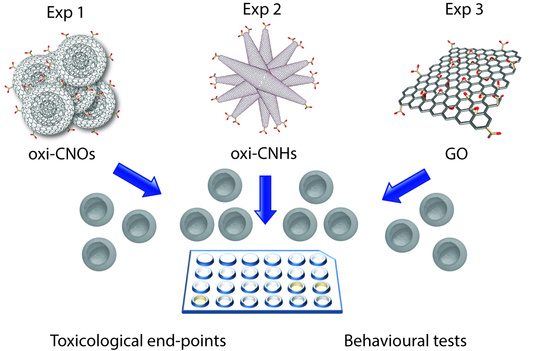
Toxicity Assessment of Carbon Nanomaterials in Zebrafish during Development
Abstract
:1. Introduction
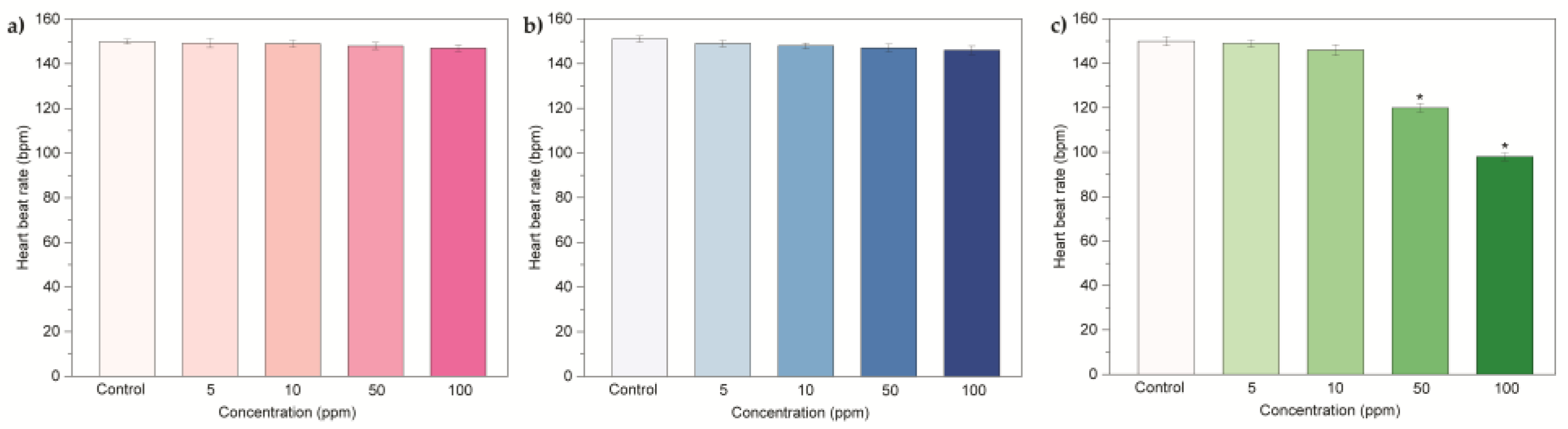
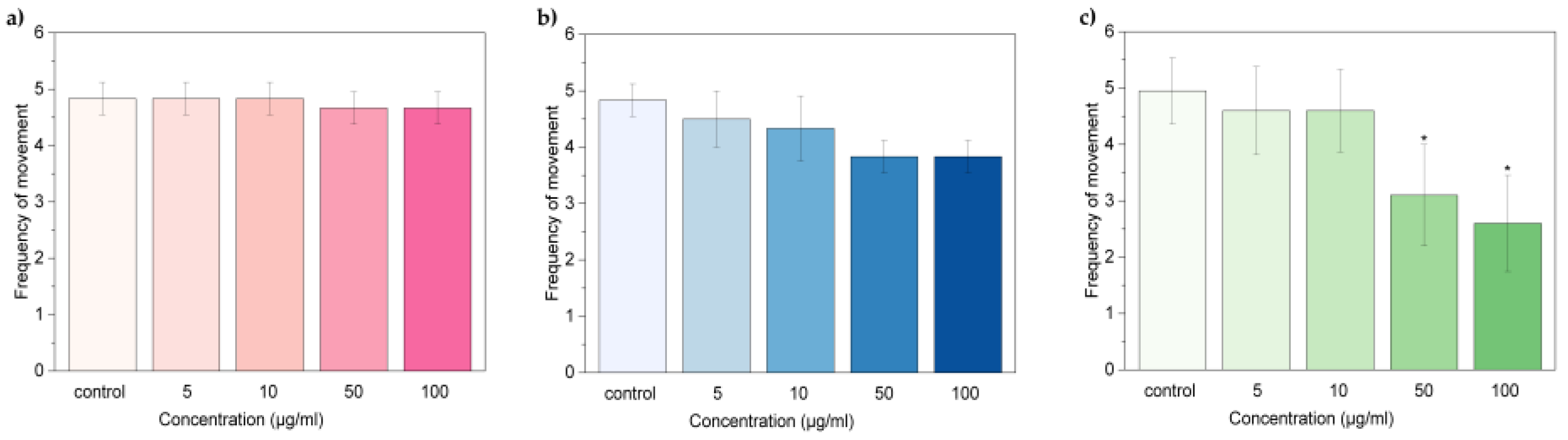
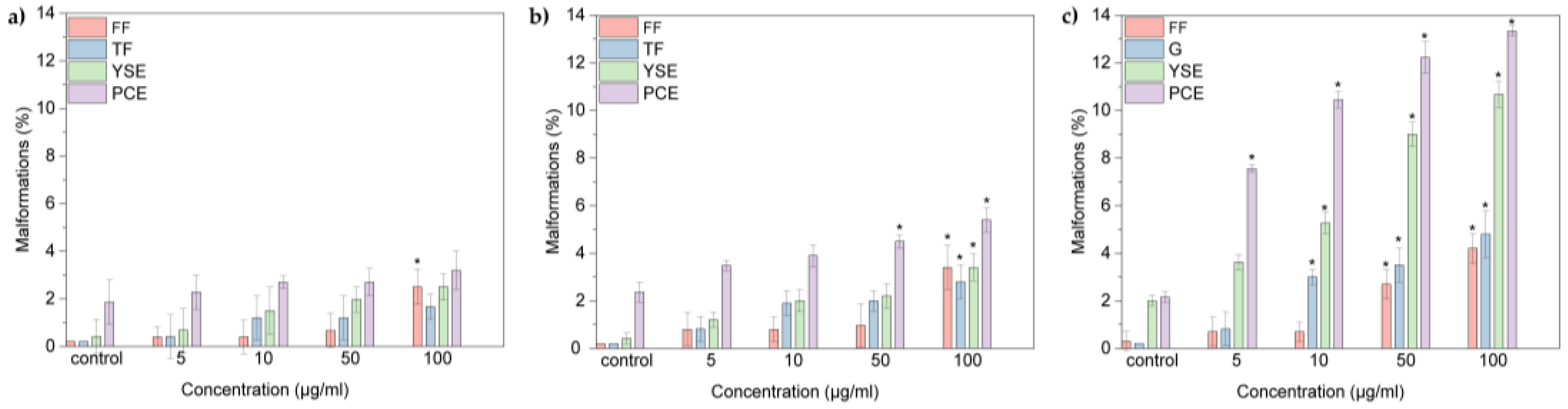
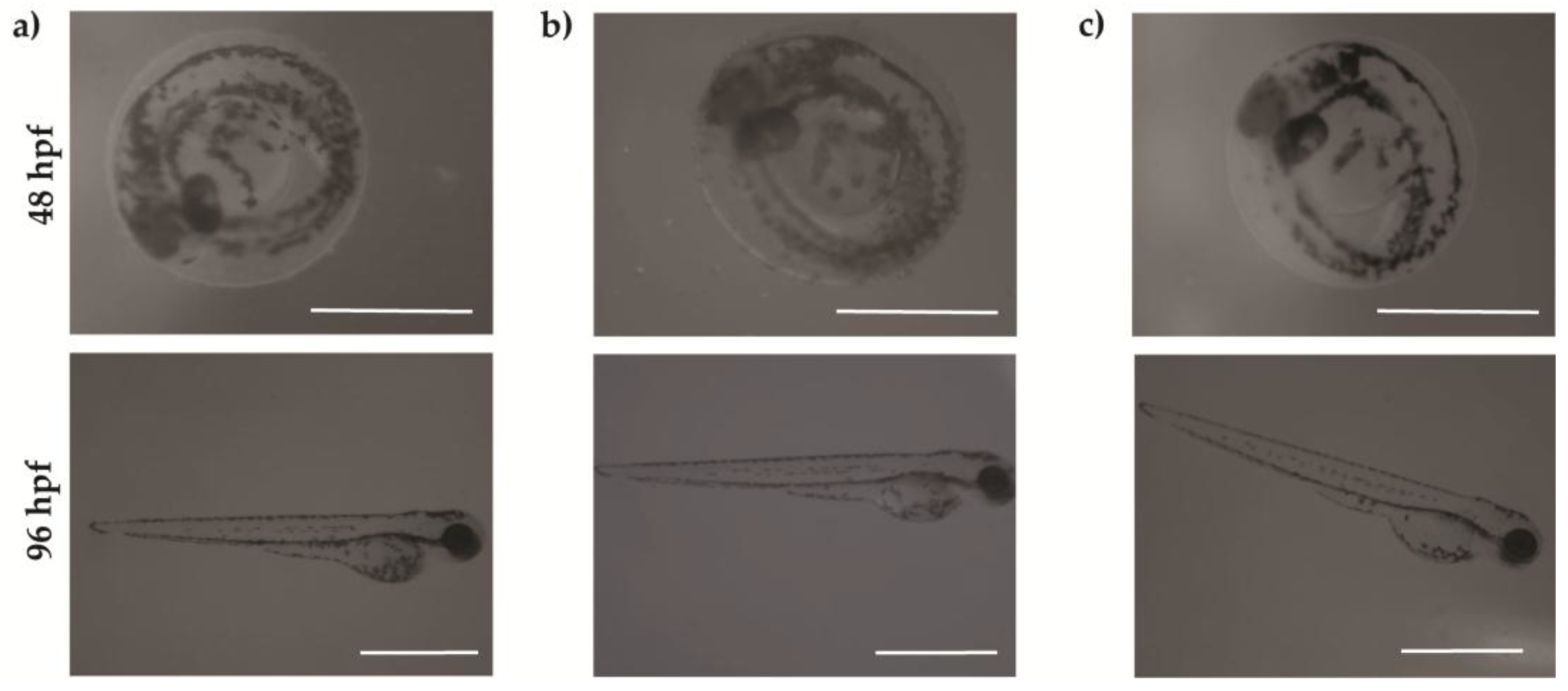
2. Results and Discussion
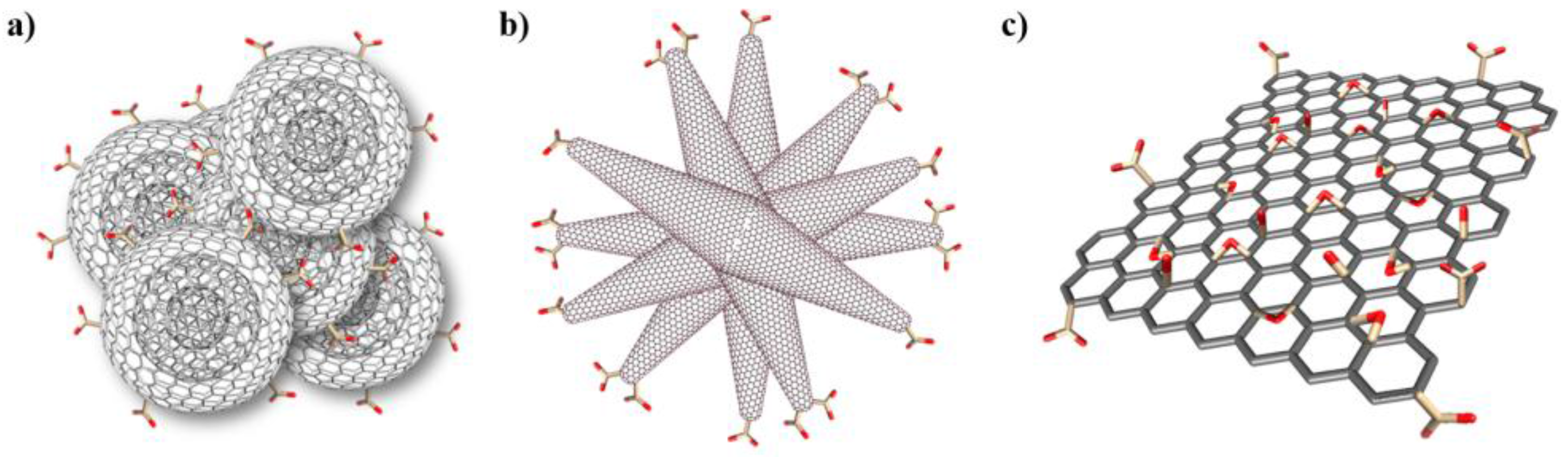
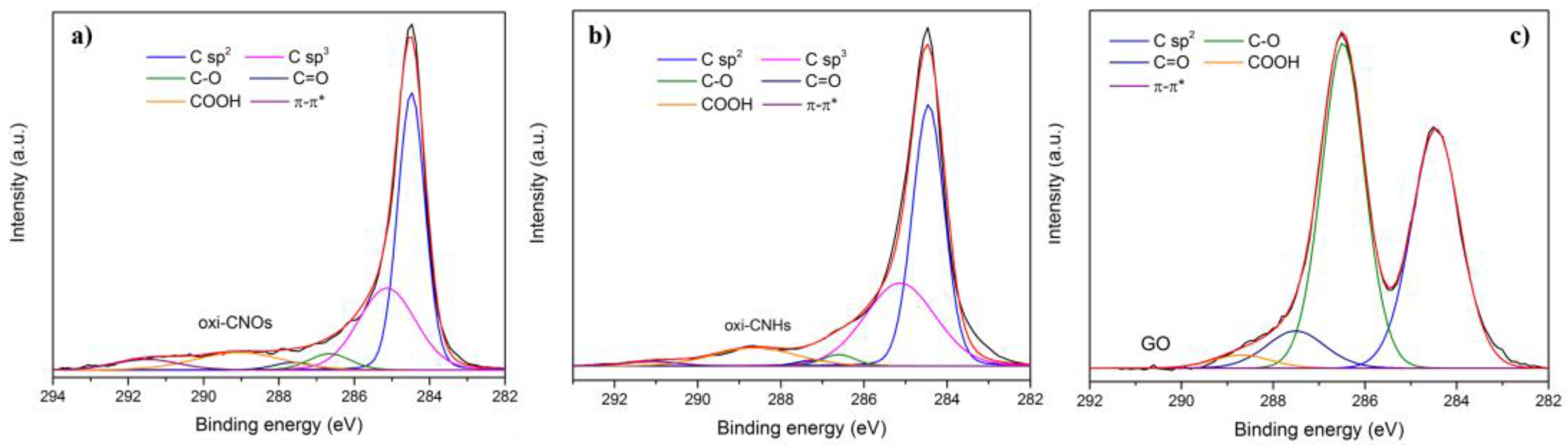
2.1 Preparation and Characterization of CNMs
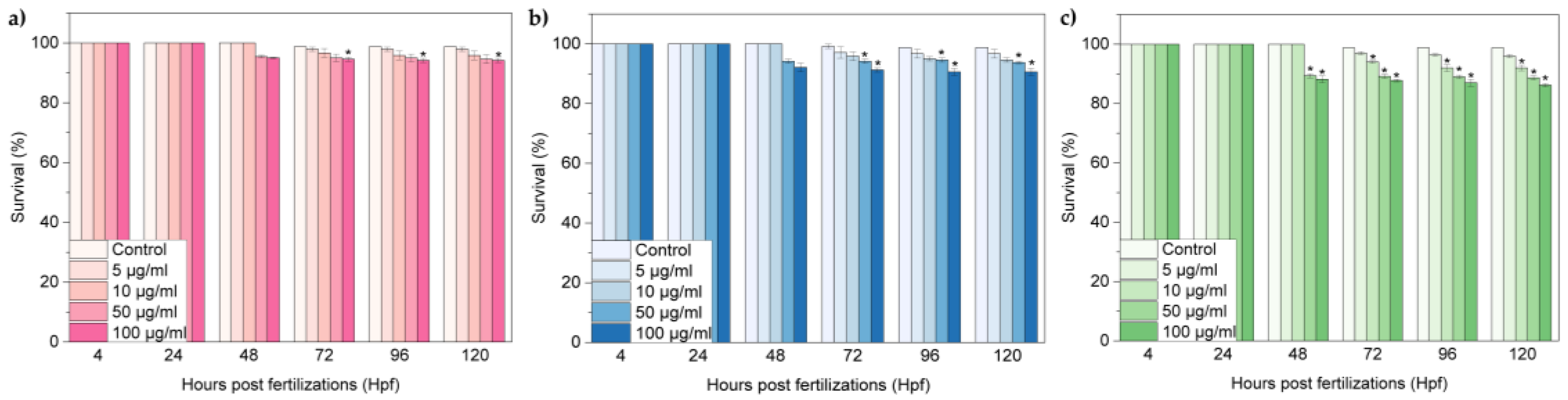
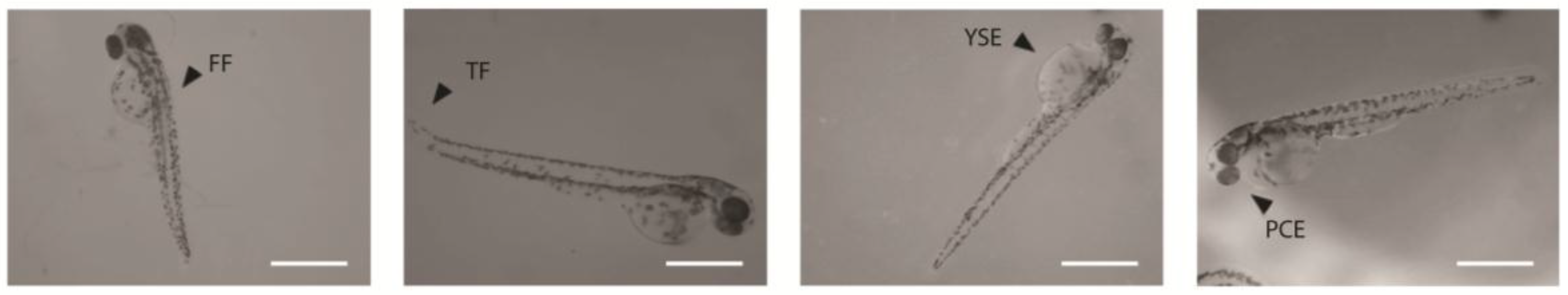
2.2 Embryonic Toxicity
3. Materials and Methods
3.1. Nanomaterials Synthesis
3.1.1. p-CNOs
3.1.2. oxi-CNOs
3.1.3. oxi-CNHs
3.2. Nanomaterials Characterization
3.2.1. Thermogravimetric Analysis (TGA)
3.2.2. Raman Spectroscopy
3.2.3. Transmission Electron Microscopy (TEM)
3.2.4. X-ray Photoelectron Spectroscopy (XPS)
3.3. In Vivo Toxicity Studies
3.3.1. Zebrafish Maintenance
3.3.2. Zebrafish Embryo Exposure to oxi-CNOs, CNHs and GO
3.3.3. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O'Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Greiner, N.R.; Phillips, D.S.; Johnson, J.D.; Volk, F. Diamonds in detonation soot. Nature 1988, 333, 440–442. [Google Scholar] [CrossRef]
- Brodie, B.C. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Qian, J.; Wang, D.; Cai, F.-H.; Xi, W.; Peng, L.; Zhu, Z.-F.; He, H.; Hu, M.-L.; He, S. Observation of multiphoton-induced fluorescence from graphene oxide nanoparticles and applications in In vivo functional bioimaging. Angew. Chem. Int. Edit. 2012, 51, 10570–10575. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, T.; Zahid, P.; Hasan, H.; Alma, R.; Trefa Mohammed, A.; Jacqueline, W.; Shaowei, Z. In vitro toxic effects of reduced graphene oxide nanosheets on lung cancer cells. Nanotechnology 2017, 28, 504001–504016. [Google Scholar]
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes. Sci. Rep. 2017, 7, 40572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Y.; Lou, Z.; Song, L.; Wu, Y.; Gu, N.; Zhang, Y. In vitro cytotoxicity evaluation of graphene oxide from the peroxidase-like activity perspective. Coll. Surf. B: Biointerfaces 2017, 151, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Mu, X.Y.; Wu, X.L.; Meng, L.X.; Guan, W.B.; Ma, Y.Q. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar] [PubMed]
- Soares, J.C.; Pereira, T.; Costa, K.M.; Maraschin, T.; Basso, N.R.; Bogo, M.R. Developmental neurotoxic effects of graphene oxide exposure in zebrafish larvae (Danio rerio). Coll. Surf. B Biointerfaces 2017, 157, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, P.; Zhang, L.; Huang, S.; Luo, L.; Huang, C. Toxicity of graphene oxide and multi-walled carbon nanotubes against human cells and zebrafish. Sci. China Chem. 2012, 55, 2209–2216. [Google Scholar] [CrossRef]
- Jeong, J.; Cho, H.-J.; Choi, M.; Lee, W.S.; Chung, B.H.; Lee, J.-S. In vivo toxicity assessment of angiogenesis and the live distribution of nano-graphene oxide and its pegylated derivatives using the developing zebrafish embryo. Carbon 2015, 93, 431–440. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A. Graphene: Safe or toxic? The two faces of the medal. Angew. Chem. Int. Edit. 2013, 52, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yudasaka, M.; Kouraba, S.; Sekido, M.; Yamamoto, Y.; Iijima, S. Single wall carbon nanohorn as a drug carrier for controlled release. Chem. Phys. Lett. 2008, 461, 189–192. [Google Scholar] [CrossRef]
- Ajima, K.; Yudasaka, M.; Murakami, T.; Maigné, A.; Shiba, K.; Iijima, S. Carbon nanohorns as anticancer drug carriers. Mol. Pharm. 2005, 2, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, M.; Bussy, C.; Iijima, S.; Kostarelos, K.; Yudasaka, M. Biodegradation of carbon nanohorns in macrophage cells. Nanoscale 2015, 7, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, J.; Yudasaka, M.; Azami, T.; Kubo, Y.; Iijima, S. Toxicity of single-walled carbon nanohorns. ACS Nano 2008, 2, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, D. Onion-like graphitic particles. Carbon 1995, 33, 989–993. [Google Scholar] [CrossRef]
- Iijima, S. Direct observation of the tetrahedral bonding in graphitized carbon black by high resolution electron microscopy. J. Cryst. Growth 1980, 50, 675–683. [Google Scholar] [CrossRef]
- Li, Y.; Kroger, M.; Liu, W.K. Shape effect in cellular uptake of pegylated nanoparticles: Comparison between sphere, rod, cube and disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Flavin, K.; Kopf, I.; Radics, G.; Hearnden, C.H.A.; McManus, G.J.; Moran, B.; Villalta-Cerdas, A.; Echegoyen, L.A.; Giordani, S.; et al. Functionalization of carbon nanoparticles modulates inflammatory cell recruitment and nlrp3 inflammasome activation. Small 2013, 9, 4194–4206. [Google Scholar] [CrossRef] [PubMed]
- Bartelmess, J.; De Luca, E.; Signorelli, A.; Baldrighi, M.; Becce, M.; Brescia, R.; Nardone, V.; Parisini, E.; Echegoyen, L.; Pompa, P.P.; et al. Boron dipyrromethene (bodipy) functionalized carbon nano-onions for high resolution cellular imaging. Nanoscale 2014, 6, 13761–13769. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Camisasca, A.; d’Amora, M.; Diaspro, A.; Uchida, T.; Nakajima, Y.; Yanagisawa, K.; Maekawa, T.; Giordani, S. Far-red fluorescent carbon nano-onions as a biocompatible platform for cellular imaging. RSC Adv. 2017, 7, 45676–45681. [Google Scholar] [CrossRef]
- Marco, F.; Viviana, M.; Juergen, B.; Luis, E.; Silvia, G. Highly surface functionalized carbon nano-onions for bright light bioimaging. Methods Appl. Fluoresc. 2015, 3, 044005. [Google Scholar]
- Frasconi, M.; Marotta, R.; Markey, L.; Flavin, K.; Spampinato, V.; Ceccone, G.; Echegoyen, L.; Scanlan, E.M.; Giordani, S. Multi-functionalized carbon nano-onions as imaging probes for cancer cells. Chem. A Eur. J. 2015, 21, 19071–19080. [Google Scholar] [CrossRef] [PubMed]
- Bartelmess, J.; Frasconi, M.; Balakrishnan, P.B.; Signorelli, A.; Echegoyen, L.; Pellegrino, T.; Giordani, S. Non-covalent functionalization of carbon nano-onions with pyrene-bodipy dyads for biological imaging. RSC Adv. 2015, 5, 50253–50258. [Google Scholar] [CrossRef]
- Lettieri, S.; d’Amora, M.; Camisasca, A.; Diaspro, A.; Giordani, S. Carbon nano-onions as fluorescent on/off modulated nanoprobes for diagnostics. Beilstein J. Nanotechnol. 2017, 8, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Giordani, S.; Bartelmess, J.; Frasconi, M.; Biondi, I.; Cheung, S.; Grossi, M.; Wu, D.; Echegoyen, L.; O’Shea, D.F. Nir fluorescence labelled carbon nano-onions: Synthesis, analysis and cellular imaging. J. Mater. Chem. B 2014, 2, 7459–7463. [Google Scholar] [CrossRef]
- Marchesano, V.; Ambrosone, A.; Bartelmess, J.; Strisciante, F.; Tino, A.; Echegoyen, L.; Tortiglione, C.; Giordani, S. Impact of carbon nano-onions on hydra vulgaris as a model organism for nanoecotoxicology. Nanomaterials 2015, 5, 1331–1350. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, M.; Rodio, M.; Bartelmess, J.; Sancataldo, G.; Brescia, R.; Cella Zanacchi, F.; Diaspro, A.; Giordani, S. Biocompatibility and biodistribution of functionalized carbon nano-onions (f-cnos) in a vertebrate model. Sci. Rep. 2016, 6, 33923. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-K.; Chen, X.; Yoon, J.; Shin, I. Zebrafish as a good vertebrate model for molecular imaging using fluorescent probes. Chem. Soc. Rev. 2011, 40, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Fako, V.E.; Furgeson, D.Y. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv. Drug Deliv. Rev. 2009, 61, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Harper, S.L.; Tanguay, R.L. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon 2007, 45, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Harper, S.L.; Tanguay, R.L. Fullerene c60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol. Appl. Pharm. 2008, 229, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-F.; Li, Y.-H.; Fang, Y.-W.; Xu, Y.; Wei, X.-M.; Yin, X.-B. Carbon quantum dots for zebrafish fluorescence imaging. Sci. Rep. 2015, 5, 11835. [Google Scholar] [CrossRef] [PubMed]
- Aryee, E.; Dalai, A.K.; Adjaye, J. Functionalization and characterization of carbon nanohorns (cnhs) for hydrotreating of gas oils. Top. Catal. 2014, 57, 796–805. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Kasuya, D.; Yudasaka, M.; Takahashi, K.; Kokai, F.; Iijima, S. Selective production of single-wall carbon nanohorn aggregates and their formation mechanism. J. Phys. Chem. B 2002, 106, 4947–4951. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Kuznetsov, V.L.; Chuvilin, A.L.; Butenko, Y.V.; Mal’kov, I.Y.; Titov, V.M. Onion-like carbon from ultra-disperse diamond. Chem. Phys. Lett. 1994, 222, 343–348. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amora, M.; Camisasca, A.; Lettieri, S.; Giordani, S. Toxicity Assessment of Carbon Nanomaterials in Zebrafish during Development. Nanomaterials 2017, 7, 414. https://doi.org/10.3390/nano7120414
D’Amora M, Camisasca A, Lettieri S, Giordani S. Toxicity Assessment of Carbon Nanomaterials in Zebrafish during Development. Nanomaterials. 2017; 7(12):414. https://doi.org/10.3390/nano7120414
Chicago/Turabian StyleD’Amora, Marta, Adalberto Camisasca, Stefania Lettieri, and Silvia Giordani. 2017. "Toxicity Assessment of Carbon Nanomaterials in Zebrafish during Development" Nanomaterials 7, no. 12: 414. https://doi.org/10.3390/nano7120414