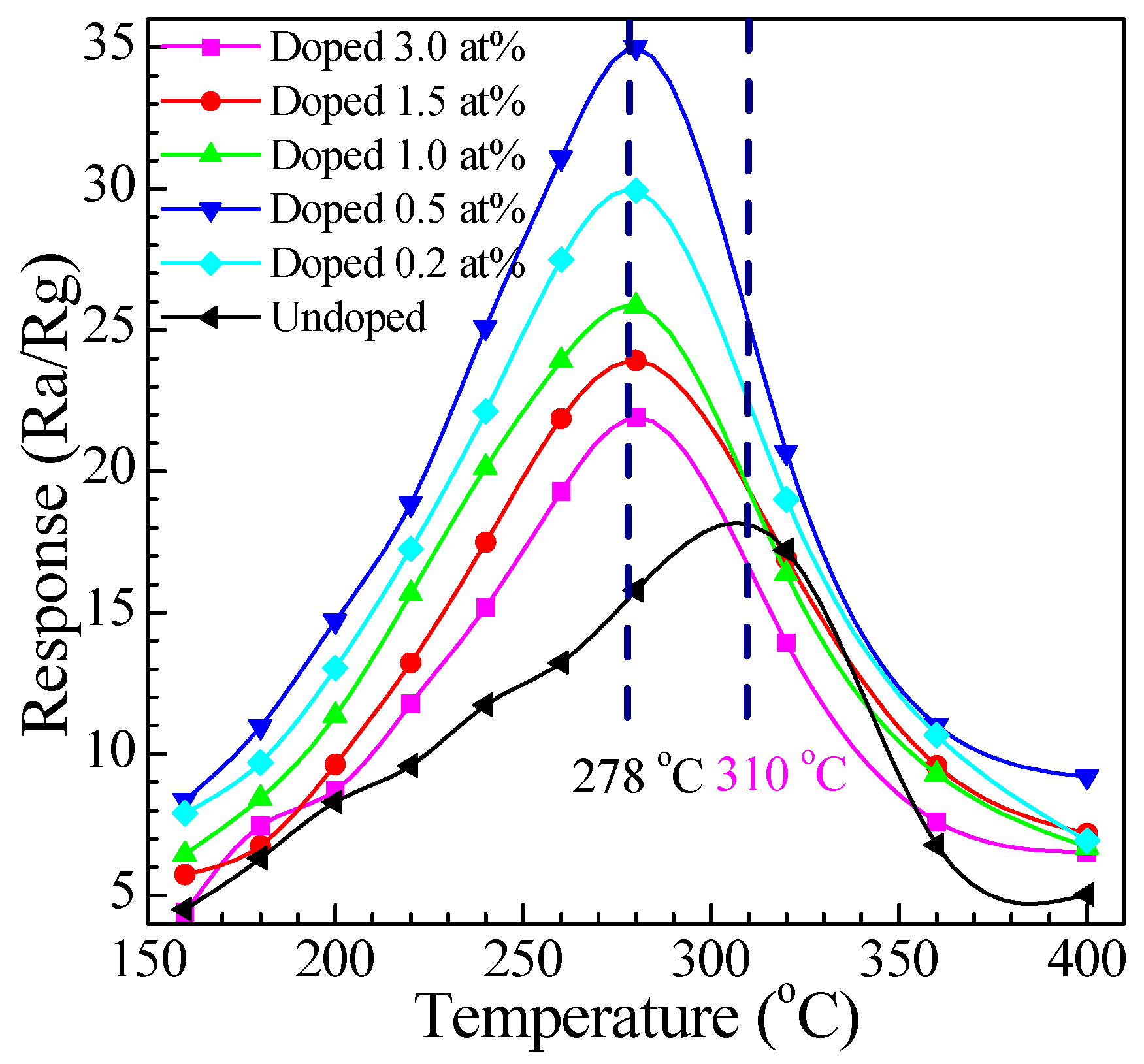
3.2.1. Relationship between Response and Operating Temperature
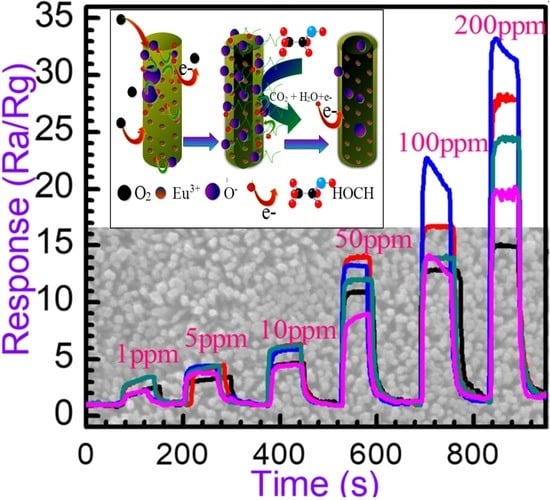
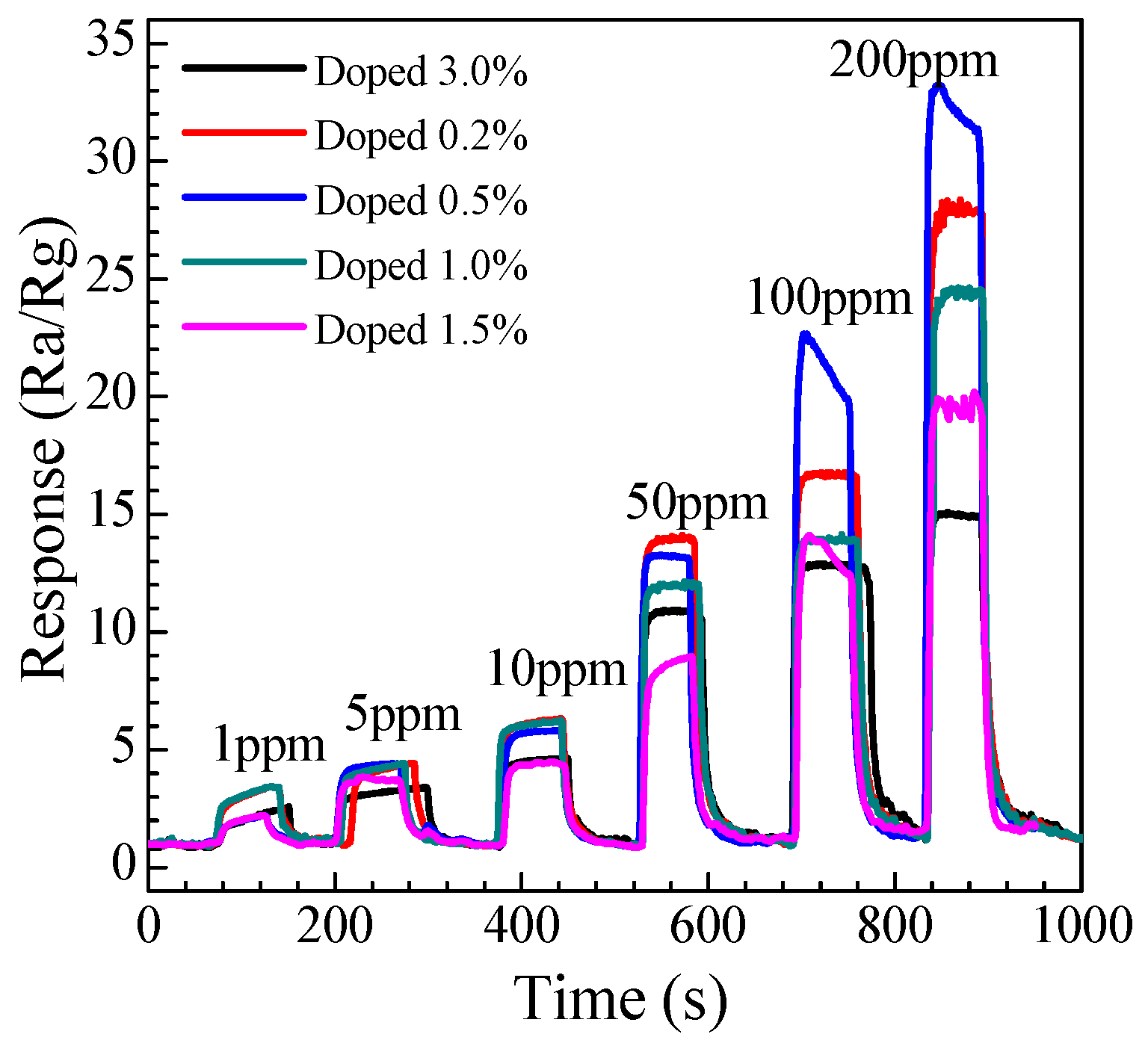
3.2.2. Dynamic Response to Different Concentration
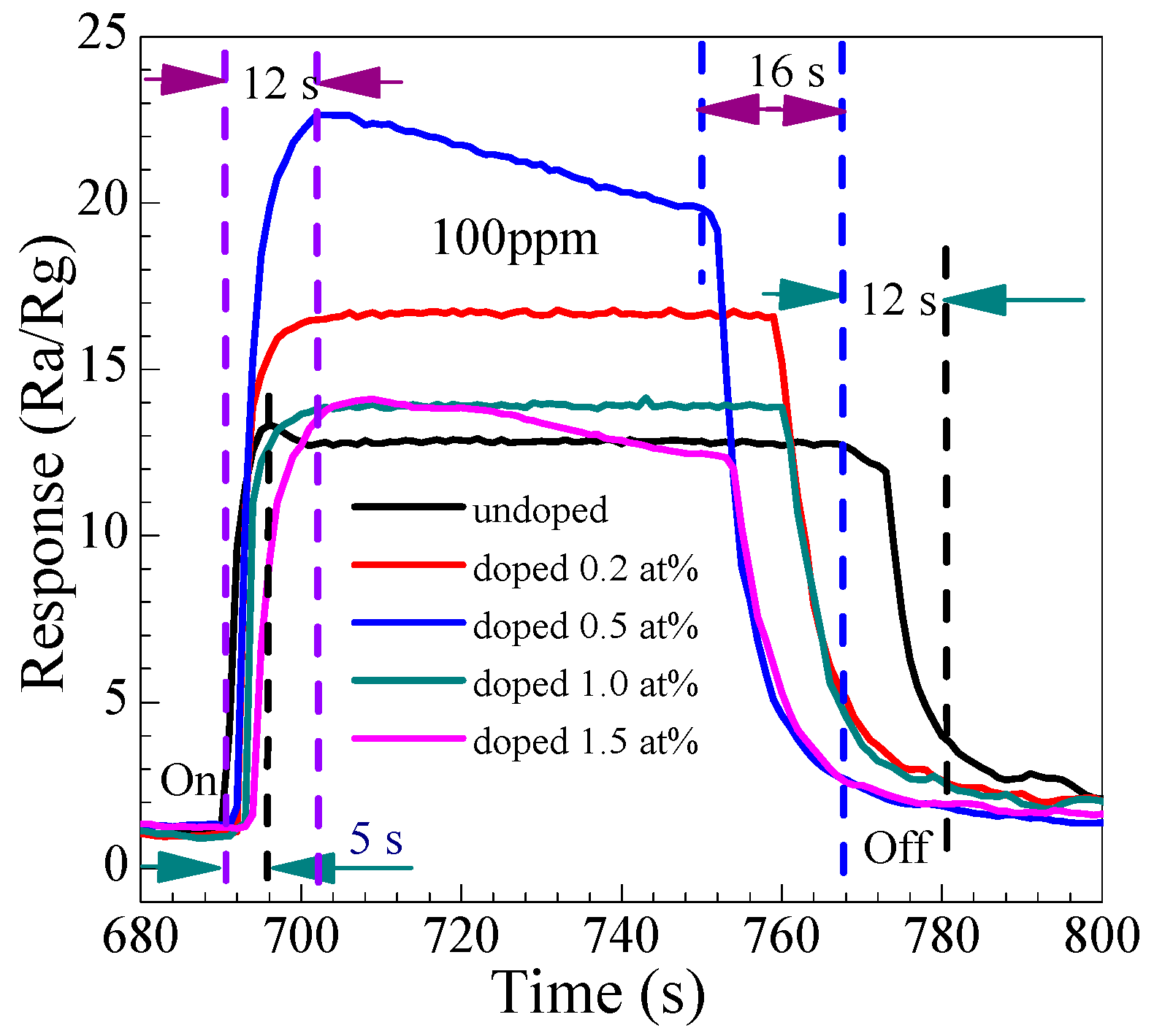
3.2.3. Gas-Sensing Response and Recovery Time
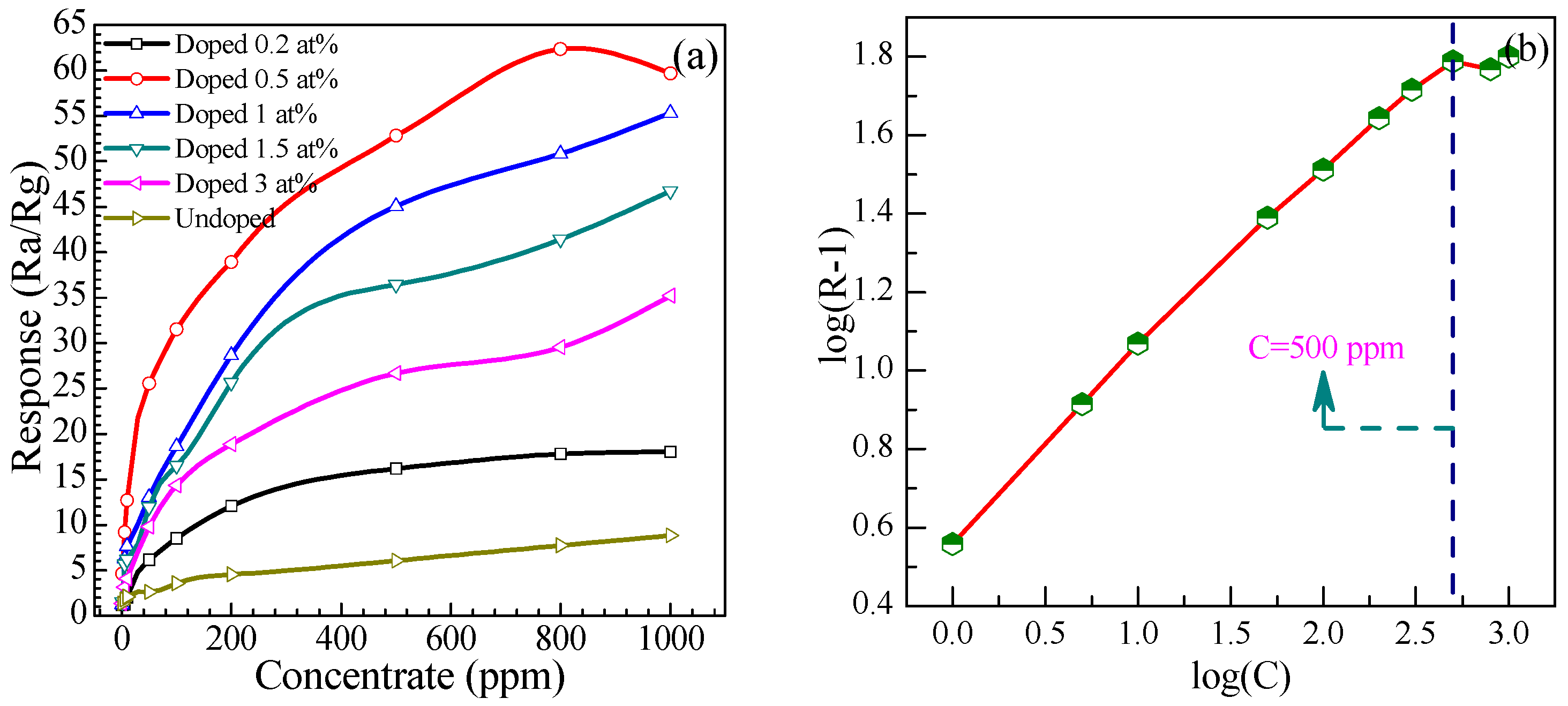
3.2.4. Relationship between Responses and Concentrations
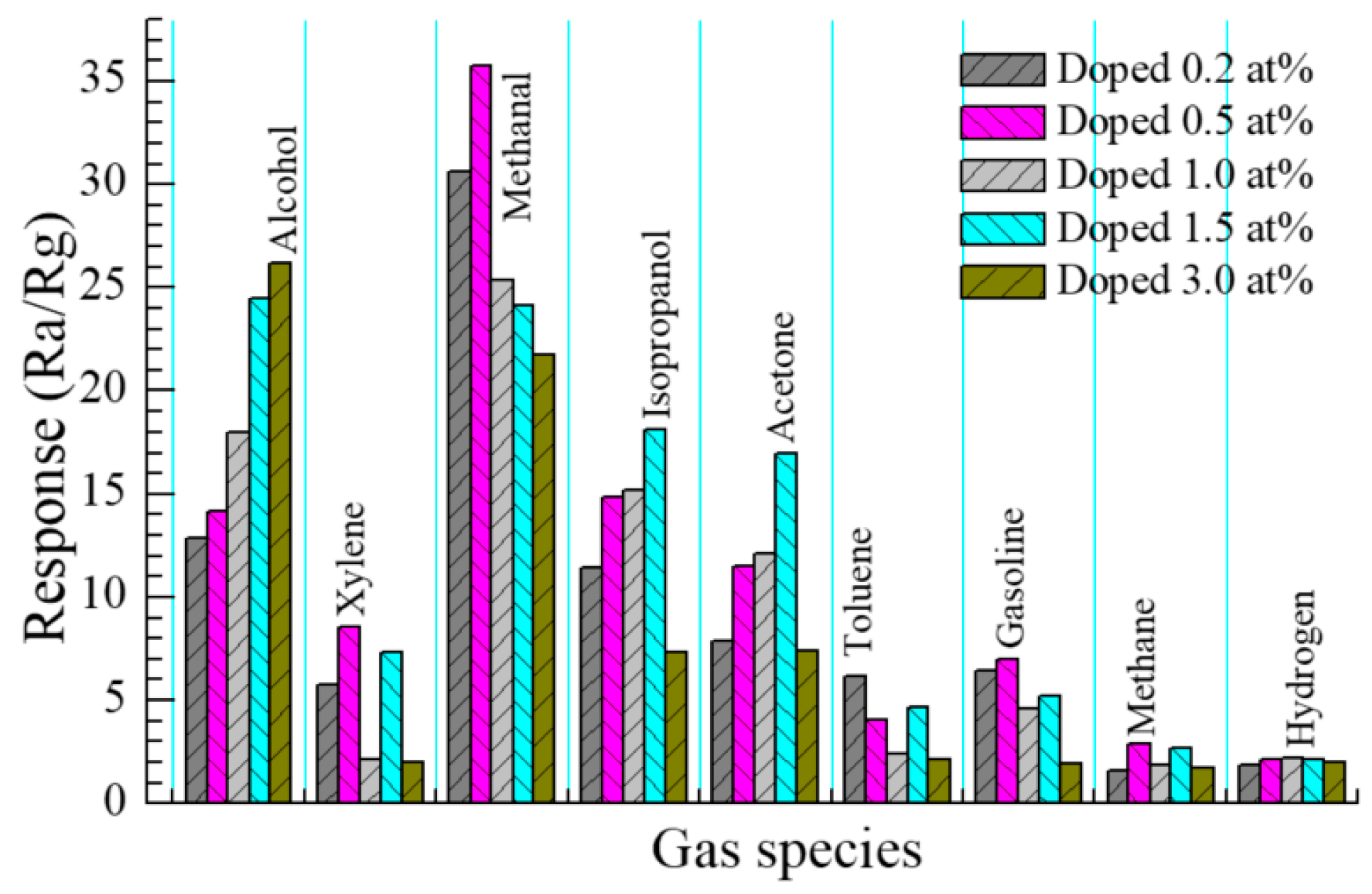
3.2.5. Selectivity of Sensor
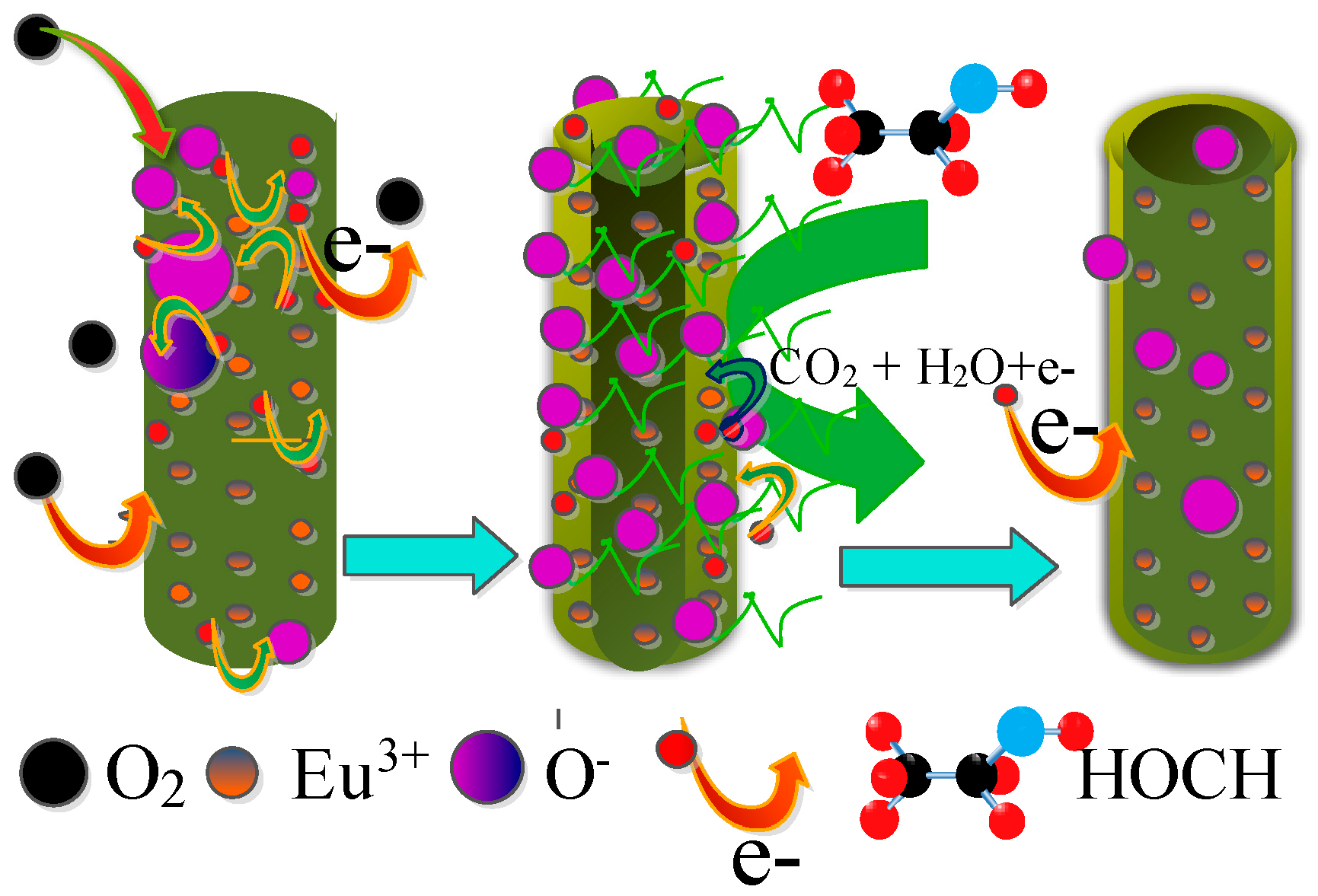
3.3. Enhanced Gas Sensing Performance Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Das, S.; Jayaraman, V. SnO2: A Comprehensive Review on Structures and Gas Sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Li, X.W.; Sun, P.; Yang, T.L.; Zhao, J.; Wang, Z.Y.; Wang, W.N.; Liu, Y.P.; Lu, G.Y.; Du, Y. Template-Free Microwave-Assisted Synthesis of ZnO Hollow Microspheres and Their Application in Gas Sensing. CrystEngComm 2013, 15, 2949–2955. [Google Scholar] [CrossRef]
- Nomani, M.W.K.; Kersey, D.; James, J.; Diwan, D.; Vogt, T.; Web, R.A.; Koley, G. Highly Sensitive and Multidimensional Detection of NO2 Using In2O3. Sens. Actuators B Chem. 2011, 160, 251–259. [Google Scholar] [CrossRef]
- Dong, C.J.; Li, Q.; Chen, G.; Xiao, X.C.; Wang, Y.D. Enhanced Formaldehyde Sensing Performance of 3D Hierarchical Porous Structure Pt-Functionalized NiO via a Facile Solution Combustion Synthesis. Sens. Actuators B Chem. 2015, 220, 171–179. [Google Scholar] [CrossRef]
- Nicolae, B.; Markus, S.; Wolfgang, G. Fundamental and Practical Aspects in the Design of Nanoscaled SnO2 Gas Sensors: A Status Report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar]
- Fratoddi, I.; Bearzotti, A.; Venditti, I.; Cametti, C.; Russo, M.V. Role of Nanostructured Polymers on the Improvement of Electrical Response-Based Relative Humidity Sensors. Sens. Actuators B Chem. 2016, 225, 96–108. [Google Scholar] [CrossRef]
- Bearzotti, A.; Macagnano, A.; Pantalei, S.; Zampetti, E.; Venditti, I.; Fratoddi, I.; Vittoria Russo, M. Alcohol Vapors Sensory Properties of Nanostructured Conjugated Polymer. J. Phys. Condens. Matter 2008, 20, 1005–1008. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Q.; Zhang, S.; Zong, P.; Yang, F. Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers. Sensors 2017, 17, 2351. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.D.; Chen, G.F.; Zhou, X.; Zeng, W.; Zhang, H. The Gas Sensitivity of Nano TiO2-SnO2 Film Doped with Cd(II) Ion. Mater. Lett. 2009, 63, 2277–2279. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, S.Y.; Li, X.B.; Luo, J.; Li, W.Q.; Li, F.M.; Mao, Y.Z.; Wang, T.T.; Li, Y.F. Highly Sensitive Acetone Sensors Based on Y-Doped SnO2 Prismatic Hollow Nanofibers Synthesized by Electrospinning. Sens. Actuators B 2014, 200, 181–190. [Google Scholar] [CrossRef]
- Cao, J.L.; Qin, C.; Wang, Y.; Zhang, H.L.; Sun, G.; Zhang, Z.Y. Solid-State Method Synthesis of SnO2-Decorated g-C3N4 Nanocomposites with Enhanced Gas-Sensing Property to Ethanol. Materials 2017, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, B.; Gong, Y.; Zhang, H.; Sun, G.; Bala, H.; Zhang, Z. Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application. Nanomaterials 2017, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.H.; Ma, S.Y.; Sun, A.M.; Zhang, Z.M.; Jin, W.X.; Wang, T.T.; Li, W.Q.; Xu, X.L.; Luo, J.; Chen, L.; et al. Hydrothermal Self-Assembly of Novel Porous Flower-Like SnO2 Architecture and Its Application in Ethanol Sensor. Appl. Surf. Sci. 2015, 355, 1192–1200. [Google Scholar] [CrossRef]
- Comini, E.; Sberveglieri, G. Metal Oxide Nanowires as Chemical Sensors. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Law, M.; Kind, H.; Messer, B.; Kim, F.; Yang, P.D. Photochemical Sensing of NO2 with SnO2 Nanoribbon Nanosensors at Room Temperature. Angew. Chem. Int. Ed. 2002, 41, 2405–2408. [Google Scholar] [CrossRef]
- Pan, J.; Ganesan, R.J.; Shen, H.; Mathur, S.J. Plasma-Modified SnO2 Nanowires for Enhanced Gas Sensing. J. Phys. Chem. C 2010, 114, 8245–8250. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Li, Y.H.; Yang, L.F.; Wu, X.H. Synthesis, Characterization and Gas-Sensing Property for C2H5OH of SnO2 Nanorods. Mater. Chem. Phys. 2008, 112, 244–248. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Pan, Z.W.; Wang, Z.L. Stable and Highly Sensitive Gas Sensor Based on Semiconducting Oxide Nanobelts. Appl. Phys. Lett. 2002, 81, 1869–1871. [Google Scholar] [CrossRef]
- Shi, L.; Lin, H.L. Preparation of Band Gap Tunable SnO2 Nanotubes and Their Ethanol Sensing Properties. Langmuir 2011, 27, 3977–3981. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yang, R.; Wang, Z.L. Growth of Horizonatal ZnO Nanowire Arrays on Any Substrate. J. Phys. Chem. C 2008, 112, 18734–18736. [Google Scholar] [CrossRef]
- Luo, L.; Jiang, Q.P.; Qin, G.H.; Zhao, K.; Du, G.F.; Wang, H.; Zhao, H.Y. Gas Sensing Characteristics of Novel Twin-Layered SnO2 Nanoarray Fabricated by Substrate-Free Hydrothermal Route. Sens. Actuators B Chem. 2015, 218, 205–214. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zou, R.J.; Song, G.S.; Yu, L.; Chen, Z.G.; Hu, J.Q. Highly Aligned SnO2 Nanorods on Graphene Sheets for Gas Sensors. J. Mater. Chem. 2011, 21, 17360–17365. [Google Scholar] [CrossRef]
- Vayssieres, L.; Prof, M.G. Highly Ordered SnO2 Nanorod Arrays from Controlled Aqueous Growth. Angew. Chem. 2004, 116, 3752–3756. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Yang, H.; Li, X.; Li, N.; Shi, R.; Zhao, H.; Yu, J. Low-Temperature Vapor–Solid Growth and Excellent Field Emission Performance of Highly Oriented SnO2 Nanorod arrays. Acta Mater. 2011, 59, 1291–1299. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, J.; Liu, M.L. Well-Aligned “Nano-Box-Beams” of SnO2. Adv. Mater. 2004, 16, 353–356. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.H.; Zhao, Y.P.; Wan, W.J.; Du, G.F.; Ren, X.P.; Zhao, H.Y. Facile Synthesis of Flower-Like Hierarchical Architecture of SnO2 Nanoarrays. J. Alloys Compd. 2017, 703, 354–360. [Google Scholar] [CrossRef]
- Costa, A.C.; Kiminami, R.H.; Santos, P.T.; Silva, J.F. ZnAl2O4 Co-Doped with Yb3+/Er3+ Prepared by Combustion Reaction: Evaluation of Photophysical Properties. J. Mater. Sci. 2013, 48, 172–177. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, B.; Jiao, L.; Cui, F.D.; Xu, H.X.; Yang, Y.T.; Yu, R.H.; Cheng, X.N. Sol-Gel Synthesis of Y2O3-Doped ZnO Thin Films Varistors and Their Electrical Properties. Trans. Nonferrous Met. Soc. China 2012, 22, s110–s114. [Google Scholar] [CrossRef]
- Li, B.; Metiu, H. DFT Studies of Oxygen Vacancies on Undoped and Doped La2O3 Surfaces. J. Phys. Chem. C 2010, 114, 12234–12244. [Google Scholar] [CrossRef]
- Deng, R.; Zhang, X.T.; Zhang, E.; Liang, Y.; Liu, Z.; Xu, H.; Hark, S.K. Planar Defects in Sn-Doped Single-Crystal ZnO Nanobelts. J. Phys. Chem. C 2007, 111, 13013–13015. [Google Scholar] [CrossRef]
- Matsushima, S.; Maekawal, T.; Tamaki, J.; Miura, N.; Yamazoe, N. Role of Additives on Alcohol Sensing by Semiconductor Gas Sensor. Chem. Lett. 1989, 18, 845–848. [Google Scholar] [CrossRef]
- Gao, F.; Qin, G.H.; Li, Y.H.; Jiang, Q.P.; Luo, L.; Zhao, K.; Liu, Y.J.; Zhao, H.Y. One-Pot Synthesis of La-Doped SnO2 Layered Nanoarrays with an Enhanced Gas-Sensing Performance Toward Acetone. RSC Adv. 2016, 6, 10298–10310. [Google Scholar] [CrossRef]
- Wang, T.T.; Ma, S.Y.; Cheng, L.; Luo, J.; Jiang, X.H.; Jin, W.X. Preparation of Yb-doped SnO2 Hollow Nanofibers with an Enhanced Ethanol-Gas Sensing Performance by Electrospinning. Sens. Actuators B Chem. 2015, 216, 212–220. [Google Scholar] [CrossRef]
- Li, W.Q.; Ma, S.Y.; Li, Y.F.; Li, X.B.; Wang, C.Y.; Yang, X.H.; Cheng, L.; Mao, Y.Z.; Luo, J.; Gengzang, D.J.; et al. Preparation of Pr-Doped SnO2 Hollow Nanofibers by Electrospinning Method and Their Gas Sensing Properties. J. Alloys Compd. 2014, 605, 80–88. [Google Scholar] [CrossRef]
- Pourfayaz, F.; Mortazavi, Y.; Khodadadi, A.; Ajami, S. Ceria-Doped SnO2 Sensor Highly Selective to Ethanol in Humid Air. Sens. Actuators B Chem. 2008, 130, 625–629. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Khodadadi, A.A.; Mortazavi, Y. CO and Ethanol Dual Selective Sensor of Sm2O3-Doped SnO2, Nanoparticles Synthesized by Microwave-Induced Combustion. Sens. Actuators B Chem. 2010, 144, 131–138. [Google Scholar] [CrossRef]
- Bazargan, S.; Leung, K.T. Nano-Environment Effects on the Luminescence Properties of Eu3+-Doped Nanocrystalline SnO2 Thin Films. J. Chem. Phys. 2012, 137, 184704. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Du, G.F.; Luo, L.; Qin, G.H.; Jiang, Q.P.; Liu, Y.J.; Zhao, H.Y. Novel Multi-Layered SnO2 Nanoarray: Self-Sustained Hydrothermal Synthesis, Structure, Morphology Dependence and Growth Mechanism. CrystEngComm 2015, 17, 2030–2040. [Google Scholar] [CrossRef]
- Tompson, P.; Cox, D.E.; Hastings, J.B. Rietveld Refinement of Debye-Scherrer Synchrotron X-ray Data from Al2O3. J. Appl. Cryst. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- McCusker, L.B.; Dreele, R.B.V.; Cox, D.E.; LoueErd, D.; Scardi, P. Rietveld Refinement Guidelines. J. Appl. Crystal. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Zaka, A.K.; Majid, W.H.A.; Abrishamib, M.E.; Yousefi, R. X-Ray Analysis of ZnO Nanoparticles by Williamson–Hall and Size–Strain Plot Methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Chikhale, L.P.; Patil, J.Y.; Rajgure, A.V.; Shaikh, F.I.; Mulla, I.S.; Suryavanshi, S.S. Structural, Morphological and Gas Sensing Properties of Undoped and Lanthanum Doped Nanocrystalline SnO2. Ceram. Int. 2014, 40, 2179–2186. [Google Scholar] [CrossRef]
- Aragón, F.H.; Coaquira, J.A.H.; Hidalgo, P.; Cohen, R.; Nagamine, L.C.; da Silva, S.W.; Morais, P.C.; Brito, H.F. Experimental Evidences of Substitutional Solution of Er Dopant in Er-doped SnO2 Nanoparticles. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Pereira, G.J.; Castro, R.H.R.; Hidalgo, P.; Gouvea, D. Surface Segregation of Additives on SnO2 Based Powders and Their Relationship with Macroscopic Properties. Appl. Surf. Sci. 2002, 195, 277–283. [Google Scholar] [CrossRef]
- Weber, I.T.; Maciel, A.P.; Lisboa-Filho, P.N.; Longo, E.; Leite, E.R.; Paiva-Santos, C.O.; Maniette, Y.; Schreiner, W.H. Effects of Synthesis and Processing on Supersaturated Rare Earth-Doped Nanometric SnO2 Powders. Nano Lett. 2002, 2, 969–973. [Google Scholar] [CrossRef]
- Freeman, C.M.; Catlow, C.R.A. A Computer Modeling Study of Defect and Dopant States in SnO2. J. Solid. State. Chem. 1990, 85, 65–75. [Google Scholar] [CrossRef]
- Oviedo, J.; Gillan, M.J. Energetics and Structure of Stoichiometric SnO2 Surfaces Studied by First-Principles Calculations. Surf. Sci. 2000, 463, 93–101. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the Gap. J. Non-Cryst. Solids 1972, 8–10, 569–585. [Google Scholar] [CrossRef]
- Sharma, A.; Prakash, D.; Verma, K.D. Optical Characterization of Hydrothermally Grown SnO2 Nanocrystals. J. Optoelectron. Adv. Mater. 2009, 3, 331–337. [Google Scholar]
- Raghupathi, P.S.; George, J.; Menon, C.S. Effect of Substrate Temperature on the Electrical and Optical Properties of Reactively Evaporated Tin Oxide Thin Films. Indian J. Pure Appl. Phys. 2005, 43, 460–466. [Google Scholar]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. Porous ZnO Polygonal Nanoflakes: Synthesis, Use in High-Sensitivity NO2 Gas Sensor, and Proposed Mechanism of Gas Sensing. J. Phys. Chem. C 2011, 115, 12763–12773. [Google Scholar] [CrossRef]
- Yao, M.S.; Ding, F.; Cao, Y.B.; Hu, P.; Fan, J.M.; Lu, C.; Yuan, F.L.; Shi, C.Y.; Chen, Y.F. Sn Doped ZnO Layered Porous Nanocrystals with Hierarchical Structures and Modified Surfaces for Gas Sensor. Sens. Actuators B Chem. 2014, 201, 255–265. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigama, J.; Kishikawa, M.; Seiyama, T. Interactions of Tin Oxide Surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Ang, G.T.; Toh, G.H.; Bakar, M.Z.A.; Abdullah, A.Z.; Othman, M.R. High Sensitivity and Fast Response SnO2 and La-SnO2 Catalytic Pellet Sensors in Detecting Volatile Organic Compounds. Process. Saf. Environ. Protect 2011, 89, 186–192. [Google Scholar] [CrossRef]
- Shi, S.L.; Liu, Y.G.; Chen, Y.J.; Zhang, J.Y.; Wang, Y.G.; Wang, T.H. Ultrahigh Ethanol Response of SnO2 Nanorods at Low Working Temperature Arising from La2O3 Loading. Sens. Actuators B Chem. 2009, 140, 426–431. [Google Scholar] [CrossRef]
- Williams, D.E. Semiconducting Oxides as Gas-Sensitive Resistors. Sens. Actuators B Chem. 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Batzill, M.T.; Diebold, U.R. Surface Studies of Gas Sensing Metal Oxides. Phys. Chem. Chem. Phys. 2007, 9, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.P.; Sun, F.Q.; Gu, F.L.; Zuo, Y.B.; Zhang, L.H.; Fang, C.F.; Yang, S.M.; Li, W.S. Photochemistry-Based Method for the Fabrication of SnO2 Monolayer Ordered Porous Films with Size-Tunable Surface Pores for Direct Application in Resistive-Type Gas Sensor. ACS Appl. Mater. Interfaces 2014, 6, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Liu, X.; Xiao, X.C.; Chen, G.; Wang, Y.D.; Djerdj, I. Combustion Synthesis of Porous Pt-Functionalized SnO2 Sheets for Isopropanol Gas Detection with a Significant Enhancement in Response. J. Mater. Chem. A 2014, 2, 20089–20095. [Google Scholar] [CrossRef]
- Wang, H.K.; Fu, F.; Zhang, F.H.; Wang, H.E.; Kershaw, S.V.; Xu, J.Q.; Sun, S.G.; Rogach, A.L. Hydrothermal Synthesis of Hierarchical SnO2 Microspheres for Gas Sensing and Lithium-Ion Batteries Applications: Fluoride-Mediated Formation of Solid and Hollow Structures. J. Mater. Chem. 2012, 22, 2140–2148. [Google Scholar] [CrossRef]
- Tian, S.Q.; Zhang, Y.P.; Zeng, D.W.; Wang, H.; Li, N.; Xie, C.S.; Pan, C.X.; Zhao, X.J. Surface Doping of La Ions into ZnO Nanocrystals to Lower the Optimal Working Temperature for HCHO Sensing Properties. Phys. Chem. Chem. Phys. 2015, 17, 27437–27445. [Google Scholar] [CrossRef] [PubMed]



| Eu (x, at %) (Nominal) | Mean Crystallite Size (D) (nm) | Residual Strain (ε) (%) | Lattice Parameters (Å) | c/a Ratio | u (Å) | Unit Cell Volume (V/Å3) | |
|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | ||||||
| 0 | 26.7 | 0.1924 | 4.7363 | 3.1852 | 0.6725 | 0.3014 | 71.45 |
| 0.2 | 14.5 | 0.2369 | 4.7391 | 3.1895 | 0.6730 | 0.2998 | 71.63 |
| 0.5 | 8.8 | 0.2682 | 4.7428 | 3.1961 | 0.6739 | 0.2991 | 71.89 |
| 1.0 | 9.7 | 0.2719 | 4.7432 | 3.1992 | 0.6745 | 0.2936 | 71.98 |
| 1.5 | 12.3 | 0.2769 | 4.7436 | 3.1984 | 0.6743 | 0.2965 | 71.97 |
| 3.0 | 16.6 | 0.2794 | 4.7439 | 3.1997 | 0.6744 | 0.2994 | 72.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, Y.; Ren, X.; Gao, F.; Zhao, H. The Effect of Eu Doping on Microstructure, Morphology and Methanal-Sensing Performance of Highly Ordered SnO2 Nanorods Array. Nanomaterials 2017, 7, 410. https://doi.org/10.3390/nano7120410
Zhao Y, Li Y, Ren X, Gao F, Zhao H. The Effect of Eu Doping on Microstructure, Morphology and Methanal-Sensing Performance of Highly Ordered SnO2 Nanorods Array. Nanomaterials. 2017; 7(12):410. https://doi.org/10.3390/nano7120410
Chicago/Turabian StyleZhao, Yanping, Yuehua Li, Xingping Ren, Fan Gao, and Heyun Zhao. 2017. "The Effect of Eu Doping on Microstructure, Morphology and Methanal-Sensing Performance of Highly Ordered SnO2 Nanorods Array" Nanomaterials 7, no. 12: 410. https://doi.org/10.3390/nano7120410