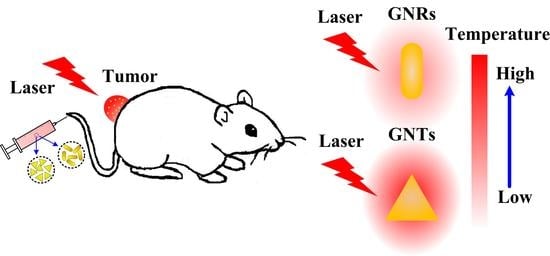
Experimental Comparison of Photothermal Conversion Efficiency of Gold Nanotriangle and Nanorod in Laser Induced Thermal Therapy
Abstract
:1. Introduction
2. Results
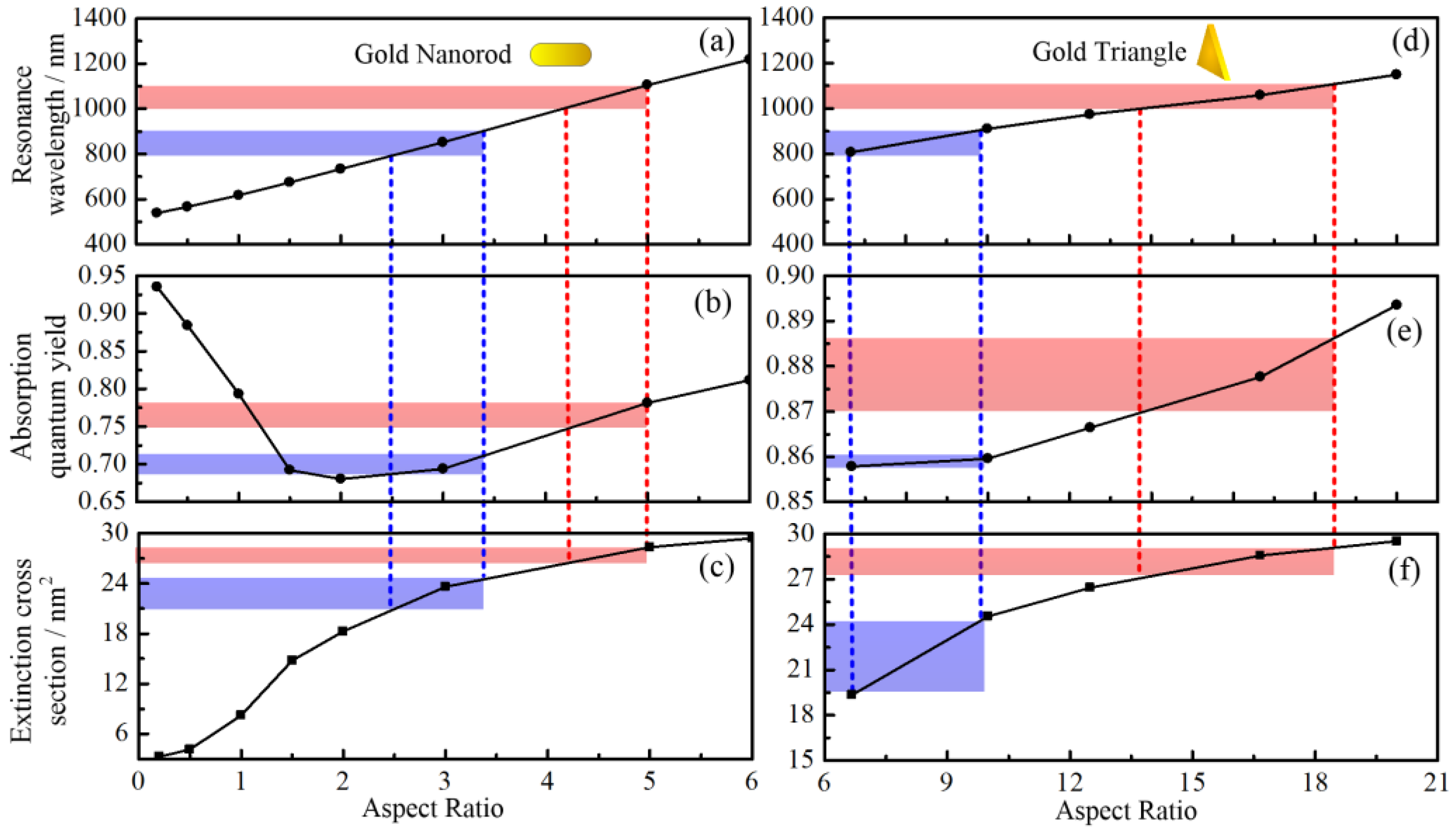
2.1. Spectral Characteristics of Gold Nanotriangles (GNTs) and Nanorods (GNRs)
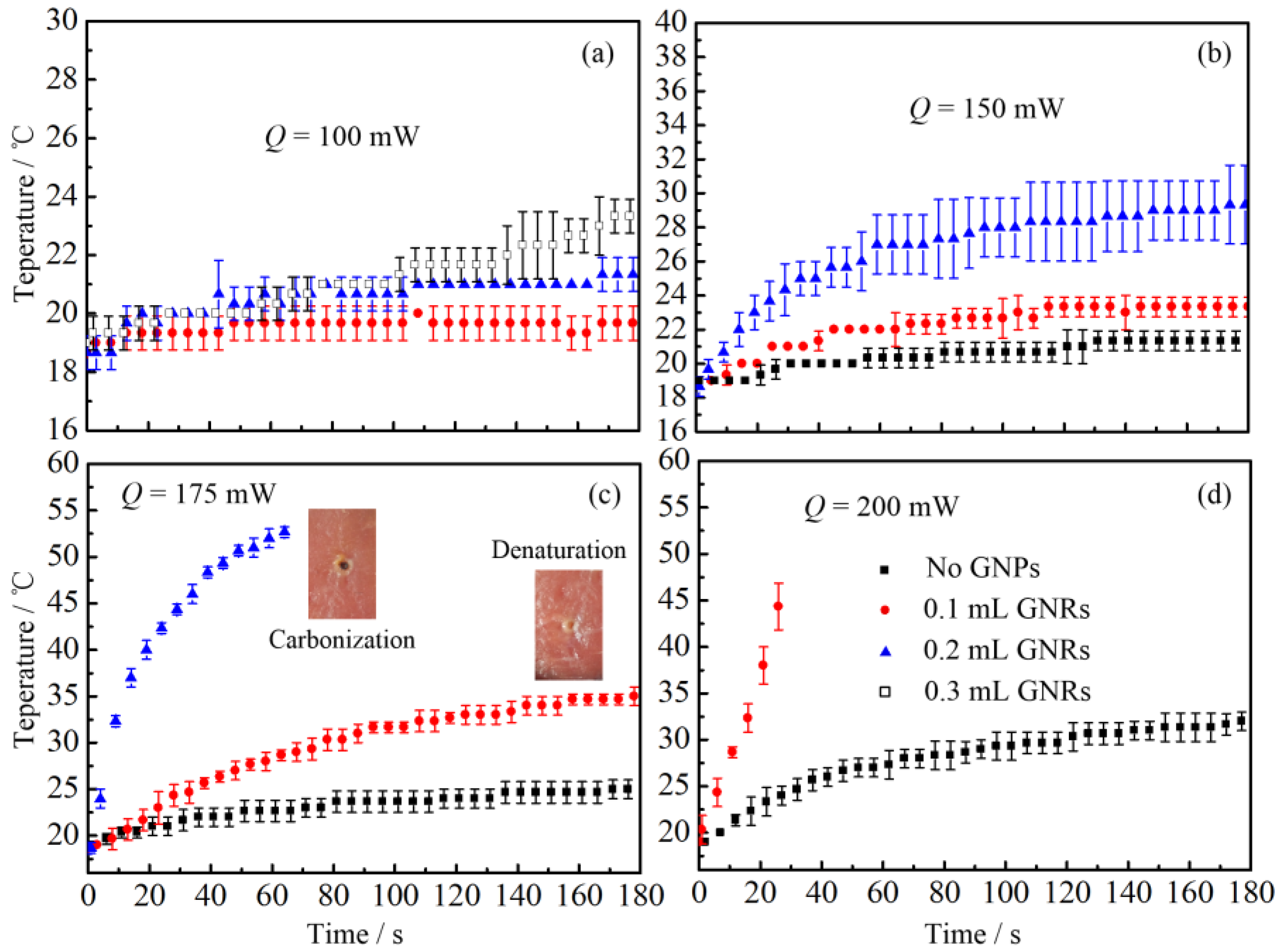
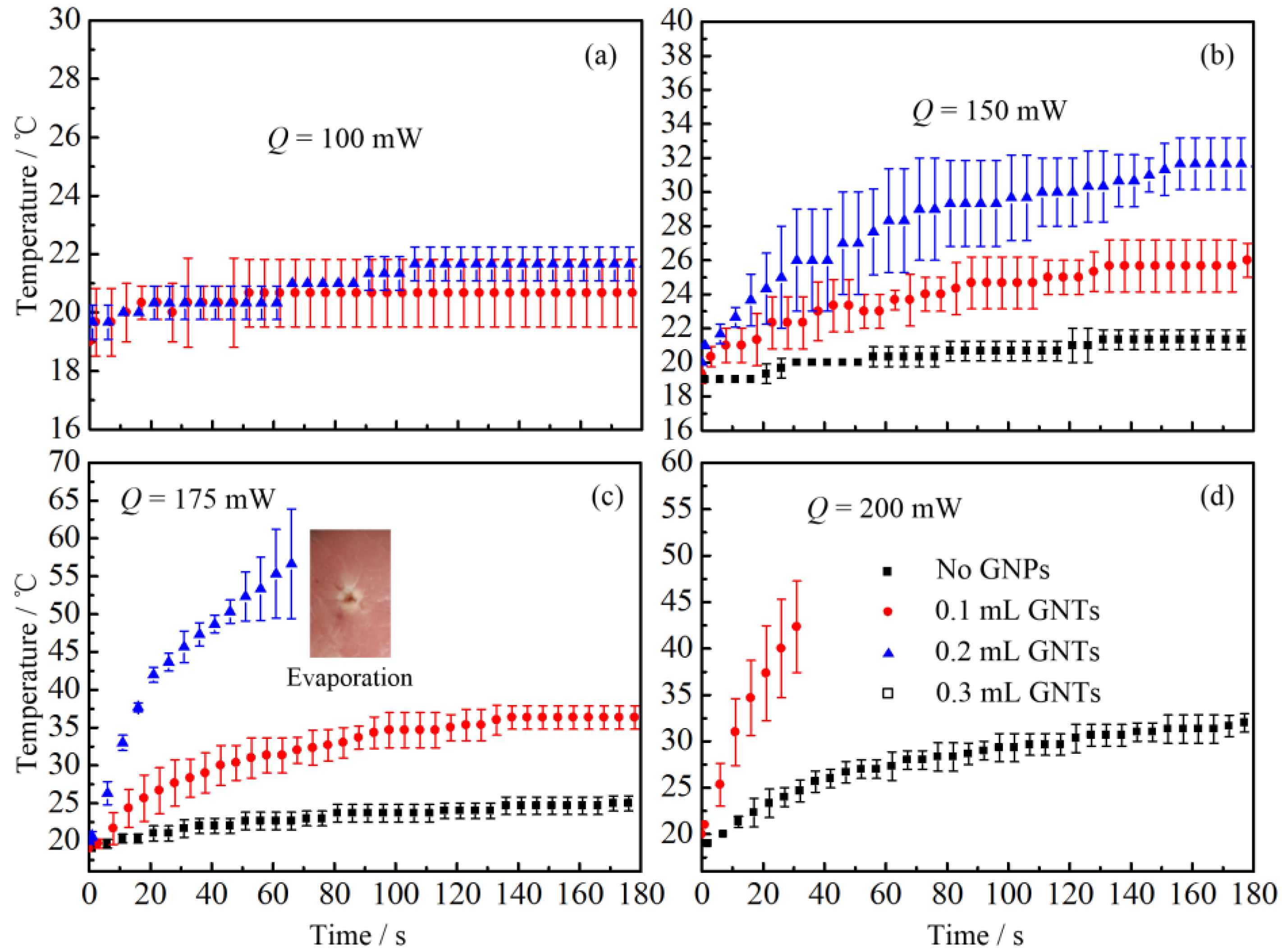
2.2. Thermal Response of Tissue In Vitro Embedded with Nanoparticles
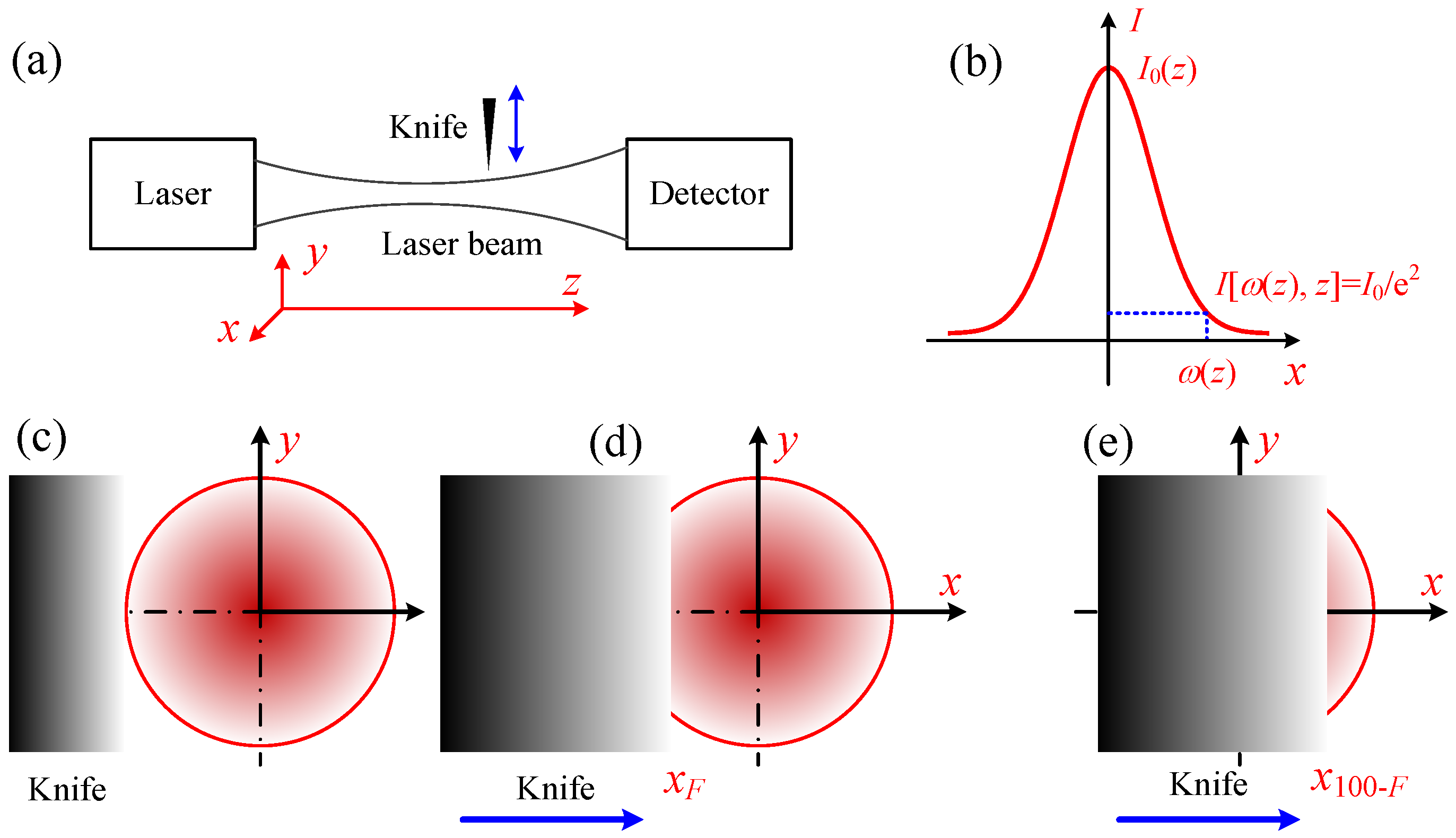
2.2.1. Laser Radius Measurement
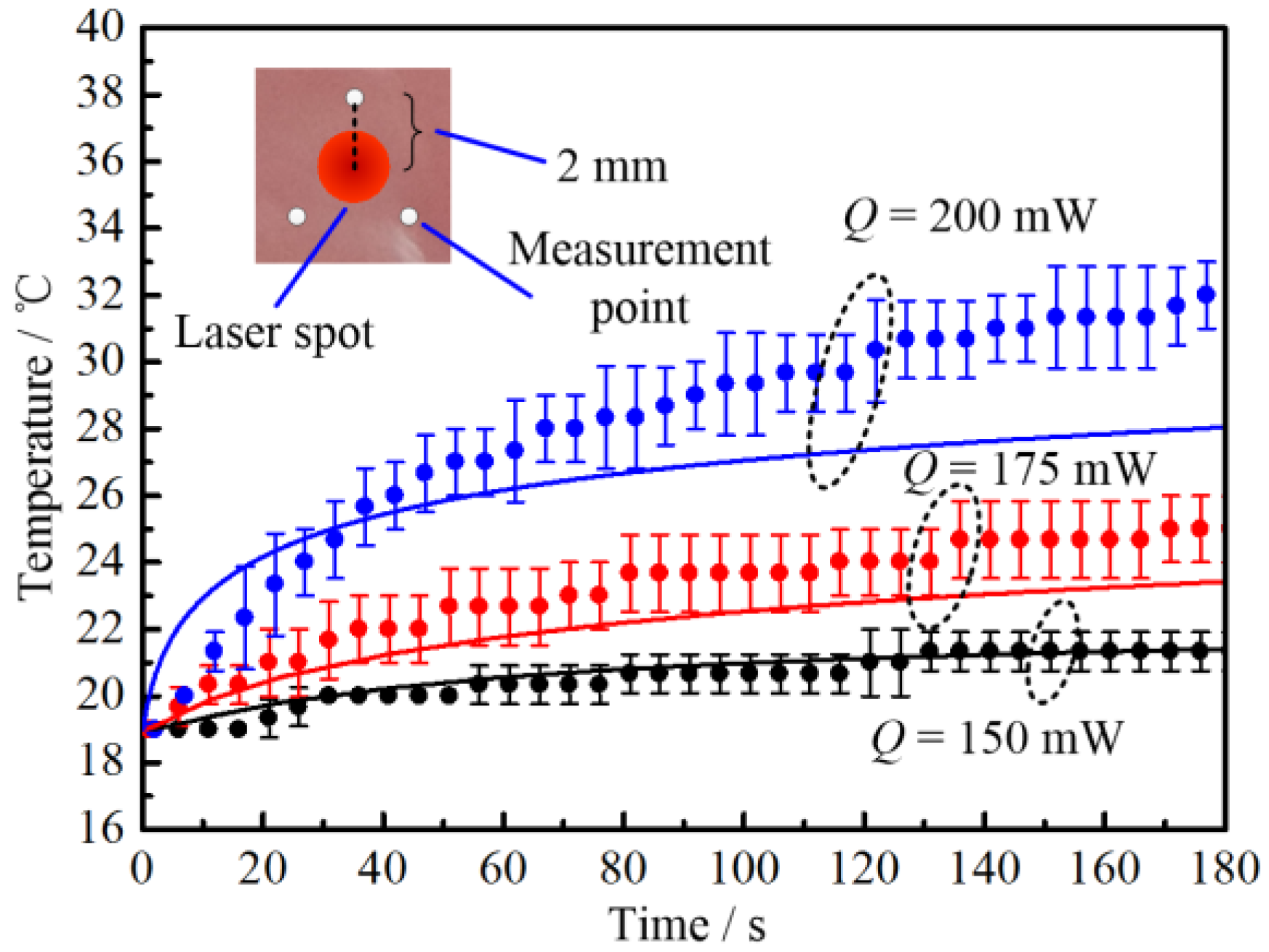
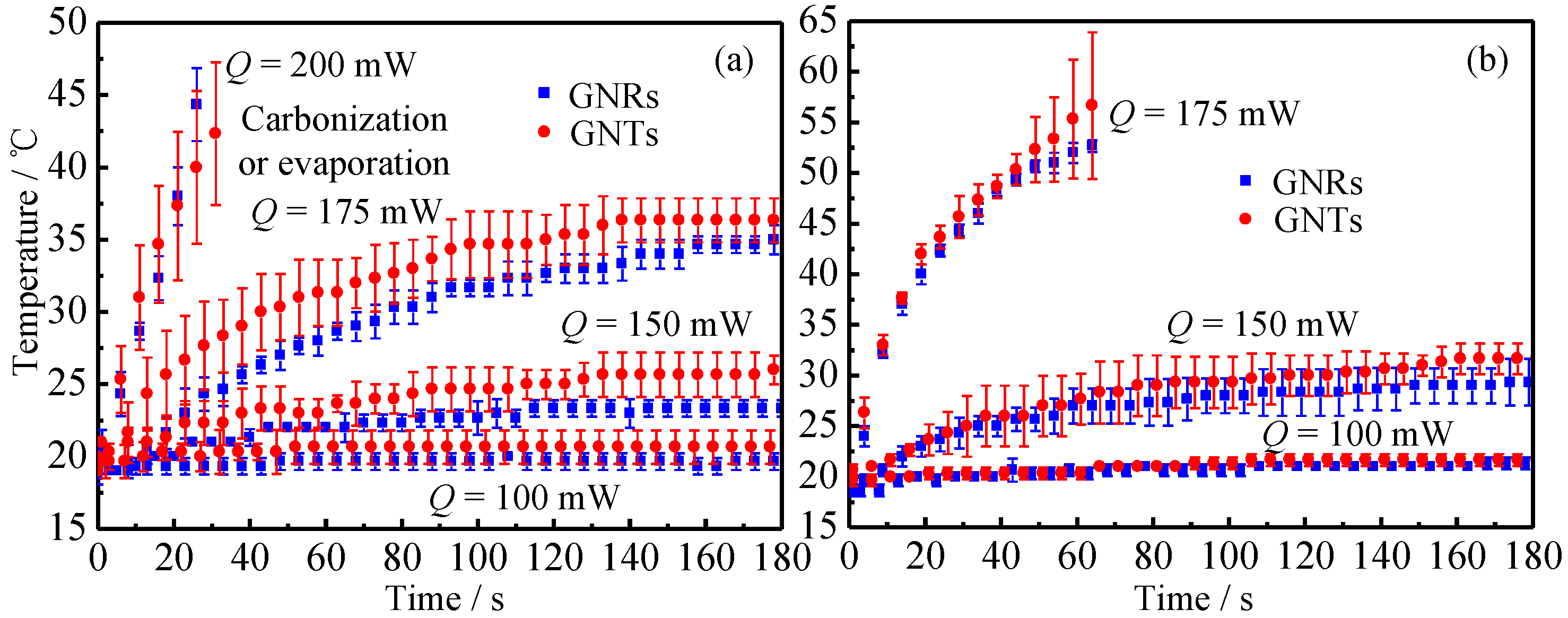
2.2.2. Laser Heating Effect
3. Discussion
3.1. Spectral Characteristic of GNRs and GNTs
3.2. Thermal Response of Tissue In Vitro Embedded with Nanoparticles
4. Materials and Methods
4.1. Gold Nanoparticles
4.2. The Discrete Dipole Approximation (DDA)
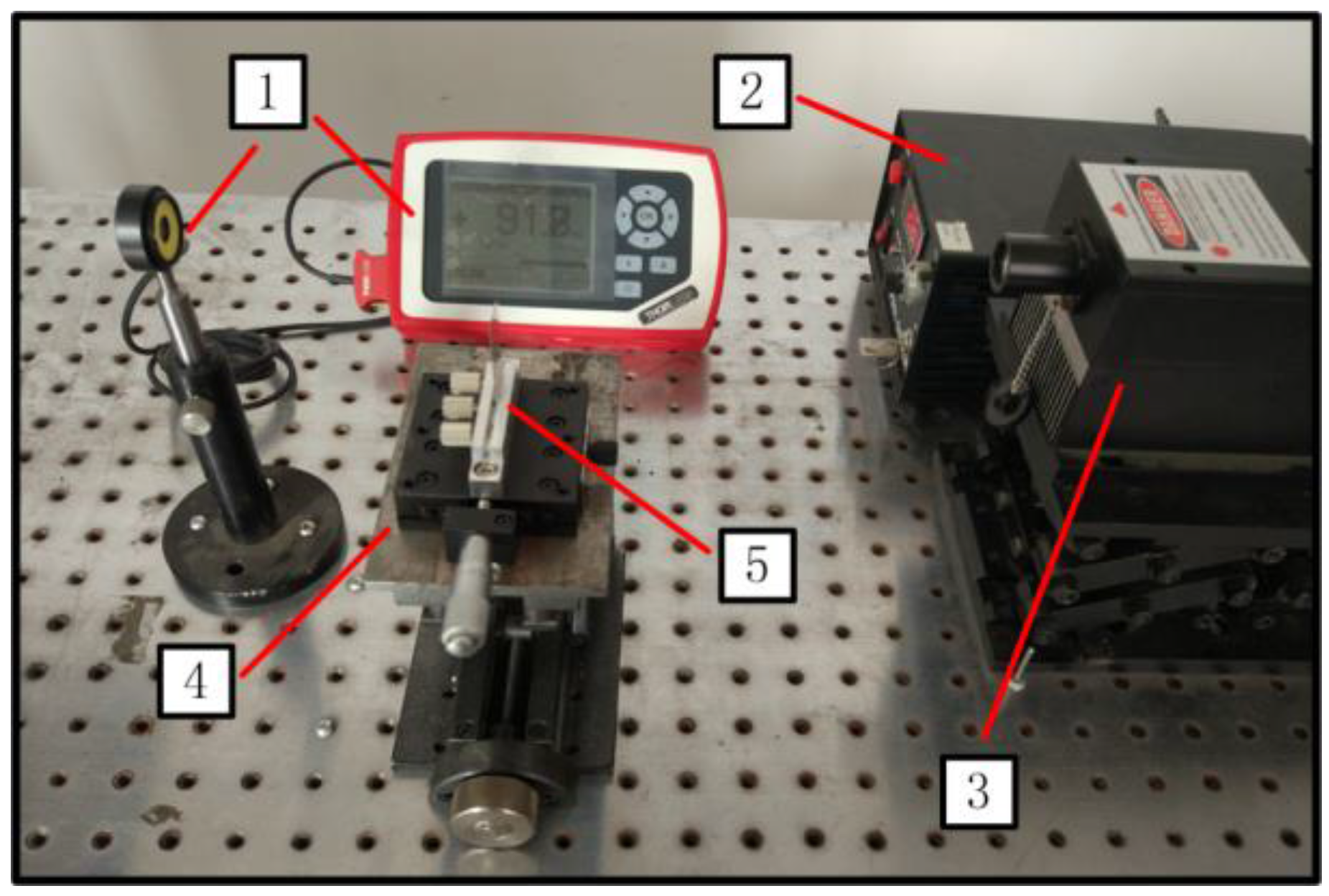
4.3. Knife-Edge Method
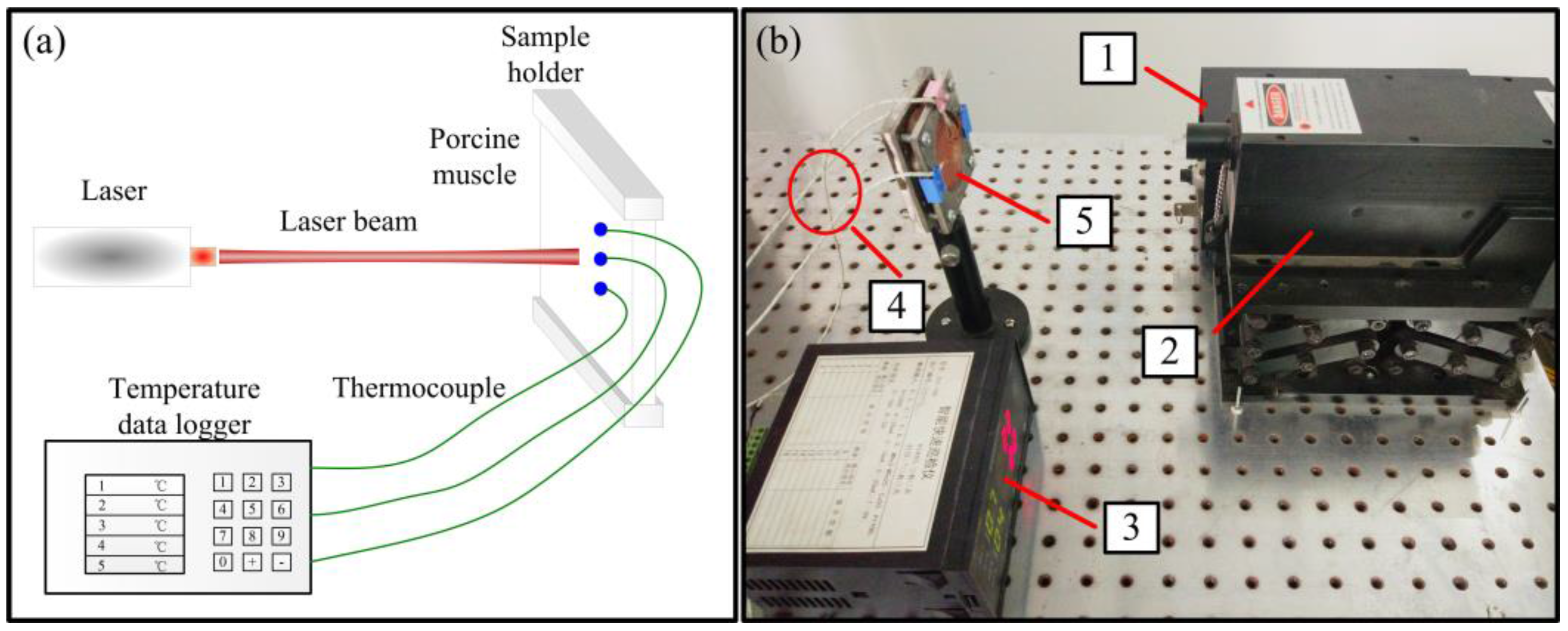
4.4. Experimental Setup
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Ren, Y.; Qi, H.; Chen, Q.; Ruan, L. Thermal dosage investigation for optimal temperature distribution in gold nanoparticle enhanced photothermal therapy. Int. J. Heat Mass Transf. 2017, 106, 212–221. [Google Scholar] [CrossRef]
- Singh, R.; Das, K.; Mishra, S.C.; Okajima, J.; Maruyama, S. Minimizing tissue surface overheating using convective cooling during laser-induced thermal therapy: A numerical study. J. Therm. Sci. Eng. Appl. 2016, 8, 011002. [Google Scholar] [CrossRef]
- Mamalis, A.; Koo, E.; Sckisel, G.D.; Siegel, D.M.; Jagdeo, J. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br. J. Dermatol. 2016, 175, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Agah, R.; Gandjbakhche, A.H.; Motamedi, M.; Nossal, R.; Bonner, R.F. Dynamics of temperature dependent optical properties of tissue: Dependence on thermally induced alteration. IEEE Trans. Biomed. Eng. 1996, 43, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.W.; Ying, H.F.; Gao, S.; Shen, Y.H.; Meng, Z.Q.; Chen, H.; Chen, Z.; Teng, W.J. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Z.B.; Chen, W.Z.; Wang, W.; Gui, Y.; Zhang, M.; Zheng, G.; Zhou, Y.; Xu, G.; Li, M.; et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview. Ultrason. Sonochem. 2004, 11, 149–154. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, W.; Ji, Z.; Li, C.; Huang, B. Microwave ablation: An experimental comparative study on internally cooled antenna versus non-internally cooled antenna in liver models. Acad. Radiol. 2010, 17, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.H.; Qian, X.; Mao, H.; Wang, A.Y.; Chen, Z.G.; Nie, S.; Shin, D.M. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int. J. Nanomed. 2008, 3, 311–321. [Google Scholar]
- Dombrovsky, L.A.; Timchenko, V.; Jackson, M. Indirect heating strategy for laser induced hyperthermia: An advanced thermal model. Int. J. Heat Mass Transf. 2012, 55, 4688–4700. [Google Scholar] [CrossRef]
- Dombrovsky, L.A.; Timchenko, V.; Jackson, M.; Yeoh, G.H. A combined transient thermal model for laser hyperthermia of tumors with embedded gold nanoshells. Int. J. Heat Mass Transf. 2011, 54, 5459–5469. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Laser Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Mackey, M.A.; Ali, M.R.K.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The most effective gold nanorod size for plasmonic photothermal therapy: Theory and in vitro experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Tyagi, H.; Taylor, R.A.; Kumar, A. Investigation on nanoparticle distribution for thermal ablation of a tumour subjected to nanoparticle assisted thermal therapy. J. Therm. Biol. 2014, 43, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Diagaradjane, P.; Cho, S.H. Nanoparticle-mediated thermal therapy: Evolving strategies for prostate cancer therapy. Int. J. Hyperth. 2010, 26, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.M.; Shetty, A.M.; Wang, J.; Hazle, J.D.; Jason Stafford, R. Use of gold nanoshells to constrain and enhance laser thermal therapy of metastatic liver tumours. Int. J. Hyperth. 2010, 26, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Von Maltzahn, G.; Park, J.H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qian, W.; El-Sayed, I.H.; El-Sayed, M.A. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Lasers Surg. Med. 2007, 39, 747–753. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Cheng, Q.; Song, J.; Si, M.; Luo, Z. Optical properties of a grating-nanorod assembly structure for solar cells. Opt. Commun. 2016, 376, 14–20. [Google Scholar] [CrossRef]
- Anderson, R.R.; Parrish, J.A. The optics of human skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Sun, I.C.; Kim, K.; Yil, D.K. Silica coated gold nanorods for imaging and photo-thermal therapy of cancer cells. J. Nanosci. Nanotechnol. 2013, 13, 3223–3329. [Google Scholar] [CrossRef]
- Tarapacki, C.; Kumaradas, C.; Karshafian, R. Enhancing laser thermal-therapy using ultrasound-microbubbles and gold nanorods of in vitro cells. Ultrasonics 2013, 53, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Grześkiewicz, B.; Ptaszyński, K.; Kotkowiak, M. Near and far-field properties of nanoprisms with rounded edges. Plasmonics 2014, 9, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Qi, H.; Chen, Q.; Wang, S.; Ruan, L. Localized surface plasmon resonance of nanotriangle dimers at different relative positions. J. Quant. Spectrosc. Radiat. Transf. 2017, 199, 45–51. [Google Scholar] [CrossRef]
- Bazán-Díaz, L.; Mendoza-Cruz, R.; Velázquez-Salazar, J.J.; Plascencia-Villa, G.; Romeu, D.; Reyes-Gasga, J.; Herrera-Becerra, R.; José-Yacamán, M.; Guisbiers, G. Gold-copper nanostars as photo-thermal agents: Synthesis and advanced electron microscopy characterization. Nanoscale 2015, 7, 20734–20742. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Yang, X.; Xia, Y. Putting gold nanocages to work for optical imaging, controlled release and cancer theranostics. Nanomedicine 2016, 11, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold nanocages as photothermal transducers for cancer treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Q.; Lee, J.Y.; Wang, D.I. The synthesis of SERS-active gold nanoflower tags for in vivo applications. ACS Nano 2008, 2, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Singh, A.; Ahmad, A.; Sastry, M. Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir 2006, 22, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Nie, C.; Du, P.; Hongwei, Z.; Wei, W.; Zhang, M.; Ambati, B.; Sun, Z. An Efficient Near-Infrared Photothermal Therapy Agent by Using Ag@ Oxides Nanoprisms in for Uveal melanoma Therapy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1540. [Google Scholar]
- Bao, C.; Conde, J.; Pan, F.; Li, C.; Zhang, C.; Tian, F.; Liang, S.; Fuente, J.M.D.L.; Cui, D. Gold nanoprisms as a hybrid in vivo cancer theranostic platform for in situ photoacoustic imaging, angiography, and localized hyperthermia. Nano Res. 2016, 9, 1043–1056. [Google Scholar] [CrossRef]
- Hodkinson, J.R.; Greenleaves, I. Computations of light-scattering and extinction by spheres according to diffraction and geometrical optics and some comparisons with the Mie theory. J. Opt. Soc. Am. 1963, 53, 577–588. [Google Scholar] [CrossRef]
- Flatau, P.J.; Draine, B.T. Fast near field calculations in the discrete dipole approximation for regular rectilinear grids. Opt. Express 2012, 20, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B. Optical constants of the noble metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, C.L.; Wang, S.M. An inverse method for flue gas shielded metal surface temperature measurement based on infrared radiation. Meas. Sci. Technol. 2016, 27, 074002. [Google Scholar] [CrossRef]
- Ren, Y.; Qi, H.; Zhao, F.; Ruan, L.; Tan, H. Simultaneous retrieval of temperature-dependent absorption coefficient and conductivity of participating media. Sci. Rep. 2016, 6, 21998. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xue, X.; Furlani, E.P. Theoretical Comparison of Optical Properties of Near-Infrared Colloidal Plasmonic Nanoparticles. Sci. Rep. 2016, 6, 34189. [Google Scholar] [CrossRef] [PubMed]
- Niemz, M.H. Laser-Tissue Interactions; Springer: Berlin/Heidelberg, Germany, 2007; pp. 29–36. [Google Scholar]
- Draine, B.T.; Flatau, P.J. Discrete-dipole approximation for scattering calculations. J. Opt. Soc. Am. A 1994, 11, 1491–1499. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, M.A. Dependence of the enhanced optical scattering efficiency relative to that of absorption for gold metal nanorods on aspect ratio, size, end-cap shape, and medium refractive index. J. Phys. Chem. B 2005, 109, 20331–20338. [Google Scholar] [CrossRef] [PubMed]
- Draine, B.T. Discrete-dipole approximation and its application to interstellar graphite grains. Astrophys. J. 1988, 333, 848–872. [Google Scholar] [CrossRef]
- Flatau, P.J. Improvements inthe discrete dipole approximation method of computing scattering and absorption. Opt. Lett. 1997, 22, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, M.A.; Silva, R.; De, L.E.; Pereira, D.P.; de Oliveira, P.C. Measurement of Gaussian laser beam radius using the knife-edge technique: Improvement on data analysis. Appl. Opt. 2009, 48, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zheng, Y.; Sun, Q.; Wang, G.; Zeng, H.; Bing, P.; Ren, H. Experimental study on measuring the beam waist of Gaussian laser beam using 90/10 knife edge method. Laser Infrared 2008, 38, 541–543. [Google Scholar]
- Khosrofian, J.M.; Garetz, B.A. Measurement of a Gaussian laser beam diameter through the direct inversion of knife-edge data. Appl. Opt. 1983, 22, 3406. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Asakura, T. A simple technique for quickly measuring the spot size of Gaussian laser beams. Opt. Laser Technol. 1976, 8, 273–274. [Google Scholar] [CrossRef]

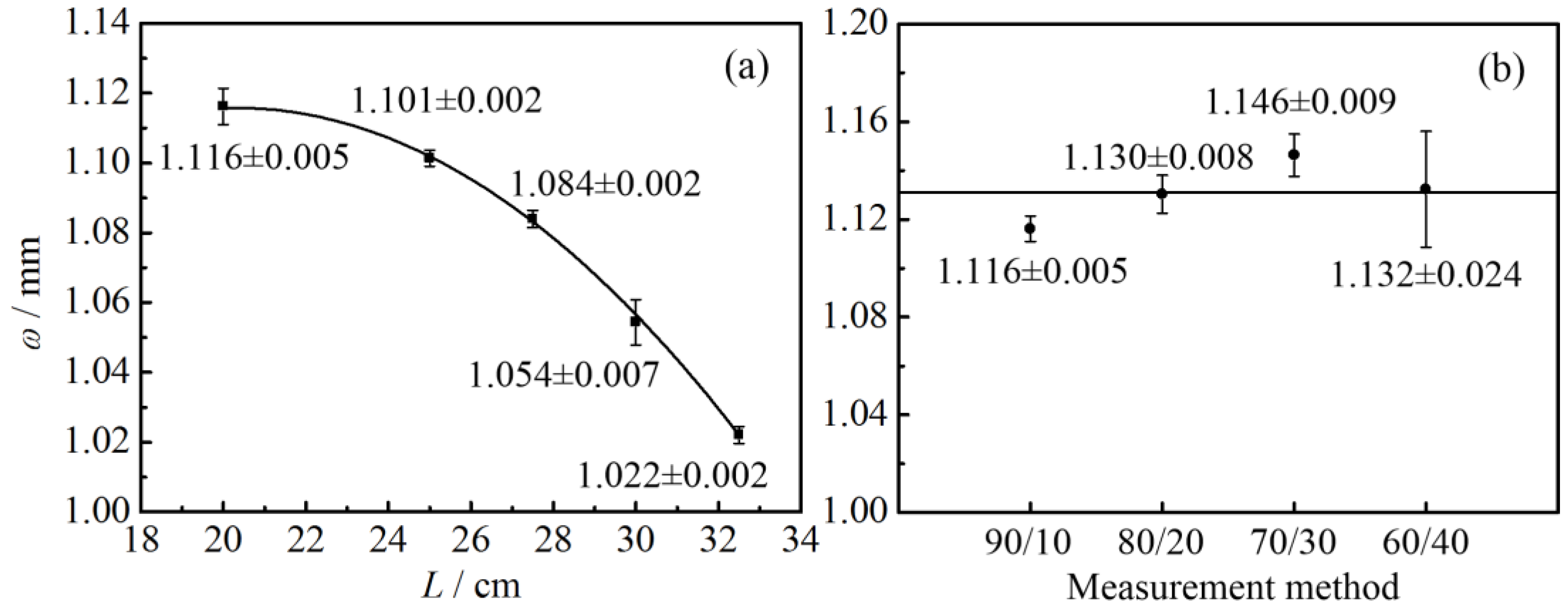

| L/cm | Measurement Position | Independent Measurement | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 20 | x90 / mm | 2.775 | 2.769 | 2.771 | 2.761 | 2.762 |
| x10 / mm | 1.341 | 1.34 | 1.330 | 1.334 | 1.338 | |
| 25 | x90 / mm | 2.628 | 2.638 | 2.639 | 2.632 | 2.637 |
| x10 / mm | 1.220 | 1.223 | 1.226 | 1.222 | 1.223 | |
| 27.5 | x90 / mm | 2.817 | 2.815 | 2.811 | 2.810 | 2.805 |
| x10 / mm | 1.429 | 1.421 | 1.421 | 1.418 | 1.419 | |
| 30 | x90 / mm | 2.825 | 2.825 | 2.86 | 2.866 | 2.863 |
| x10 / mm | 1.978 | 1.976 | 2.029 | 2.031 | 2.032 | |
| 32.5 | x90 / mm | 2.709 | 2.709 | 2.705 | 2.708 | 2.706 |
| x10 / mm | 1.402 | 1.398 | 1.392 | 1.401 | 1.391 | |
| F | 90 | 80 | 70 | 60 |
|---|---|---|---|---|
| A | 1.560 | 2.375 | 3.817 | 7.874 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Chen, Q.; Qi, H.; Ruan, L.; Ren, Y. Experimental Comparison of Photothermal Conversion Efficiency of Gold Nanotriangle and Nanorod in Laser Induced Thermal Therapy. Nanomaterials 2017, 7, 416. https://doi.org/10.3390/nano7120416
Chen Q, Chen Q, Qi H, Ruan L, Ren Y. Experimental Comparison of Photothermal Conversion Efficiency of Gold Nanotriangle and Nanorod in Laser Induced Thermal Therapy. Nanomaterials. 2017; 7(12):416. https://doi.org/10.3390/nano7120416
Chicago/Turabian StyleChen, Qin, Qin Chen, Hong Qi, Liming Ruan, and Yatao Ren. 2017. "Experimental Comparison of Photothermal Conversion Efficiency of Gold Nanotriangle and Nanorod in Laser Induced Thermal Therapy" Nanomaterials 7, no. 12: 416. https://doi.org/10.3390/nano7120416